ldentification of genes involved in regulating MnSOD2 production and root colonization in Bacillus cereus 905
2021-05-23GAOTantanDlNGMingzhengLlYanZENGQingchaoWANGQi
GAO Tan-tan,DlNG Ming-zheng,Ll Yan,ZENG Qing-chao,WANG Qi
1 Key Laboratory for Northern Urban Agriculture of Ministry of Agriculture and Rural Affairs,Beijing University of Agriculture,Beijing 102206,P.R.China
2 Department of Plant Pathology,College of Plant Protection,China Agricultural University,Beijing 100193,P.R.China
Abstract sodA2-encoding manganese-containing superoxide dismutase (MnSOD2) in Bacillus cereus 905 plays an essential role in antioxidative stress,nutrition utilization,rhizosphere and phyllosphere colonization. However,the genes involved in regulating the sodA2 expression have not been clearly elucidated in B.cereus. In this study,a genome-wide random insertion mutagenesis was constructed by using transposon TnYLB-1 to identify novel genes regulating the sodA2 expression. Seven mutants that changed the sodA2 expression at both mRNA and protein levels were finally obtained. Sequence analysis and BLAST data showed that the genes disrupted by TnYLB-1 in B.cereus 905 shared high conservations with those in the B.cereus type strain,ATCC 14579. These genes encode heat-inducible transcription repressor,chloride channel protein,recombinase A,ferrous iron transport protein,nucleoside diphosphate kinase,and histidine ammonia-lyase. Besides,we also provided the evidence that the genes regulating the sodA2 expression could influence colonization ability of B.cereus 905 on wheat roots. Specifically,those genes downregulating the sodA2 expression significantly reduced bacterial colonization on wheat roots,and mutants with increased MnSOD2 activities could enhance bacterial population densities on wheat roots to a certain degree. Our work provided information that multiple genes are involved in MnSOD2 production and wheat root colonization. The molecular regulatory pathways through which these genes modulate the sodA2 expression and root colonization need to be investigated extensively in the future.
Keywords:Bacillus cereus,sodA2,TnYLB-1 transposon,colonization
1.lntroduction
In the aerobic bacteria,superoxide dismutases (SODs)have been demonstrated to be the key enzymes defending against oxidative stresses by specifically catalyzing the dismutation of superoxide anions () to form hydrogen peroxide (H2O2) and molecular oxygen (O2),and thus scavengingspecies (Fridovich 1995;Miller 2012).SODs,metalloenzymes that contain specific mental cofactors at the catalytic center,are classified into SOD with Mn2+or Fe2+(MnSOD/FeSOD) and SOD with Cu2+plus Zn2+(Cu/ZnSOD) (Fridovich 1995,2013;Wanget al.2011). All three SODs have been identified inEscherichia coliand determined to function at different locations in cells. With few exceptions,both FeSOD and MnSOD are present in cytoplasmic compartments,while Cu/ZnSOD is largely localized in the periplasmic space (Beyeret al.1991;Benovet al.1995;Fridovich 1995;Wanget al.2011;Miller 2012). ThesodC-encoding Cu/ZnSOD is expressed at a low level in bacteria but is particularly important for eliminating exogenous superoxides (Benovet al.1995;Keyer and Imlay 1996;Miller 2012).sodB,which encodes FeSOD,is constitutively expressed and therefore thought to be a house-keeping gene (Cortezet al.1998;Fridovich 2013). The expression of MnSOD,encoded by thesodAgene,fluctuates in response to the internallevels throughout the whole growth phases (Wanget al.2007,2011). Many reports illustrated that SODs play important roles during multiple physiological processes in bacteria.For example,SodA and SodM promote the survival ofStaphylococcus aureusduring oxidative stressful environment and contribute toS.aureusvirulence (Treffonet al.2020). Thesodgene is involved in biofilm formation and regulation of expression of other stress-related genes,such assigBandrecA,inListeria monocytogenes(Suoet al.2014;Huanget al.2018). Exogenous Mn2+increases the activity of MnSOD ofB.thuringiensisand the enhanced MnSOD production is related to oxidative stress (Suet al.2017). Thakuret al.(2018) reported that the purified thermostable Fe/Mn SOD fromB.licheniformisshowed an antioxidant potential and could promote cell viability by 30%. The produced MnSOD fromB.amyloliquefaciensprotects cells against oxidative stresses (Kanget al.2018).SODs also play important roles during plant-microbe interactions,especially for bacterial colonization of plants.This is because many environmental signals,such as acid,oxygen,transition from anaerobiosis to aerobiosis,heat/cold shock,interferon-γ and membrane-binding drugs(chlorpromazine or procaine),could trigger induction of thesodAgene expression (Gregory and Firdovic 1973;Zhang and Yonei 1991;Kargalioglu and Imlay 1994;Kimet al.2005;Chenet al.2009). It was reported that,whenB.subtilisstrains that have good biocontrol efficacy were sprayed,SOD associated with disease resistance characteristics was induced in rice plants affected byMagnaporthe oryzae(Shaet al.2016).
Genetic analyses have been key to unraveling the biology of microorganisms,with transposon mutagenesis being a powerful tool in these analyses. Transposons can generate insertion mutations that are readily mapped and,depending on the particular transposon used,can also create reporter gene fusions at the site of their insertion(Le Bretonet al.2006). The commonly used TnYLB-1 transposon is contained in anE.coli/Bacillusshuttle vector pMarA,which also carries a hyperactive allele of themariner-Himar1transposase driven from a promoter recognized by σAcontaining RNA polymerase (Le Bretonet al.2006). In recent reports,the TnYLB-1 transposon mutant libraries were constructed inB.subtilisandB.amyloliquefaciensto screen genes related to biocontrol function (Fanet al.2016;Sunet al.2018,2019).
