Differences of aroma development and metabolic pathway gene expression between Kyoho and 87-1 grapes
2021-05-23
Research Institute of Pomology,Chinese Academy of Agricultural Sciences/Key Laboratory of Biology and Genetic Improvement of Horticultural Crops (Germplasm Resources Utilization),Ministry of Agriculture/Key Laboratory of Mineral Nutrition and Efficient Fertilization for Deciduous Fruits,Liaoning Province,Xingcheng 125100,P.R.China
Abstract Aroma is an important quality trait of grapes and often the focus of consumers,viticulturists and grapevine breeders. Kyoho is a hybrid between Vitis vinifera and Vitis labrusca with a strawberry-like scent,while 87-1 is an early-ripening mutant of Muscat hamburg,belonging to Vitis vinifera, with a rose scent. In this study,we compared their aroma compositions and concentrations during berry development by headspace-SPME combined with gas chromatography-mass spectrometry(GC-MS),and analyzed the expression differences of enzyme-encoding genes in the LOX-HPL,MEP and MVA metabolic pathways by qRT-PCR. Twelve esters were detected in Kyoho during the whole berry development and they were abundant after veraison,but no esters were detected in 87-1 berries. Linalool was the dominant terpene among the 14 terpenes detected in 87-1 berries,while limited amounts of terpenes were detected in Kyoho berries. qRT-PCR analysis indicated that the low expression of VvAAT might explain the low content of ester volatiles in 87-1 berries,and the low expression of coding genes in the MEP pathway,especially VvPNLinNer1,might be the reason for the low content of volatile terpenes in Kyoho berries. The results from this work will promote our understanding of aroma metabolic mechanisms of grapes,and offer some suggestions for grape aromatic quality improvement.
Keywords:grape,Kyoho,87-1,aroma,LOX-HPL,MEP,gene expression
1.Introduction
Aroma is an important factor contributing to grape quality. The stronger the aroma,the more popular the cultivars. Numerous studies have shown that grape aroma arises from a mixture of many volatile organic compounds,such as alcohols,aldehydes,esters,terpenoids,methoxypyrazines,furan derivatives,volatile sulfur compounds and phenylpropanoids (Vilanovaet al.2012;Gilet al.2013;Wanget al.2015;Aubert and Chalot 2018;Alemet al.2019). In aromatic varieties,esters and terpenoids play key roles in the charming sweet smell (Mateo and Jiménez 2000;Yanget al.2011;Wuet al.2016;Costaet al.2018). Evaluation of volatiles at the germplasm level indicated that terpenoids were abundant inVitisviniferawith a muscat aroma,while esters were dominant inVitis labruscaand its hybrids with eitherVitisviniferaorVitis amurensis(Yanget al.2009). This suggests that there may be some differences in the aroma biosynthesis pathways among grape varieties with different genetic backgrounds and distinct aromatic characteristics.
In plants,most alcohol,aldehyde and ester volatiles are biosynthesized through the lipoxygenase/hydroperoxide lyase (LOX-HPL) pathway (Wanget al.2001;Schwabet al.2008;Linet al.2019) (Fig.1). In this pathway,fatty acid desaturase (FAD) first catalyzes the production of unsaturated fatty acids,such as linoleic and linolenic acids,and then peroxidation of the polyunsaturated fatty acids by LOX produces the corresponding hydroperoxides. The hydroperoxides can be subsequently metabolized into various C6 and C9 aldehydes by hydroperoxide lyase(HPL). The aldehydes are further metabolized by alcohol dehydrogenase (ADH) to form alcohols. Lastly,alcohol acyl transferase (AAT),which belongs to the BAHD acyltransferase family,catalyzes the esterification of low molecular weight carboxylic acids and alcohols into esters.In grapes,the coding genes of FAD,LOX,HPL,ADH and AAT have been isolated and identified (Tesnière and Verriès 2000;Wang and Luca 2005;Podolyanet al.2010;Leeet al.2012;Zhuet al.2012). In the grape genome,they mostly occur in small multigene families. Among them,VvFAD2-1,VvFAD2-2,VvLOXA,VvLOXC,VvLOXD,VvLOXO,VvHPL1,VvHPL2,VvADH1,VvADH2andVvAATare involved in the synthesis of berry aroma (Xuet al.2015).
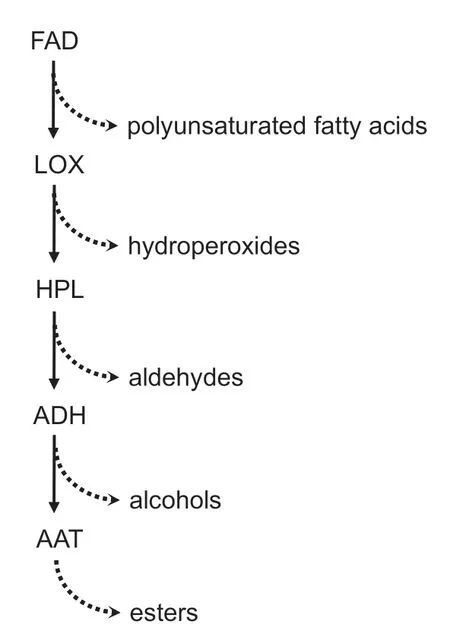
Fig.1 Schematic diagram of lipoxygenase/hydroperoxide lyase(LOX-HPL) pathway. The solid arrows indicate the stepwise catalysis of the enzymes in the metabolic pathway,while the dashed arrows indicate the metabolites formed. FAD,fatty acid desaturase;ADH,alcohol dehydrogenase;AAT,alcohol acyl transferase.