Bacillus cereus905,a plant growth-promoting rhizobacterium (PGPR) isolated from soil,could colonize wheat rhizosphere with a large population size,exhibiting an effective function in enhancing plant growth and development in greenhouse (Wanget al.2007;Gaoet al.2019). Two main SODs existing inB.cereus905 are MnSODs,termed MnSOD1 and MnSOD2,which are encoded bysodA1andsodA2,respectively (Wanget al.2007,2011).sodA1is constitutively expressed,whereas the expression ofsodA2is induced notably by environmental factors,and MnSOD2 plays a very important role in physiological processes ofB.cereus905,especially in oxidative stresses resistance,carbon source metabolism,and colonization of rhizosphere and phyllosphere (Wanget al.2007,2009;Gaoet al.2017,2019). Sufficient rhizosphere and/or plant colonization is an important step required for exhibiting beneficial effects of biocontrol agents (Lugtenberg and Kamilova 2009;Compantet al.2010). Thus,the SOD production is tightly related to the colonization ability and thus biocontrol effects ofB.cereus905 (Wanget al.2007,2009;Gaoet al.2019). In this work,we aimed to identify the genes that regulate thesodA2gene expression in theB.cereus905 strain. Therefore,we constructed a genome-wide random insertion mutagenesis in 905 by using a TnYLB-1-based transposon system. We have screened seven previously uncharacterized genes controlling thesodA2expression and wheat root colonization for the first time. Our results showed that many genes could regulate the MnSOD production and wheat root colonization ofB.cereus905. We provided evidence in the regulatory mechanisms of thesodA2gene expression and wheat root colonization in theB.cereusstrains,formulated a research direction to study the colonization mechanisms in the future,and proposed a potential effective strategy to enhance the biocontrol efficacy of plant growth-promoting rhizobacteria(PGPR) strains.
2.Materials and methods
2.1.Bacterial strains,plasmids,and growth conditions
The bacterial strains and plasmids used in this study are summarized in Table 1.Bacillus cereus905 was used for producing a library of random insertion mutants by the use of transposon TnYLB-1 (Le Bretonet al.2006). For general purpose,B.cereus905 and its derivatives were routinely cultured in Luria-Bertani (LB) medium (Sambrook and Russell 2001) or on solid LB medium supplemented with 1.5% (w/v) agar at 37°C. The medium used for flow cytometry analysis was M9 medium (Sambrook and Russell 2001). Just before use,0.2% glucose (w/v) was added as sole carbon source.Escherichia coliDH5α (Table 1;purchased from TransGen Biotech Co.Ltd.,Beijing,China),growing in LB medium at 37°C,was used as a host for constructing plasmids and molecular cloning. Primers used in this study are listed in Table 2. When required,antibiotics were supplemented at the following concentrations:5 μg mL–1of erythromycin (Erm),10 μg mL–1of tetracycline(Tet),and 50 μg mL–1of kanamycin (Kan) for the growth ofB.cereusstrains,100 μg mL–1of ampicillin (Amp) for the growth ofE.colicells. All antibiotics were purchased from Sigma (USA).
2.2.Construction of transposon random insertion mutant library
Transposition of TnYLB-1 intoB.cereus905 genome was performed using the thermo-sensitive plasmid pMarA as a delivery vector (Le Bretonet al.2006). The reporter plasmid pSodA2,carrying the fusion of PsodA2-gfpwhere the expression ofgfpwas controlled by the promoter of thesodA2gene,was introduced intoB.cereus905 cells by electroporation (1.2 kV,MicroPulser Electroporator,Bio-Rad,USA).This generated asodA2expression reporter strain that was applied for transposon mutagenesis. Briefly,about 500 ng of pMarA was transformed into the reporterstrain by electroporation (1.2 kV),followed by a selection for KanRand ErmRat 30°C on LB agar plates supplemented with Kan and Erm. The antibiotics-resistant transformants were inoculated into LB medium and grown at 37°C with shaking at 220 r min–1for 12 h. Cells were diluted and then spread on LB agar plates containing 50 μg mL–1of Kan,followedby incubation at 46°C (non-permissive temperature for replication of the pMarA plasmid in Gram-positive strains)for 10 h to select for colonies containing a transposon insertion. Subsequently,the colonies were verified to be KanRand ErmS.
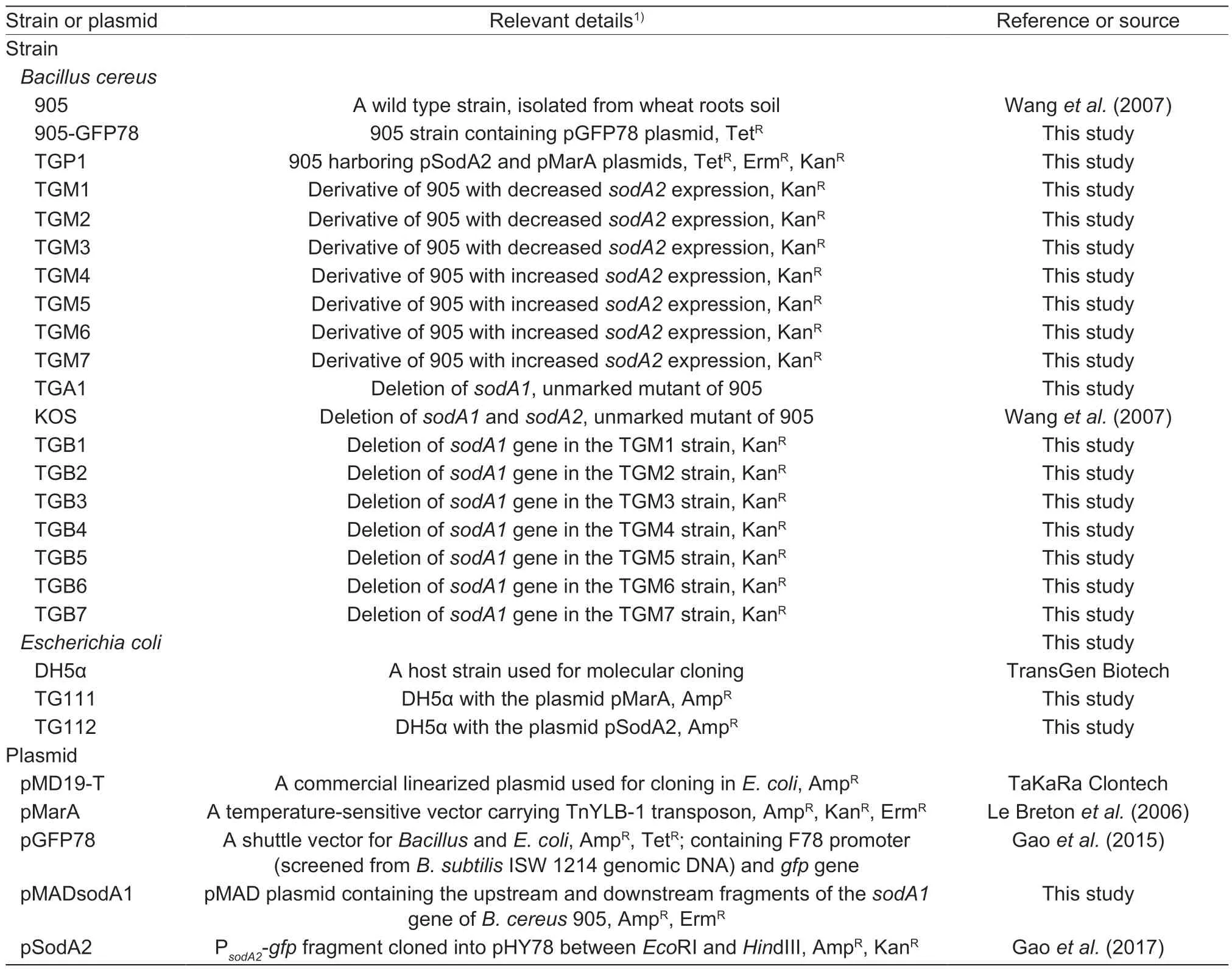
Table 1 Bacterial strains and plasmids used in this study
2.3.Determination of the sodA2 gene expression by flow cytometry analysis
The flow cytometry analysis was carried out to screen cells with altered expression ofsodA2following the protocol published previously (Penget al.2006;Wanget al.2009;Gaoet al.2017). The transcriptional activity of thesodA2gene promoter in each transposon insertion mutant was monitored by a FACS caliber flow cytometer (BD Biosciences,San Jose,CA). Briefly,bacterial cells were grown in LB medium containing Kan at 37°C overnight. The cells were diluted as 1:1 000 into fresh M9 medium (a lownutrient medium for induction of MnSOD2 expression) and were grown to the exponential phase (the optical density at 600 nm (OD600) of 0.5). The samples were harvested and washed once with 1× phosphate-buffered saline (PBS)at 4 000 r min–1for 5 min,and resuspended in PBS to the OD600of 0.1. The bacterial cells were then run through the FACS caliber flow cytometer. Samples were subjected to flow cytometry until 20 000 fluorescent particles had been examined. The flow cytometry results were analyzed by Cell Quest software (BD Biosciences,San Jose,CA). All the experiments were repeated in triplicate.
2.4.ldentification of transposon insertion sites
The mapping of transposon insertion sites was performed as described previously (Le Bretonet al.2006). Briefly,10 μg of genomic DNA of TnYLB-1 insertional mutants was extracted using the published method of Gaoet al.(2017),digested withTaqI,and circularized in a ligation reaction using the T4 DNA ligase (TaKaRa,Beijing,China) at a DNA concentration of 5 ng μL–1. Ligation products were purified using E.Z.N.A.TMCycle-Pure Kit (OMEGA Bio-Tek,Beijing,China) and resuspended in double-distilled water at 10 ng μL–1. Inverse PCR (IPCR) was performed on 150 ng of ligated DNA with theEx TaqDNA polymerase (TaKaRa,Beijing,China) using the primers oIPCT1 and oIPCR2 (Table 2),which face outward from the transposon sequence. The PCR products were then cloned into pMD19-T plasmid (TaKaRa,Beijing,China) after purification. And the generating plasmids were sequenced using the common primers for pMD19-T.
For Southern blot analysis,5 μg of genomic DNA of transposon insertion mutant cells were extracted and digested withEcoRI,electrophoresed on 0.8% agarose gels,and then transferred to Hybond-N+membrane(Amersham Biosciences,Amersham,UK). A Kan fragment(389-bp) amplified from the TnYLB-1 transposon element was labelled with a Random Prime DNA Labelling System(DIG High Prime DNA Labelling and Detection Starter Kit II,Roche,Mannheim,Germany) and used as the hybridization probe. The Southern blot was then performed following the manufacturer’s instructions of DIG High Prime DNA Labeling and Detection Starter Kit II using Hybond-N+membranes and DIG-labelled DNA probe.
Table 2 Oligonucleotide primers used in this study
2.5.DNA sequencing and nucleotide sequence analysis
Plasmids or fragments were sequenced by the TSINGKE Biological Technology Firm (Beijing,China). The nucleotide BLAST search of theB.cereus905 genome (the wholegenome shotgun project of strain 905 has been deposited in DDBJ/ENA/GenBank under the accession number LSTW00000000) (Dinget al.2016) was conducted to identify the target genes. To identify homologs of known TnYLB-1 inserted genes ofB.cereus905,the BLASTX Program (http://www.ncbi.nlm.nih.gov/BLAST/) was used for database analysis inB.cereusATCC 14579 (NCBI accession no.NC_004722.1). The percentage of theamino acid sequence identity in the corresponding proteins was calculated by the DNAMAN Software.
2.6.Construction of sodA1 deletion mutant
To construct thesodA1deletion mutant in the wild-type (WT)and each TnYLB-1 insertional mutant,the pMADsodA1 plasmid containing the upstream and downstream fragments of thesodA1gene was introduced into the WT and TGM1–7,respectively,by electroporation (0.1 cm cuvette,1.8 kV,MicroPulser Electroporator,Bio-Rad,USA). ThesodA1deletion mutant was obtained through double crossover recombination (Gaoet al.2017). The transformants obtained on LB agar plates were confirmed by PCR amplification and DNA sequencing that verified the deletion of thesodA1gene.