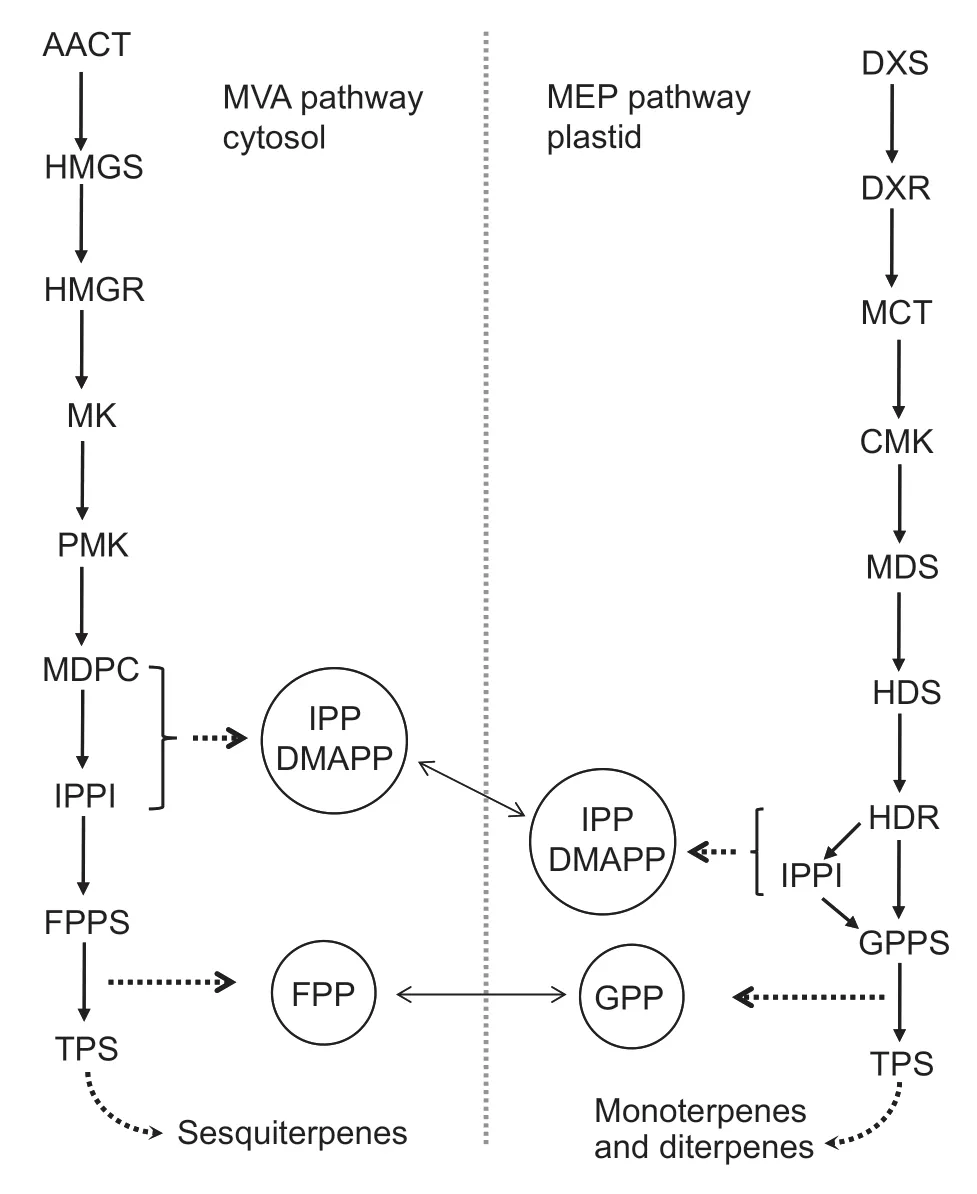
Fig.2 Schematic diagram of the methylerythritol phosphate(MEP) and mevalonic acid (MVA) metabolic pathway. Solid-line arrows indicate that the stepwise catalysis of the enzymes in the metabolic pathway,the dashed arrows indicate the metabolites formed,and the two-way arrows indicate the exchange of intermediates between the two pathways. AACT,acetyl-CoA C-acetyltransferase;HMGS,3-hydroxy-3-methylglutaryl-CoA synthase;HMGR,3-hydroxy-3-methylglutaryl-CoA reductase;MK,MVA kinase;PMK,phospho-MVA kinase;MPDC,diphospho-MVA decarboxylase;IPPI,isopentenyl diphosphate Δ-isomerase;FPPS,farnesyl diphosphate synthase;TPS,terpene synthase;DXS,1-deoxy-D-xylulose 5-phosphate synthase;DXR,1-deoxy-D-xylulose 5-phosphate reductoisomerase;MCT,2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase;CMK,4-(cytidine 5´-diphospho)-2-Cmethyl-D-erythritol kinase;MDS,2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase;HDS,4-hydroxy-3-methylbut-2-enyl-diphosphate synthase;HDR,4-hydroxy-3-methylbut-2-enyl diphosphate reductase;GPPS,geranyl diphosphate synthase;IPP,isopentenyl diphosphate;DMAPP,dimethylallyl diphosphate;FPP,farnesyl diphosphate;GPP,geranyl diphosphate.
Terpenoids are the most abundant plant secondary metabolites,and serve multiple physiological and ecological roles,such as flavor volatiles (Dudarevaet al.2004;Abbaset al.2017). They are biosynthesized through two independent and complementary pathways:the methylerythritol phosphate (MEP) pathway in plastids and the mevalonic acid (MVA) pathway in the cytosol (Vranováet al.2013) (Fig.2). The MEP pathway is the primary route for the biosynthesis of monoterpenes (C10) and diterpenes(C20),while the MVA pathway is the predominant route for the biosynthesis of sesquiterpenes (C15) (Eisenreichet al.2004). Monoterpenes and sesquiterpenes represent the volatile terpenoids,while diterpenoids are generally nonvolatile (Maffeiet al.2011). Isopentenyl diphosphate (IPP)and its isomer dimethylallyl diphosphate (DMAPP) are the 5-carbon building blocks for terpenoids,which are derived from either acetyl-CoA through the MVA pathway or pyruvate and glyceraldehyde-3-phosphate through the MEP pathway.Geranyl diphosphate synthase (GPPS) and farnesyl diphosphate synthase (FPPS) catalyze the condensation of one IPP molecule and one DMAPP molecule into geranyl diphosphate (GPP) and farnesyl diphosphate(FPP),respectively. The metabolic intermediates IPP,DMAPP,GPP and FPP can be shared by the MEP and MVA metabolic pathways through cross-membrane transport(Mayet al.2013;Pazouki and Niinemets 2016). Lastly,terpene synthases (TPS) are responsible for catalyzing the production of monoterpenes and sesquiterpenes from the substrates GPP and FPP,respectively.