2.7.qRT-PCR
Quantitative real-time RT-PCR (qRT-PCR),performed according to the previously published method (Gaoet al.2017),compared thesodA2transcripts of the WT and transposon insertional mutants. Specifically,total RNA from bacterial cells of the exponential growth phase (OD600=0.8)was extracted usingTransZol(TransGen Biotech,Beijing,China) reagent method.cDNA was synthesized using theTransScriptAll-in-One First-Stand cDNA Synthesis SuperMix for qPCR (TransGen Biotech,Beijing,China). cDNA levels of thesodA2gene in different strains were quantified by qRT-PCR using SYBR PremixEx Taq(TaKaRa,Beijing,China). qRT-PCR data were analyzed with therpoAgene ofB.cereus905 being an endogenous control for data analysis (Salvettiet al.2011;Gaoet al.2017).
2.8.Assays of SOD activities
Bacterial cells of the exponential growth phase (OD600=1.0)were pelleted and washed with 1× PBS (pH 7.0) for three times,followed by a disruption using sonication (200 W,run 3 s,pause 5 s,10 min) (JY88-IIN,Ningbo,China).After centrifugation (20 000×g,4°C,30 min),supernatant was used to measure SOD activities following the protocol published previously (Wanget al.2007;Gaoet al.2017).One unit of activity was calculated as the quantity of proteins necessary to inhibit the rate of superoxide-dependent reduction of cytochromecby 50% (Wanget al.2007).
2.9.Assays of wheat root colonization
The stains of WT with a Tet resistant plasmid pGFP78(Table 1) and the TnYLB-1 insertional mutants were used for evaluating colonization ability on wheat roots.Specifically,wheat seeds (Triticum aestivum90-3) were surface sterilized,washed thoroughly with sterile water,and put into an incubator for germination at 24°C for 48 h with 80% humidity. The bacterial cells in LB broth (OD600=1.0)were harvested and resuspended in PBS (pH=7.0) to an OD600=0.8 (~108CFU mL–1). The germinated seeds were soaked into the above bacterial suspension for 2 h. Then the seeds were sown into the plastic pots (6 seeds per pot),which were filled with autoclaved substrates consisting of commercial nutrition soil and peat moss (2:1,v/v),in a growth chamber with the growth condition of 22°C,14 h/10 h(day/night),and 60% humidity. Seedlings were collected at 7,14,21,and 28 days post-incubation from each group and the roots were excised for the determination of bacterial amount. The exact experimental procedures were the same as those of Gaoet al.(2019). The colonization assay was repeated independently three times.
3.Results
3.1.Production,isolation and characterization of mutants with altered sodA2 expression
ThesodA2-encoding MnSOD2 is essential for resistance to oxidative stress,carbon resource utilization,and colonization of rhizosphere and phyllosphere inB.cereus905 (Wanget al.2007,2009;Gaoet al.2017). With the aim of identifying the genes that alter thesodA2expression inB.cereus,we constructed a random insertional library of strain 905 (WT) by using the plasmid pMarA carrying a TnYLB-1 element (Le Bretonet al.2006). Using the WT as the baseline control,green fluorescent protein (GFP)was determined on gated populations of bacterial cells by flow cytometry. Finally,26 mutants producing significantly different GFP intensity from those of the WT were screened out from approximately 2 000 KanRand ErmScolonies. It has been shown that the growth of ΔsodA2in LB medium without oxidative stress was similar to that of the WT strain under the same condition (Wanget al.2007). In this study,we aimed to screen the genes which could regulate thesodA2expression without the effects of growth defects;thus 10 mutants showing significant growth defects (prolonged lag phase) in liquid LB medium under normal growth conditions were not analyzed further. Among the remaining 16 mutants,some strains subsequently demonstrated to contain identical transposon insertions and therefore were likely to be siblings.Some strains contained two or three transposon insertions in the Southern blot analysis (data not shown). Leaving these strains aside,our mutant collection consisted of seven strains.These mutants showed overlapping growth curves (data not shown),but remarkably different GFP intensity with the WT(Fig.1). The results showed that thesodA2expression was reduced to 53.7,62.6,and 15.1% in the mutants of TGM1,TGM2,and TGM3,respectively,compared with the WT;while in the TGM4,TGM5,TGM6,and TGM7 strains,thesodA2expression was increased to 2.48-,2.02-,5.72-,and 3.12-fold of that in the WT,respectively (Fig.1).
In parallel,to confirm the regulation ofsodA2expression in these seven TnYLB-1 insertional mutants,thesodA2transcripts in the WT and mutants were compared by qRT-PCR. To exclude the effect of thesodA1gene,we introduced a deletion of thesodA1gene to WT and the TnYLB-1 insertional mutants of TGM1–7,generating strains of TGA1 and TGB1–7,respectively. In the TGB1,TGB2,and TGB3 strains,the amount ofsodA2mRNAs was 0.51-,0.58-,and 0.23-fold of that of TGA1,respectively;whereas,the amount ofsodA2mRNAs of TGB4,TGB5,TGB6,and TGB7 was increased to 2.27-,2.17-,5.33-,and 3.46-fold of that of TGA1 (Fig.2). The results were consistent with our previous findings on GFP intensity changes (Fig.1),suggesting that the genes disrupted by TnYLB-1 could change the transcription of thesodA2gene.
Fig.1 Expression of the sodA2 gene in the wild-type (WT)and the TnYLB-1 insertional mutants of Bacillus cereus 905.The sodA2 promoter activities of bacterial cells grown to OD600 of 0.5 were measured. Mean fluorescence intensity (MFI) of green fluorescent protein (GFP) expressed under the sodA2 promoter was determined for gated populations of bacterial cells by flow cytometry (FACS). Asterisks indicate statistically significant differences in GFP MFI between the WT and each transposon insertional mutant (P<0.05),Student’s t-test. The error bars represent standard error of the mean value (n=3).
Next,we compared total SOD activities from the WT and each mutant to determine whether thesodA2expression at the translational level was regulated or not. The results showed that MnSODs accounted for 95% of the total SODs inB.cereus905,as ΔsodA1sodA2had much lower SOD activities (1.2 U mg–1) than the WT (23.9 U mg–1). We also deleted thesodA1gene in the WT and each TnYLB-1 insertional mutant to exclude the MnSOD1 activity. The SOD activity in each mutant was dramatically different from that in the ΔsodA1strain (Fig.3). TGB1,TGB2,and TGB3 showed decreased SOD activity,at 9.8,11.1,and 6.8 U mg–1,respectively;while TGB4,TGB5,TGB6,and TGB7 displayed enhanced SOD activity,at 39.1,33.0,84.2,and 39.0 U mg–1,respectively (Fig.3). Among these strains,TGB3 and TGB6 had the lowest and highest SOD activity,about 0.3-and 4.4-fold of that in ΔsodA1(19.1 U mg–1),respectively (Fig.3).It can be speculated that the altered SOD activities in the mutants are MnSOD2,because transcriptions of thesodA2gene were specifically regulated (Fig.2).The above results confirmed that the MnSOD2 production was indeed changed by the TnYLB-1 insertion.
Fig.2 Determination of regulation of the sodA2 by TnYLB-1 insertion in each mutant by qRT-PCR. Relative mRNA levels of sodA2 in the ΔsodA1 and each TnYLB-1 insertional mutant containing a deletion of the sodA1 gene. The rpoA gene was used as an endogenous control for data analysis.Asterisks indicate statistically significant differences in the mRNA level of the mutants compared with the ΔsodA1 (P<0.05),Student’s t-test. The error bars represent standard error of the mean value (n=3).
Fig.3 Activities of superoxide dismutase (SOD) in the wildtype (WT),ΔsodA1,ΔsodA1sodA2 and various insertional mutants with TnYLB-1 containing a deletion of the sodA1 gene(TGB1–7) of Bacillus cereus 905. Different bacterial cells grown to exponential growth phase (OD600 =1.0) were harvested,and disrupted by sonication,followed by centrifugation. The SOD activities in the supernatant were determined with the standard SOD assay. Asterisks indicate statistically significant differences in SOD activities of the mutants compared with the WT (P<0.05),Student’s t-test. The error bars represent standard error of the mean value (n=3).
3.2.ldentification of genes disrupted in mutants with changed sodA2 expression
As noEcoRI restriction site is present within the transposon TnYLB-1 itself,the sevensodA2expression-altered mutants were analyzed for the transposon presence by Southern hybridization of chromosomal DNA digested withEcoRI using 389-bp PCR fragment containing theKangene of TnYLB-1 as a probe. The results showed that each of the seven mutants had a single reactive band that was different in size (Fig.4),indicating that each mutant contained a unique and different genomic insertion. SinceTaqI does not cut the TnYLB-1 transposon,the genomic DNA was extracted from each of the mutants and subject to digestion withTaqI. The digested DNA fragment flanking the TnYLB-1 was cloned into pMD19-T (TaKaRa,Beijing,China) and transformed toE.coliDH5α. The recombinant plasmids were used as templates to determine the nucleotide sequence of the chromosomal DNA adjacent to the TnYLB-1 insertion site. TheHimar1transposase of themarinertransposon excises the transposable element TnYLB-1 at the inverted terminal repeat (ITR),and a“TA”dinucleotide insertion site in the target DNA serves as a tag to identify the disrupted gene (Le Bretonet al.2006).
The sequences obtained from each mutant were submitted to theB.cereus905 genomic DNA sequence database to search for the corresponding genes inB.cereus905. Then we used the same nucleotide sequences of the genes interrupted by TnYLB-1 inB.cereus905 to identify the closest homologs in theB.cereustype strain ATCC 14579. The search results revealed that the genes disrupted by TnYLB-1 were highly conserved among the strains belonging toB.cereusgroup,with the nucleotide identities all above 99% (Table 3),implying that homologs of these genes may also regulate thesodA2expression in other strains ofB.cereus,likeB.cereusATCC 14579.
Through mapping the mutants,expression of thesodA2gene was reduced by TnYLB-1 located withinBC4314(hrcA,heat-inducible transcription repressor),BC5407(clc,chloride channel protein) andBC3779(recA,recombinase A) in the strain of TGM1,TGM2 and TGM3,respectively(Table 3;Fig.5). The remaining 4 TnYLB-1 insertional mutants with increasedsodA2expression were those with disruptedBC0707(feoB2,ferrous iron transport protein),BC1515(ndk,nucleoside diphosphate kinase),BC3652(hutH,histidine ammonia-lyase),andBC0708(feoB1,ferrous iron transport protein) in the strain of TGM4,TGM5,TGM6,and TGM7,respectively (Table 3;Fig.5).
3.3.Different root colonization ability of TnYLB-1 insertional mutants
Fig.4 Southern blot analyses of the TnYLB-1 insertional sites in seven mutants of Bacillus cereus 905. The TnYLB-1 insertion sites were determined by Southern blot using the DIG-labeled probe,with WT as the negative control.