Enzymes and coding genes in the MEP and MVA pathways have been comprehensively explored inArabidopsis thaliana(Vranováet al.2013)and partly studied in grapes.For example,the MEP metabolic pathway has been shown to be the dominant metabolic route for monoterpene synthesis in berries (Luan and Wüst 2002);GPPS and FPPS have been purified and characterized in berries (Feronet al.1990;Clastreet al.1993);grape genome analysis revealed a large gene family of monoterpene synthases (mono-TPS)and sesquiterpene synthases (sesqui-TPS) (Jaillonet al.2007;Martinet al.2010);QTL mapping revealed thatDXSgene affects the monoterpene content of berries (Battilanaet al.2009;Duchêneet al.2009),which might be due to an amino acid substitution at position 284 (Emanuelliet al.2010;Battilanaet al.2011;Costaet al.2018).
Kyoho and 87-1 are two table grape varieties with different aroma characteristics and genetic backgrounds.Kyoho is a hybrid betweenVitis viniferaandVitis labruscawith a strawberry-like scent,while 87-1 is an early-ripening mutant of Muscat hamburg belonging toVitisviniferawith a rich rose scent. In this study,we compared their aroma compositions and concentrations during berry development by headspace-SPME combined with gas chromatography-mass spectrometry (GCMS),and analyzed the expression differences of the enzyme-encoding genes in the LOX-HPL,MEP and MVA metabolic pathways by qRT-PCR. The results from this work will promote our understanding of aroma metabolic mechanisms of grapes,and offer some suggestions for grape aromatic quality improvement.
2.Materials and methods
2.1.Plant materials and sampling
Six-year-old vines of Kyoho and 87-1 grapes,using Beta as rootstock,were used as experimental materials in this study. The vines were cultivated in the same vineyard in Xingcheng,Liaoning Province in the northeast of China.Vines were arranged in north-south oriented rows,and spaced 1.5 m apart within and 4 m apart between the rows,with a horizontal trellis system. The vineyard management practices were identical. Thirty vines of each variety were selected for the experiments. According to findings of a preliminary experiment,berries were sampled at 2 weeks before veraison,at veraison,2 weeks after veraison,and in the mature period,which corresponded to 42,56,70 and 84 DAFs for Kyoho and 30,44,58,and 72 DAFs for 87-1,respectively. Each sample contained 50 clusters of approximately 200 berries and was randomly divided into three biological replicates. The berries and exocarp were frozen with liquid nitrogen and stored at–75°C for aroma determination and RNA extraction.
2.2.GC-MS analysis of volatile compounds
Headspace-SPME combined with GC-MS were used to measure grape volatile composition and concentration,as described by Jiet al.(2019). Samples of 200 g of berries were homogenized,and 10 g of the homogenate was transferred into a 20-mL solid-phase microextraction vial,added with 1 g NaCl and 5 μL internal standard (0.4 g L–13-nonanone) at the same time. The vial was heated in a water bath at 37°C for 30 min to allow equilibration between the solution and headspace,and then headspace extraction was performed with the fibre (50 μm/30 μm,DVB/CAR/PDMS;Supelco,Bellefonte) for 30 min. The fibre was then inserted into the injection port of the 7890A gas chromatograph at 250°C for 3 min in splitless mode. The capillary column used in this study was an HP-5MS (30 m×0.25 mm×0.25 μm). The temperature program was 40°C for 3 min;2°C min–1to 70°C and held for 2 min;3°C min–1to 120°C;5°C min–1to 150°C;and finally 10°C min–1to 220°C and held for 2 min. High purity helium was used as carrier gas with a flow rate at 1.3 mL min–1. The EI (electronic impact) of the 5975C mass spectrograph was 70 eV and the scanned area was 50–550 m/z at 2.88 scans s–1. The components were identified by comparing the mass spectra with the NIST 11 library. All compounds were quantified as 3-nonanone equivalents.
2.3.RNA extraction and real-time RT-PCR assay
Grape skin was peeled off with a blade and ground into a powder with liquid nitrogen.Then,0.2 g of the powder was used for RNA extraction. Total RNA was isolated by the RNAprep pure Plant Kit (TianGen,Beijing,China)according to the standard protocol. The quality of the RNA was tested by agarose gel and A260/A280 using a UV spectrophotometer. Then,cDNA was synthesized by FastKing One Step RT-PCR Kit (TianGen,Beijing,China)according to the standard protocol.
SuperReal PreMix Color (SYBR Green) Kit was used to perform Real-time RT-PCR on an CFX96 Touch system(Bio-Rad,California,USA). Each reaction (20 μL) contained 10 μL 2×SuperReal Color PreMix,1 μL cDNA,0.6 μL forward primer (10 μmol L–1),0.6 μL reverse primer (10 μmol L–1),and 7.8 μL ddH2O. The real-time qPCR program used was 95°C for 15 min,followed by 40 cycles of amplification at 95°C for 10 s and 60°C for 30 s,and followed by a melt cycle from 60 to 95°C.
The primers used in this study are shown in Table 1.The primers ofVvActin,VvGAPDH,VvFAD2-1,VvFAD2-2,VvLOXA,VvLOXC,VvLOXD,VvLOXO,VvHPL1,VvHPL2,VvADH1,VvADH2andVvAATare from Xuet al.(2015),and the primers ofVvDXS,VvDXR,VvHDR,VvGPPS,VvPNLinNer1,VvCSLinNer,VvCSbOci,VvCSbOciMandVvGwGerare from Zhanget al.(2017). We screened the homologous gene sequences ofVvMCT,VvCMK,VvMDS,VvHDS,VvIPPI,VvAACT,VvHMGS,VvHMGR,VvMK,VvPMK,VvMPDC,andVvFPPStoArabidopsis(Vranováet al.2013) in the Grape Genome databases (http://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/) by using the tBLASTn program and designed specific primers using Primer Premier 6. The sequences ofVvPNCuCadandVvGwGerAare fromMartinet al.(2010),and specific primers were designed using Primer Premier 6.The specification of the primers was detected by their melt curves and gel electrophoresis.VvGAPDHandVvActinwere used as internal controls. For all analyses,the signals obtained for the target genes were normalized against the mean of the Ct values of the two reference genes,and relativequantification of the target genes was calculated by the cycle threshold (Ct) 2–ΔΔCtmethod. All samples were tested in three biological replicates.