Table 3 Determination of the transposon insertional sites that alter the sodA2 gene expression
In our study,it has been shown that the mutants with a TnYLB-1 insertion affect thesodA2expression on both mRNA and protein levels (Figs.2 and 3). Since MnSOD2 inB.cereus905 plays an indispensable role in environmental stress resistance and colonization and also contributes to biofilm formation and bacterial mobility (Wanget al.2007;Gaoet al.2019),we sought to determine the root colonization ability of the mutants. Here,we chose the WT (905-GFP78) and seven TnYLB-1 insertional mutants,3 producing the reduced MnSOD2 (TGM1,TnΩHrcA;TGM2,TnΩclc;and TGM3,TnΩrecA) and 4 generating the increased MnSOD2 (TGM4,TnΩfeoB1;TGM5,TnΩndk;TGM6,TnΩhutH;and TGM7,TnΩfeoB2),as the target strains for analyzing their colonization competence.The results are shown in Table 4,compared with the WT strain,therecAmutant,which produced the lowest MnSOD,showed about a 100-fold reduction in wheat root colonization during all growth phases,and thehrcAandclcmutants with half of the MnSOD activities displayed 10–20-fold decrease in wheat root colonization (Table 4).As predicted,thehutHmutant with increased MnSOD2(6-fold of the WT) exhibited an enhanced colonization ability as the bacterial population density of TGM6 was 2-to 5-fold of that of the WT strain (Table 4). ThefeoB1,feoB2,andndkmutants,which harbored 2–3-fold MnSOD of that in the WT,showed similar bacterial population densities to that of the WT in the early growth stage;however,the colonization ability increased to~2-fold of that of the WT at the end of the growth stage (Table 4). Our results indicated that the genes that regulated expression of thesodA2gene also played important roles in wheat root colonization ofB.cereus905.
Table 4 Root colonization of Bacillus cereus wild-type (905-GFP78) and its 7 TnYLB-1 insertional mutants (TGM1–7)
4.Discussion
4.1.Regulation of sod gene expression in bacteria
Regulation of thesodgene expression in some Gramnegative bacteria,especiallyE.coli,has been clearly elucidated. The oxidative stress-responsive transcription factors,SoxRS,OxyR and Fur,are the key regulators of SODs production. SoxR,a member of the MerR family of transcriptional regulators,plays a central role in sensing and regulating gene expression in response to superoxide and nitrosative stresses (Fee 1991;Pomposiello and Demple 2001). Superoxide anions or reactive nitrogen molecules can oxidize SoxR,which in turn activatessoxS,an AraC-type transcriptional regulator (Reizeret al.1988).The oxidized SoxS can activate expression of at least 15 genes in the SoxRS regulon,including thesodAgene(Bruce 1991;Zhenget al.1999;Pomposiello and Demple 2001;Eiamphungpornet al.2006). The redox-sensing transcription activator OxyR is a tetrameric DNA-binding protein that is activated under oxidative stresses by forming a disulphide bond between two Cys residues (Greenberg and Demple 1989).Thesodgenes are positively regulated by SoxRS or OxyR in several bacteria,such asE.coli,Porphyromonas gingivalisandAgrobacterium tumefaciens(Touati 1988,1989;Zhenget al.1999;Eiamphungpornet al.2006;Wuet al.2008). Specifically,upon exposure to superoxide stress,the oxidized SoxRS or OxyR binds to the specific region located within thesodpromoter,activating the transcription of thesodgene. The third regulator for SOD expression inE.coliis Fur (ferric uptake regulation)protein,which could be activated by SoxRS and OxyR(Zhenget al.1999). Fur negatively regulates transcription of thesodAgene in the classical manner of Fe-dependent repression,through binding to the iron box located in the untranslated 5´-region of thesodAgene,while positively regulatessodBexpression at both transcriptional and post-transcriptional levels not in a classical Fe2+-dependent manner (Niederhofferet al.1990;Hassan and Sun 1992).In Gram-positive bacteria,studies on the regulation ofsodexpression are relatively less. The alternative sigma factor σB,encoded by thesigBgene,is involved in regulating the expression ofsodinL.monocytogenes. It was reported that SigB regulated thesodgene expression inL.monocytogenesunder acid stress and oxidative stress (van der Veen and Abee 2010b). Suoet al.(2014) elucidated that expression of thesodgene was relevant to the stress-relatedrecAgene. Besides,it was also proved that transcription level of thesodgene was significantly down-regulated during the biofilm state compared with that of the planktonic cell state in some strains ofL.monocytogenes(Huanget al.2018).InS.aureus,SOD was found to be important in starvation survival,stress response and pathogenesis,and the levels ofsodAandsodMtranscription were markedly enhanced in thesarAmutant (Clementset al.1999;Ballal and Manna 2009;Shrihari and Singh 2012). The PerR-regulated manganese ion uptake activates MnSOD and protectsStreptococcus oligofermentansfrom(Wanget al.2014). The regulation of active MnSOD production inBacillusspp.,however,remains unclear. One report proved that MnSOD is posttranslationally regulated by the manganesein vivoandin vitroinB.subtilis(Inaokaet al.1999). Induction of thesodAgene expression under salt stress (4% NaCl) inB.subtiliswas σBdependent (Petersohnet al.2001). Although mostB.subtilisandB.cereusstrains possess Fur and Fur homologs,it is not clear whether and how Fur is involved insodAregulation (Bsatet al.1998;Baichooet al.2002;Harvieet al.2005).Bacillus cereus0-9 strain has 4 SODs,but the regulatory pathways were unknown (Zhanget al.2020). In this study,we identified that seven new genes,BC4314(hrcA),BC5407(clc),BC3779(recA),BC0707(feoB1),BC0708(feoB2),BC1515(ndk),andBC3652(hutH),could regulate thesodA2gene expression inB.cereus905.
4.2.BC4314,a gene encoding heat-inducible transcription repressor
The mutant TGM1 contains a TnYLB-1 insertion located within theBC4314(hrcA) homologous gene,which encodes a heat-inducible transcription repressor (NP_834026.1) in bacteria (Fig.5-A;Table 3). HrcA,containing a typical helixturn-helix (HTH) motif,is a DNA-binding protein (Hitomiet al.2003). In Gram-positive bacteria,such asB.subtilisandS.pneumoniae,HrcA directly represses Class I heat shock genes by binding to an operator-element named CIRCE.This precedes the heptacistronicdnaKand the bicistronicgroEoperon (Reischlet al.2002;Kimet al.2008),encoding for the heat-shock proteins DnaK and GroEL,respectively.HrcA is indirectly involved in up-regulation of 36 genes inL.monocytogenes,including those encoding proteins with functions in virulence and stress response (relAandperR,as well as the PerR-regulatedfurandtrxB),acid stress resistance (gadCandgadB),heat stress (clpX),cold stress resistance (cspD),and proteins with unknown functions.It is also important for static and continuous-flow biofilm formation and disinfectant resistance (Huet al.2007;van der Veen and Abee 2010a). ThesodA2inB.cereus905 is greatly induced in response to a variety of environmental stress conditions including exposure to oxygen,TiO2,redox cycling compounds such as paraquat which exacerbates the level of intracellular superoxide radicals,iron chelation (i.e.,iron deprivation),and oxidants (Wanget al.2007,2009).The previous observations provide a hint that HrcA is tightly connected to environmental stress resistance,implying that HrcA may indirectly up-regulate thesodA2expression through regulating some regulators that could bind to thesodA2promoter.In this regard,MnSOD2 plays a major role in oxidative stress resistance inB.cereus905.
4.3.BC0707 and BC0708,genes coding for ferrous iron transport protein B
The genes interrupted by TnYLB-1 in mutants TGM4 and TGM7 both encoding proteins showed high sequence similarity (99 and 100%) in ferrous transport protein B ofB.cereusATCC 14579 (Fig.5-D;Table 3). The Feo (Ferrous iron transport) system is the major route for bacteria to uptake ferrous iron from environment. Thefeooperon ofE.coliandVibrio choleraeconsists of 3 genes,feoA,feoBandfeoC,encoding the FeoA,FeoB,and FeoC proteins,respectively. The components of FeoB can simultaneously associate with both FeoA and FeoC to form a large complex,working together for transporter activities (Cartronet al.2006;Weaveret al.2013;Stevensonet al.2016). The Feo systems also have been identified in the genomes of various Gram-positive bacteria,with different organizations for thefeooperon (Lauet al.2016). In theB.cereus905 strain,thefeoloci are made up of onefeoAand twofeoBgenes (Fig 5-D).
The key regulatory protein Fur (ferric uptake regulator)senses and regulates cytoplasmic iron concentrations inE.coliand many other bacteria (Hantke 1981;Bagg and Neilands 1987;Jeonet al.2008;Lauet al.2016).Overexpression or induction of FeoB,a ferrous iron transporter,enables bacterial cells to import more external Fe2+,which elevates the levels of Fe2+-Fur complex and consequently binds to the Fur box of target genes and enhances this Fur-dependent repression (Jeonet al.2008;Lauet al.2016). Fur is inactivated by the release of Fe2+and the genes under Fur control are induced,such as the MnSOD-encoding genesodAofE.colithat is repressed by Fur (Niederhofferet al.1990). The Fur homologues have been identified inB.subtilis(Bsatet al.1998). In our study,it is assumed that disruption of thefoeBgenes leads to the inactivation of Fur inB.cereus905,and the expression ofsodA2is therefore induced greatly (Figs.2 and 3).
4.4.BC5407,a gene putatively encoding chloride channel protein
The transposition TnΩA88 inactivated a gene that putatively encodes chloride channel protein (Table 3;Fig.5-B) and down-regulates thesodA2gene expression (Figs.2 and 3). Chloride channels (CLCs) belong to a superfamily of ion channels that permit passive passage of anions,mainly chloride,across the cell membrane (Jentschet al.2002;Duranet al.2010). To now,CLCs form a large gene family and have been found in bacteria,archaea,eukaryotes,nematodes,plants,and mammals (Hechenbergeret al.1996;Estevez and Jentsch 2002;Jentsch 2008;Zhouet al.2014).CLCs play a variety of important physiological roles in regulation of cytosolic pH,cell volume homeostasis,organic solute transport,cell migration,cell proliferation,and cell differentiation (Jentschet al.2002;Duranet al.2010;Zhouet al.2014). Bulk flow of chloride is important for cell volume regulation,and changes in intracellular Cl–1concentration may have a regulatory role in cells (Jentschet al.2002).BC5407(clc) gene encodes a putative chloride protein channel inB.cereus905. The TnYLB-1 insertion inactivated theclcgene and enhanced the MnSOD2 activity,which implies that CLCs play a role in regulating thesodA2gene expression through a regulatory pathway that deserves rigorous investigations in the future.
4.5.BC3779,a gene encoding recombinase A
Strain TGM3 harbors a TnYLB-1 insertion in the gene homologous withBC3779(recA) gene ofB.cereusATCC 14579,which encodes recombinase A (RecA) (Fig.5-C;Table 3). RecA is an important factor in DNA repair and furthermore the activator of the SOS response,which is an inducible pathway involved in DNA repair and restart of stalled replication forks (van der Veenet al.2010;van der Veen and Abee 2011). The SOS response is activated under certain stress conditions,such as mild heat exposure forL.monocytogenes(van der Veenet al.2007,2010).For pathogens ofL.monocytogenesandMycobacterium tuberculosis,RecA also plays an important role in the evaluation of antibiotic resistanceviastress-induced DNA repair mechanism. And duringB.subtilissporulation,RecA is active for protecting sporulating cells from DNA damage(Nautiyalet al.2014;Ramírez-Guadianaet al.2016;Ojha and Patil 2019). RecA is activated in continuous-flow biofilms which experience oxidative stress and radicalinduced DNA damage inL.monocytogenes(van der Veen and Abee 2011). Bacterial cells have developed complex antioxidant systems including genes which encode enzymes that scavenge reactive oxygen,such assod,and DNA repair genes that repair damaged DNA,such asrecA.WhenL.monocytogeneswas treated with paraquat to generate reactive oxygen species (ROS) in cells and lead to oxidative stress,the resistance generecAwas induced to be expressed (Suoet al.2014). Compared to the WT,recAwas not influenced in Δsodin the absence of paraquat. However,the transcription level ofrecAin Δsodwas induced after the treatment of 1 mmol L–1paraquat (Suoet al.2014). Our results showed that in low nutrient medium,the inactivation ofrecAresulted in a significantly lower expression of thesodA2gene,compared to the WT (Figs.2 and 3).It can be deduced from the previous reports and our data that there is a connection betweenrecAandsodA2,although the exact regulatory mechanisms need to be studied further.