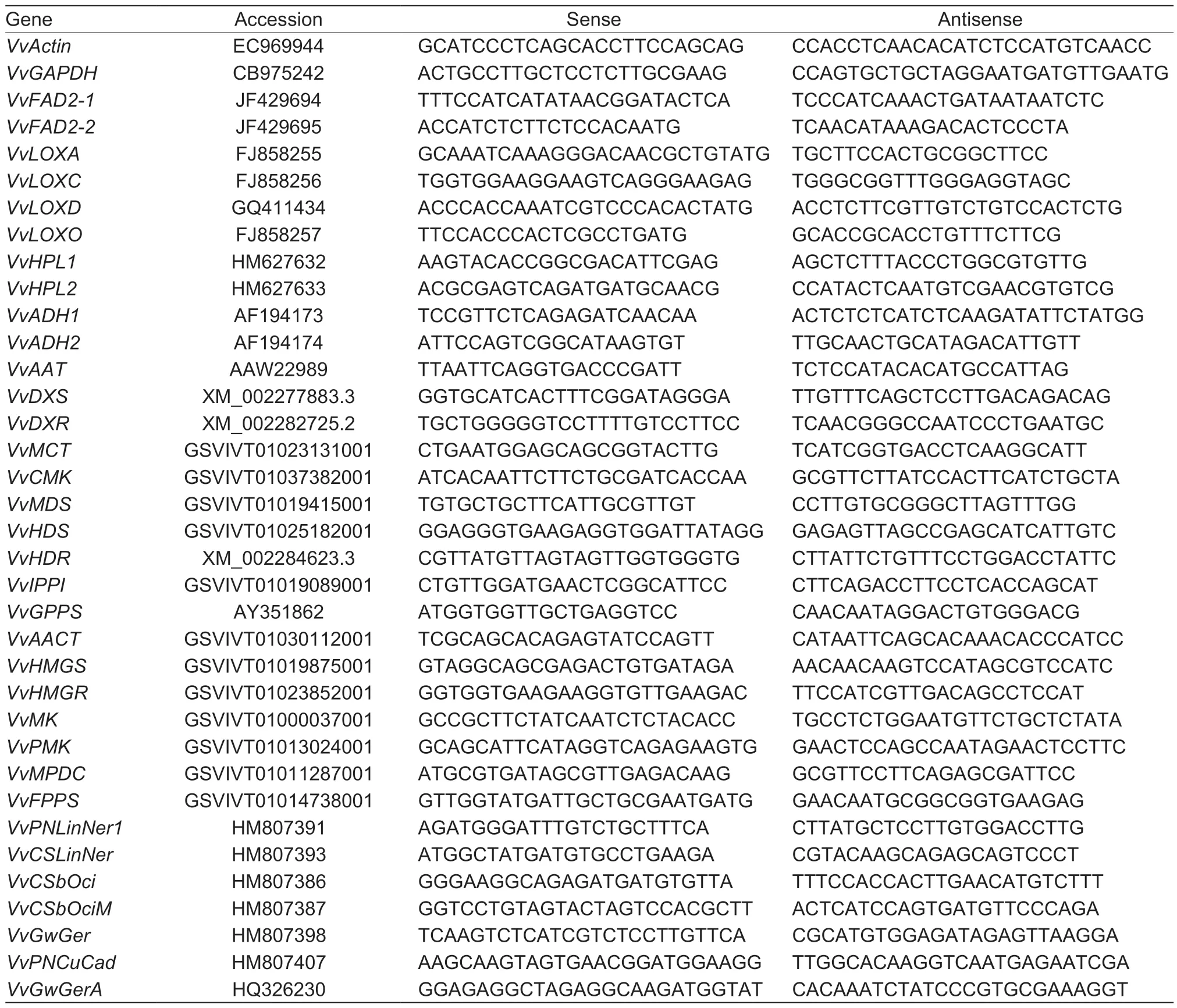
Table 1 Primers used in real-time PCR
2.4.Data analysis
One-way analysis of variance (ANOVA),Pearson’s correlation analysis and principal component analysis (PCA)were performed by SPSS 20.0 for windows (SPSS Inc.,Chicago,IL,USA). The variance analysis in Table 2 used Tukey test,and the variance analysis in Figs.4–7 usedt-test.t-test was performed for each time point separately.The Tukey test andttest for ANOVA were each performed at a level ofP<0.05. Correlation analysis was performed by SPSS 20.0 between average gene relative expression levels and average metabolite contents. Data were normalized by SPSS 20.0 for the principal component analysis (PCA).Excel 2010 software was used for drawing graphs.
3.Results
3.1.Differences in aroma compositions and concentrations between Kyoho and 87-1 berries
A total of 41 aroma components were detected during the whole berry development of Kyoho and 87-1 (Table 2).They could be divided into four groups:aldehydes,esters,terpenes and alcohols. Aldehydes were abundant in both Kyoho and 87-1 berries.Both hexanal and 2-hexenal,the dominating aldehydes,had high contents throughout the development of the berries. The alcohols were more abundant in Kyoho berries than in 87-1 berries. 2-Hexen-1-ol and 1-octen-3-ol were the main alcohols in Kyoho berries,while only a small amount of 1-octen-3-ol was detected in 87-1 berries. Twelve different esters,which were more abundant after veraison,were found in Kyoho berries,but none was found in 87-1 berries. Ethyl acetate was dominant among the esters. Fourteen terpenes were detected in 87-1 berries,but only seven were found in Kyoho berries. Almost all the terpenes were monoterpenes. Linalool,which was the dominant terpene in 87-1 berries and most abundant near maturity,was not found in Kyoho berries.
In order to simplify and visually display the aroma development of Kyoho and 87-1 berries,a principal component analysis was conducted. Two principal components were obtained,explaining 86% of the total variances. The first principal component (PC1) and the second principal component (PC2) accounted for 57.29%and 28.71% of the total variability,respectively. Esters and alcohols had high loadings to PC1,while aldehydes and terpenoids had high loadings to PC2 (Fig.3-A). The points representing the berry developmental stages of Kyoho were concentrated in the first and fourth quadrants,while the points representing those of 87-1 were concentrated in the second and third quadrants (Fig.3-B). This visually shows that the aroma development in Kyoho berries significantly differ from that in 87-1 berries.
The total amount of LOX-HPL pathway products in Kyoho berries was always higher than that in 87-1 berries,and it gradually increased with the berry ripening,and decreased slightly at the maturity stage (Fig.4-A). The contents and development trends of aldehydes in Kyoho and 87-1 berries were similar (Fig.4-B),while there were significant differences in both ester and alcohol contents between Kyoho and 87-1 during the whole berry development,as almost no esters or alcohols were detected in 87-1 berries(Fig.4-C and D). The contents of terpenoids were low in both Kyoho and 87-1 berries before veraison. After veraison,they increased sharply in 87-1 berries but only slightly in Kyoho berries (Fig.4-E). Therefore,terpenoids were significantly higher in 87-1 berries than in Kyoho berries at the maturity stage.
3.2.Gene expression differences in the LOX-HPL pathway
Fatty acid desaturase (FAD) catalyzes the synthesis of unsaturated fatty acid precursors for the LOX-HPL metabolic pathway. The expression abundances ofVvFAD2-1andVvFAD2-2in Kyoho berries were significantly higher than those in 87-1 berries (Fig.5-A and B). The expression abundance ofVvFAD2-1was relatively stable,with only a slight increase between the mature stage and the young fruit stage. The expression level ofVvFAD2-2increased sharply before veraison,and decreased slightly at the maturity stage,which was consistent with the change of the total content of aldehydes,alcohols and esters. Correlation analysis showed that the gene expressions ofVvFAD2-1andVvFAD2-2had significant correlations with the ester content and the total content of aldehyde,alcohol and ester(Table 3).
Lipoxygenase (LOX) catalyzes the synthesis of hydroperoxides,which are direct precursors of aldehyde synthesis. The expression ofVvLOXAin Kyoho and 87-1 berries both peaked at the veraison stage,but its expression in 87-1 berries was slightly higher than that in Kyoho berries(Fig.5-C). The expression ofVvLOXCin Kyoho and 87-1 berries showed opposite trends,with a downward trend for the former while an upward trend for the latter (Fig.5-D).The expression ofVvLOXDin Kyoho and 87-1 berries both showed downward trends,and its expression in Kyoho berries was higher than that in 87-1 berries (Fig.5-E). The expression ofVvLOXOin Kyoho berries was significantly higher than that in 87-1 berries.The expression decreasedbefore veraison and then increased near ripening in Kyoho berries,but was low and stable in 87-1 berries (Fig.5-F).Correlation analysis showed that the gene expression levels ofVvLOXDandVvLOXOwere significantly correlated with alcohol content (Table 3).

Table 2 Aroma compositions and concentrations in Kyoho and 87-1 during berry development (ng g–1 FW)1)
Hydroperoxide lyase (HPL) catalyzes the synthesis of aldehydes. The expression ofVvHPL1in Kyoho and 87-1 berries showed overall upward trends. Its expression peaked at the veraison stage in 87-1 berries and the maturity stage in Kyoho berries (Fig.5-G),which was consistent with the development of aldehydes. The expression ofVvHPL2during berry development in Kyoho and 87-1 showed fluctuations and its expression levels were basically the same between young fruits and mature fruits (Fig.5-H).Correlation analysis showed that the gene expression level ofVvHPL2had a higher correlation with alcohol content than that ofVvHPL1,but none of the correlations reached a significant level (Table 3).
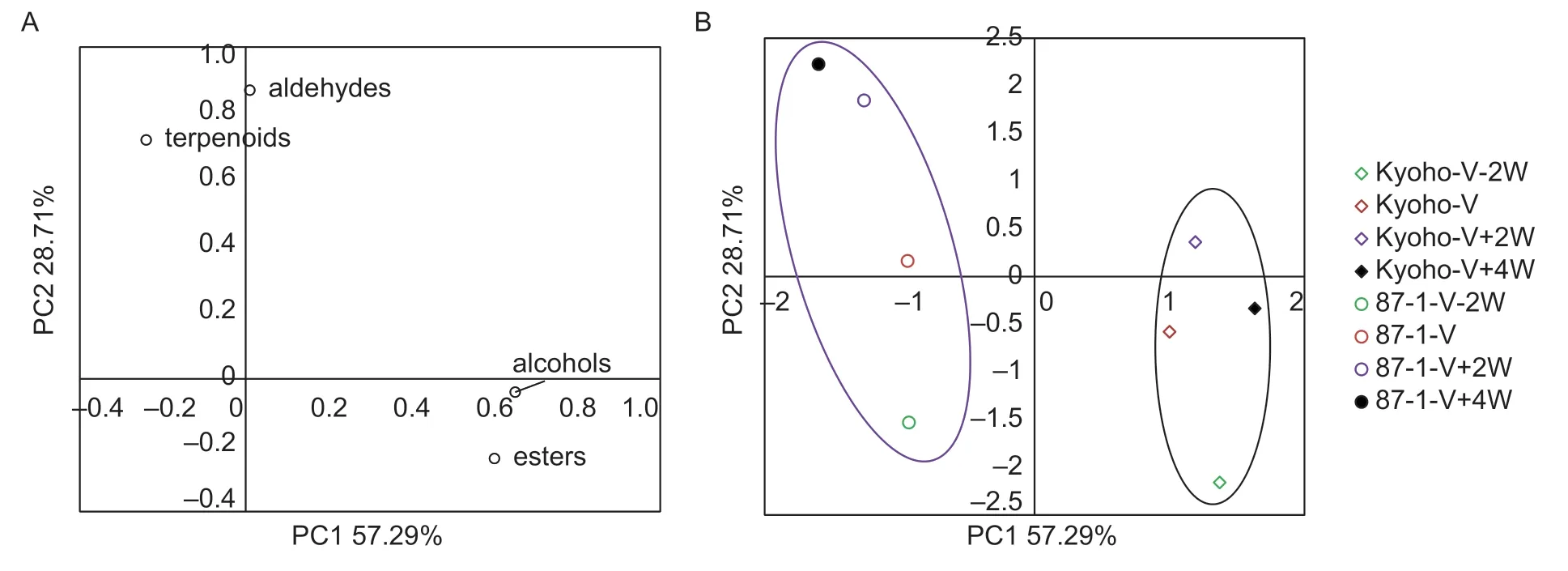
Fig.3 Principal component analysis (PCA) of aroma development in Kyoho and 87-1 grape berries. A,loading plot. B,scores scatter plot. For both Kyoho and 87-1 cultivars:V-2W,two weeks before veraison;V,at veraison stage;V+2W,two weeks after veraison;V+4W,four weeks after veraison.