4.6.BC1515,a gene predicted to encode nucleoside diphosphate kinase (Ndk)
The transposition TnΩB63 in strain TGM5 inactivated a gene that encodes nucleoside diphosphate kinase (Ndk),showing 99.78% similarity in amino acid sequence to that encoded byBC1515 gene fromB.cereusATCC 14579 (Fig.5-E;Table 3).Ndk is a pleiotropic effector playing a role in nucleotide metabolism,protein histidine phosphorylation,DNA cleavage/repair,and gene regulation,such as manipulating bacterial virulence,motility and adaptive responses (Yuet al.2017). In the process of bacteria-host interactions,Ndk regulates bacterial adaptation in hosts,including inhibition of phagocytosis,disruption of ROS generation,and regulation of cell survival and colonization in the host(Yuet al.2017). Besides,inBacillusspp.,Ndk is adapted to perform under stress conditions and can be co-expressed with MnSOD encoded by thesodAgene (Chenet al.2003;Misraet al.2009). In our study,disruption of thendkgene significantly increased thesodA2gene expression in both mRNA and protein levels (Figs.2 and 3). Since Ndk is a pleiotropic effector regulating gene expression and is also connected with stress response and ROS elimination,it can be supposed that such an enzyme is involved in regulating thesodA2gene expression in a particular mechanism.
4.7.BC3652,a gene encoding histidine ammonialyase
TGM6 contains a TnYLB-1 insertion located in theBC3652homologous gene (hutH) (Fig.5-F;Table 3),which encodes histidine ammonia-lyase (HAL,EC 4.3.1.3) inB.cereusATCC 14579. HAL exists in many organisms like bacteria,animals,fungi,and plants,and catalyzes the degradation ofL-histidine that forms urocanic acid and ammonia (Asanoet al.2009). HAL exerts optimal activity when a divalent cation (Mn2+,Fe2+,Zn2+,or Cd2+) is added (Klee 1972;Iwunze and Adeyemo 2005). HAL activity is stimulated 2-to 5-fold by MnCl2and is biologically important in the dismutation of superoxide radical () by Cu/ZnSOD (Taineret al.1983),implying that HAL may influence MnSOD production (Klee 1972). It is likely that disruption of the HAL-encoding gene could promote MnSOD to ligate more Mn2+,and hence increases MnSOD production.
4.8. MnSOD’s essential role in wheat root colonization of B.cereus
Root-associated bacteria,especaillyBacillusspp.strains,have received considerable attention in China.This is because effective application of the bacteria provides a safe and efficient alternative solution for managing crop diseases. Successful colonization of PGPR is considered as the first step for efficient biocontrol efficacy. Many studies have illustrated that theBacillusspp.strains have evolved diverse adaptations,such as biofilm formation,swarming motility,surfactin production,and production of metabolic enzymes,to establish sufficient colonization in the rhizosphere (Compantet al.2010;Chenet al.2013;Fanet al.2017). It has been demonstrated that SODs significantly contribute to the competitive root colonization and strong environmental persistence for bacteria (Antoine and Daniele 1986;Kimet al.2000;Santoset al.2000;Takahashiet al.2003;Wanget al.2007;Alquéreset al.2013). In the process of root colonization by PGPR,the toxic by-products of reactive oxygen species (ROS),such as H2O2and,are generated by PGPR itself and plant roots(Glick 1995;Wanget al.2007). In response to oxidative stresses,PGPR produce SODs as the first line of defense to eliminate,and thus successfully colonize plant roots and rhizosphere (Glick 1995;Kimet al.2000). InB.cereus905,thesodA2gene is crucial for wheat root colonization(Wanget al.2007,Gaoet al.2017). In our study,thehrcA,clc,andrecAmutants ofB.cereus905 with reduced SOD activities showed remarkable decreases in root colonization abilities,while thefeoB1,feoB2,hutH,andndkmutants ofB.cereus905 produced higher MnSOD than the WT and enhanced bacterial population densities on wheat roots to a certain degree (Table 4). Based on previous studies and our results,we proposed that the above seven genes influence the abilities ofB.cereus905 to colonize wheat roots at least partially due to thier regulatory roles in thesodA2gene expression. However,the detailed root colonization mechanisms ofB.cereus905 regulated by these seven genes need to be studied in depth. Nevertheless,we found novel genes regulating thesodA2gene expression and provided information for studying the root colonization by the beneficialB.cereusstrain in the future.
5.Conclusion
This study reported that seven genes involved in different physiological processes,hrcA,clc,recA,feoB1,feoB2,ndk,andhutH,could regulate thesodA2gene expression on both mRNA and protein levels in the PGPR strainB.cereus905 for the first time. Moreover,these genes also showed regulatory effects on the colonization ability on wheat roots. Our findings suggested that maybe there are multiple regulatory mechanisms on MnSOD production and wheat root colonization ofB.cereus905,and provided a potential genetic strategy for improving the biocontrol efficacy ofB.cereusstrains. In the future,our studies should emphasize on:(I) the mechanisms through which the seven genes regulate thesodA2gene expression,and the genetic intermediates that connect the seven genes and thesodA2gene;and (II) the mechanisms of wheat root colonization ofB.cereus905 regulated by the seven genes through resistance to environmental stresses,utilization of nutrition,biofilm formation,and bacterial motility.
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China (31701860) and the Program of Science and Technology of Beijing,China(Z191100004019025).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Low glycemic index:The next target for rice production in China?
- Effect of side deep placement of nitrogen on yield and nitrogen use efficiency of single season late japonica rice
- Advancements in plant regeneration and genetic transformation of grapevine (Vitis spp.)
- Indica rice restorer lines with large sink potential exhibit improved nutrient transportation to the panicle,which enhances both yield and nitrogen-use efficiency
- Effects of nitrogen management on the ratoon crop yield and head rice yield in South USA
- Response of grain-filling rate and grain quality of mid-season indica rice to nitrogen application