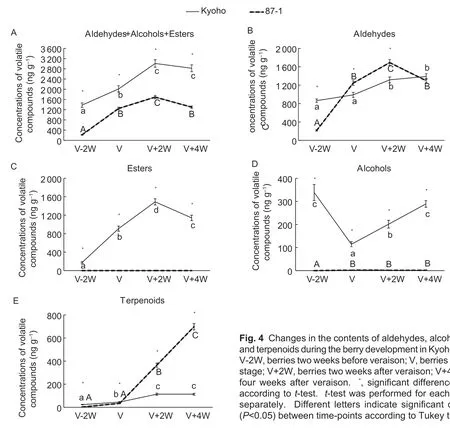
Fig.4 Changes in the contents of aldehydes,alcohols,esters and terpenoids during the berry development in Kyoho and 87-1.V-2W,berries two weeks before veraison;V,berries at veraison stage;V+2W,berries two weeks after veraison;V+4W,berries four weeks after veraison. *,significant difference (P<0.05)according to t-test. t-test was performed for each time point separately. Different letters indicate significant differences(P<0.05) between time-points according to Tukey test.
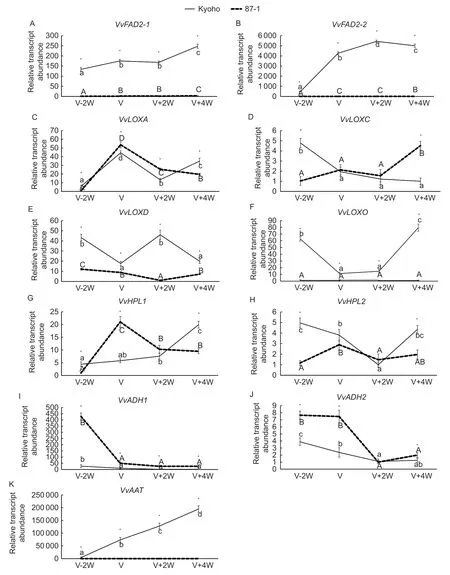
Fig.5 Transcription level analysis of genes in the lipoxygenase-hydroperoxide lyase (LOX-HPL) metabolic pathway during berry development in Kyoho and 87-1. VvFAD2-1 and VvFAD2-2,coding genes of fatty acid desaturase;VvLOXA,VvLOXC,VvLOXD and VvLOXO,coding genes of lipoxygenase;VvHPL1 and VvHPL2,coding genes of hydroperoxide lyase;VvADH1 and VvADH2,coding genes of alcohol dehydrogenase;VvAAT,coding gene of alcohol acyl transferase. V-2W,berries two weeks before veraison;V,berries at veraison stage;V+2W,berries two weeks after veraison;V+4W,berries four weeks after veraison. *,significant difference (P<0.05) according to t-test. t-test was performed for each time point separately. Different letters indicate significant differences (P<0.05) between time-points according to Tukey test.
Alcohol dehydrogenase (ADH) catalyzes the synthesis of alcohols. The expressions ofVvADH1andVvADH2in Kyoho and 87-1 berries showed similar downward trends,and their expression levels were lower in Kyoho berries than in 87-1 berries (Fig.5-I and J). Correlation analysis showed that their expressions had negative correlations with alcohol content (Table 3).
Alcohol acyl transferases (AAT) catalyze the esterification of low molecular weight carboxylic acids and alcohols into esters. The expression ofVvAATin Kyoho and 87-1 berries showed significant differences. Its expression displayed a ripening-induced expression pattern in Kyoho berries,but was very low during the whole berry development in 87-1(Fig.5-K),which was significantly correlated with ester content (Table 3).
3.3.Gene expression differences in MVA pathway
The MVA metabolic pathway uses acetyl-CoA as a substrate and catalyzes a succession of transformations through acetyl-CoA C-acetyltransferase (AACT),3-hydroxy-3-methylglutaryl-CoA synthase (HMGS),3-hydroxy-3-methylglutaryl-CoA reductase (HMGR),MVA kinase(MK),phospho-MVA kinase (PMK),and diphospho-MVA decarboxylase (MPDC) to form Isopentenyl diphosphate(IPP). Isopentenyl diphosphate Δ-isomerase (IPPI) then catalyzes the isomerization of IPP and DMAPP. The expression abundances ofVvAACT,VvHMGS,VvHMGR,VvMK,VvPMKandVvMPDCwere different between Kyoho and 87-1 berries,but their expression trends were basically the same (Fig.6-A–F). The expression level ofVvIPPIwas dramatically higher in 87-1 berries than in Kyoho berries.The expression increased rapidly before veraison and then decreased gradually after veraison in 87-1 berries (Fig.6-G). Farnesyl diphosphate synthase(FPPS) catalyzes the synthesis of farnesyl diphosphate,which is the direct precursor of sesquiterpene synthesis.The expression trends ofVvFPPSwere basically the same in Kyoho and 87-1 berries,with both decreasing before veraison and then increasing near ripening. The expression level ofVvFPPSwas slightly higher in Kyoho berries than in 87-1 berries (Fig.6-H). Two sesquiterpene synthase coding genes,VvPNCuCadandVvGwGerA,were used for quantitative analysis in this study. No signal was detected in either Kyoho or 87-1 berries,which was consistent with the lack of detection of sesquiterpenoids in Kyoho and 87-1 berry aromas.
3.4.Gene expression differences in MEP pathway
The MEP metabolic pathway is the main route for monoterpene biosynthesis. It uses pyruvate and glyceraldehyde-3-phosphate as substrates and generates IPP and DMAPP through continuous catalysis by 1-deoxy-Dxylulose 5-phosphate synthase (DXS),1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR),2-C-methyl-Derythritol 4-phosphate cytidylyltransferase (MCT),4-(cytidine 5´-diphospho)-2-C-methyl-D-erythritol kinase (CMK),2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase(MDS),4-hydroxy-3-methylbut-2-enyl-diphosphate synthase(HDS) and 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR). Most of the coding genes for these enzymes,such asVvDXS,VvMCT,VvMDSandVvHDS,were highly expressed in 87-1 berries but poorly expressed in Kyoho berries.However,the expressions ofVvCMKandVvHDRwere higher in Kyoho berries than in 87-1 berries (Fig.7-A–G). DMAPP is converted by geranyl diphosphate synthase (GPPS) into geranyl diphosphate(GPP),which is a direct precursor of monoterpene synthesis.The expression ofVvGPPSin 87-1 increased gradually during berry development,while it was maintained at a low level in Kyoho berries (Fig.7-I). Terpene synthetases are responsible for catalyzing the production of monoterpenes.There are 89 putative terpenoid synthase genes in the grape genome. The expression of five mono-TPSs were detected:VvPNLinNer1,VvCSLinNer,VvCSbOci,VvCSbOciMandVvGwGer. Among them,VvCSLinNer,VvCSbOci,VvCSbOciMandVvGwGerhad no signals in either Kyoho or 87-1 berries. The expression ofVvPNLinNer1in 87-1 berries increased sharply after veraison,but was not detected in Kyoho berries (Fig.7-J). Correlation analysis showed that,except for the expression ofVvCMKandVvHDRthat had negative correlations with terpene content,the expressions of the other genes had positive correlations with terpene content,with onlyVvGPPSandVvPNLinNer1reaching significant levels (Table 4).
4.Discussion
Aroma,an important quality characteristic of grapes,is a common focus of consumers,viticulturists and grapevine breeders. Kyoho and 87-1,two table grape cultivars popular with consumers in China,have pleasant but different scents.In this study,we analyzed their aroma components and contents,and found dramatic differences. Esters were abundant in Kyoho berries but not detected in 87-1 berries,while terpenes were abundant in 87-1 berries but scarce in Kyoho berries. This was similar to the aroma differences of Kangtai and Xiangfei reported by Yanget al.(2009). In addition,Fujiminori,Zuijinxiang,Yoho,Kyoho,Suiho,Jingya and Black Beet were rich in ester aromas but low in terpene aromas,while Italian,Shine Muscat,Red Alexandria,High Bailey and Tamina were rich in terpene aromas but low in ester aromas (Wuet al.2016). Parentage analysis indicated that Kangtai,Fujiminori,Zuijinxiang,Yoho,Kyoho,Suiho,Jingya and Black Beet all had the genetic background ofVitis labrusca,while Xiang fei,87-1,Italian,Shine Muscat,Red Alexandria,High Bailey and Tamina all had the genetic background ofVitisvinifera.Vitis viniferaandVitislabruscaare two commercially important species in genusVitis. Both are widely grown for the production of wine and table grapes around the world. They are also important backbone parents for grape breeding. Numerous studies indicated that terpene aromas,such as linalool,geraniol,and nerol,are abundant inVitisvinifera,especially Muscat varieties (Mateo and Jiménez 2000;Aubert and Chalot 2018),and ester aromas are abundant inVitislabrusca(Wang and Luca 2005;Leeet al.2016). So,we speculated that the ester aroma and terpene aroma trait of grapes might be derived fromVitis labruscaandVitisvinifera,respectively.
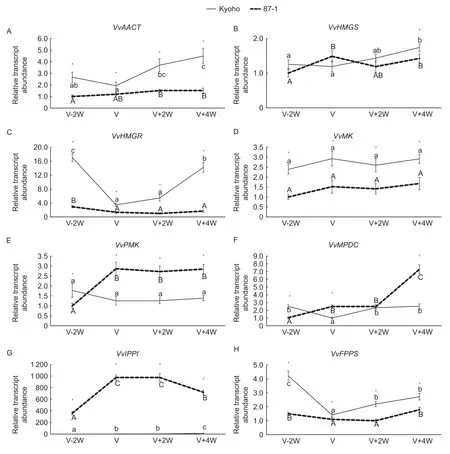
Fig.6 Transcription level analysis of genes in the mevalonic acid (MVA) metabolic pathway during the berry development in Kyoho and 87-1. VvAACT,acetyl-CoA C-acetyltransferase;VvHMGS,3-hydroxy-3-methylglutaryl-CoA synthase;VvHMGR,3-hydroxy-3-methylglutaryl-CoA reductase;VvMK,MVA kinase;VvPMK,phospho-MVA kinase;VvMPDC,diphospho-MVA decarboxylase;VvIPPI,isopentenyl diphosphate Δ-isomerase;VvFPPS,farnesyl diphosphate synthase. V-2W,berries two weeks before veraison;V,berries at veraison stage;V+2W,berries two weeks after veraison;V+4W,berries four weeks after veraison. *,significant difference (P<0.05) according to t-test. t-test was performed for each time point separately. Different letters indicate significant differences (P<0.05) between time-points according to Tukey test.
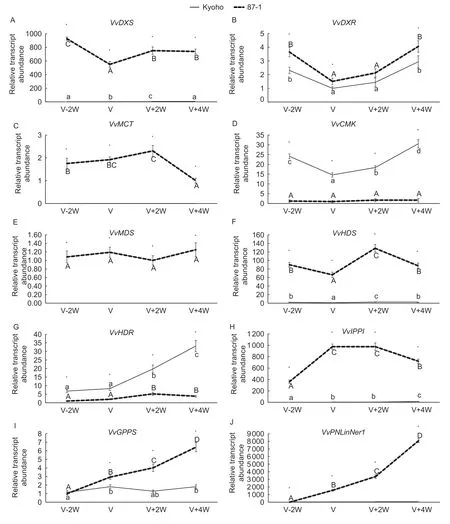
Fig.7 Transcription level analysis of genes in the methylerythritol phosphate (MEP) metabolic pathway during the berry development in Kyoho and 87-1. VvDXS,1-deoxy-D-xylulose 5-phosphate synthase;VvDXR,1-deoxy-D-xylulose 5-phosphate reductoisomerase;VvMCT,2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase;VvCMK,4-(cytidine 5´-diphospho)-2-C-methyl-D-erythritol kinase;VvMDS,2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase;VvHDS,4-hydroxy-3-methylbut-2-enyl-diphosphate synthase;VvHDR,4-hydroxy-3-methylbut-2-enyl diphosphate reductase;VvIPPI,isopentenyl diphosphate Δ-isomerase;VvGPPS,geranyl diphosphate synthase;VvPNLinNer1,coding genes of monoterpene synthase. V-2W,berries two weeks before veraison;V,berries at veraison stage;V+2W,berries two weeks after veraison;V+4W,berries four weeks after veraison. *,significant difference(P<0.05) according to the t test. t-test was performed for each time point separately. Different letters indicate significant difference(P<0.05) between time-points according to Tukey test.
Volatile esters are formed by the esterification of alcohols and carboxylic acids catalyzed by alcohol acyltransferase,and the LOX-HPL metabolic pathway provides substrates such as alcohols for ester biosynthesis. In this study,gene expression analysis of the LOX-HPL pathway indicated that the coding genes of LOX,HPL,and ADH were each expressed at a high level in Kyoho and 87-1 berries,but the gene encoding alcohol acyltransferase (VvAAT) had a significant difference between Kyoho and 87-1 berries. The expression ofVvAATin Kyoho berries increased sharply after veraison and maintained a high level at the ripening stage,but only limited such expression was detected in 87-1 berries,which coincided with the dynamic change of the ester aroma content. This indicated that alcohol acyltransferase was essential for ester aroma biosynthesis in Kyoho and 87-1 berries,and the low expression ofVvAATmight be the reason for the low content of ester volatiles in 87-1 berries. A strong positive correlation between alcohol acyltransferase activity and volatile ester content has been found in many fruits,such as strawberry (Pérezet al.1993,1996),banana (Haradaet al.1985),melon (Shalitet al.2001) and apple (Liet al.2006). Further,genes encoding alcohol acyltransferase have been cloned and identified,and genetic differences inAATlocus have been reported.(Aharoniet al.2000; Yahyaouiet al.2002;Beekwilderet al.2004;Souleyreet al.2005;Liet al.2006). Two alcohol acyltransferase encoding genes (CM-AAT1andCM-AAT2) with strong sequence homology have been isolated from melon fruit. CM-AAT1 protein exhibited alcohol acyl-transferase activity while no such activity could be detected for CM-AAT2(Yahyaouiet al.2002). In apple,multipleAAT1gene variants were identified in Royal Gala (high ester production) and Granny Smith (low ester production),but only two variants were functional (Souleyreet al.2014). Therefore,we speculated that there might be multiple alleles in theVvAATlocus,which is the genetic basis of the ester aroma trait in genusVitis. In addition,we found that the expressions of the alcohol dehydrogenase coding genes,VvADH1andVvADH2,were negatively correlated with the volatile alcohol content. This was in contrast to previous studies(Tesnière and Verriès 2000;Tesnièreet al.2006) where the expression ofVvADH2was found to be up regulated during the onset of ripening in the grape skin. We inferred that other homologous genes or mechanisms for alcohol synthesis might be involved in the berry development.
Terpenes are biosynthesized from two independent pathways:MEP pathway in plastids and MVA pathway in the cytosol. The gene expression of MEP metabolic pathway was significantly different between Kyoho and 87-1 berries,which was consistent with the difference in terpene contents.Nevertheless,the gene expression levels in MVA metabolic pathway was similar in Kyoho and 87-1 berries. These results indicated that MEP was the key metabolic pathway for terpene synthesis in berries,which was consistent with a previous report (Luan and Wüst 2002). 1-deoxy-D-xylulose 5-phosphate synthase (DXS)is the first enzyme in the MEP pathway. This study found the expression ofVvDXSwas dramatically lower in Kyoho berries than in 87-1 berries,but for both Kyoho and 87-1 the expression was relatively stable during berry development.This may be becauseDXSis a key gene of the terpene backbone metabolism pathway,which not only synthesizes monoterpenes,but also synthesizes hormones (gibberellins and abscisic acid),photosynthetic pigments (phytol and carotenoids),electron carriers (ubiquinone),and structural components of membranes (phytosterols),and thus have important effects on plant growth and development(McGarvey and Croteau 1995). Therefore,the stable expression ofDXShas biological significance for plants.Geranyl diphosphate (GPP),the precursor of monoterpene synthesis,is synthesized from isopentenyl diphosphate and dimethylallyl diphosphate by geranyl diphosphate synthase (GPPS). Our research found that the expression ofVvGPPSwas positively correlated with terpene contents.The expression increased rapidly in 87-1 after veraison,which was significantly higher than that in Kyoho,indicating thatVvGPPSis a key gene for monoterpene synthesis that has different expression mechanisms in Kyoho and 87-1.Terpene synthases (TPS) are responsible for the production of monoterpenes and sesquiterpenes. There is a highly expandedVvTPSgene family in the grapevine genome,including 69 putatively functionalVvTPS,20 partialVvTPS,and 63VvTPSprobable pseudogenes. Among them,two sesquiterpene synthase coding genes (VvPNCuCadandVvGwGerA) and five monoterpene synthase coding genes(VvPNLinNer1,VvCSLinNer,VvCSbOci,VvCSbOciMandVvGwGer) were used for quantitative analysis in this study. The expression ofVvPNLinNer1was high in 87-1 berries after veraison,but not detected in Kyoho berries.The expression ofVvPNCuCad,VvGwGerA,VvCSLinNer,VvCSbOci,VvCSbOciMandVvGwGerwas limited in both Kyoho and 87-1 berries.These results were in line with the findings in a previous report (Zhanget al.2017). Therefore,it is inferred that the low expression ofVvPNLinNer1might be the main reason for the low content of volatile terpenes in Kyoho berries.

Table 3 Correlation analysis of gene expression and metabolite contents in the lipoxygenase-hydroperoxide lyase (LOX-HPL)pathway

Table 4 Correlation analysis between gene expression of methylerythritol phosphate (MEP) metabolic pathway and terpenoid content
5.Conclusion
This research studied differences within the LOX-HPL,MEP and MVA pathways between Kyoho and 87-1 berries from the transcriptional level. qRT-PCR analysis indicated that the low expression ofVvAATmight be the reason for the low content of ester volatiles in 87-1 berries,and the low expression of encoding genes in the MEP pathway,especiallyVvPNLinNer1,might be the reason for the low content of terpene volatiles in Kyoho berries.
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFD1000200),the Fundamental Research Funds for Central Non-profit Scientific Institution,the Agricultural Science and Technology Innovation Program,Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2015-RIP-04) and the earmarked fund for China Agriculture Research System (CARS-29-zp).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Low glycemic index:The next target for rice production in China?
- Effect of side deep placement of nitrogen on yield and nitrogen use efficiency of single season late japonica rice
- Advancements in plant regeneration and genetic transformation of grapevine (Vitis spp.)
- Indica rice restorer lines with large sink potential exhibit improved nutrient transportation to the panicle,which enhances both yield and nitrogen-use efficiency
- Effects of nitrogen management on the ratoon crop yield and head rice yield in South USA
- Response of grain-filling rate and grain quality of mid-season indica rice to nitrogen application
