锂嵌入型化合物-氢气电池
2021-05-17朱正新王明明
朱正新,张 翔,王明明,陈 维,3
(1.中国科学技术大学化学与材料科学学院应用化学系,2.化学系,
3.微尺度物质科学国家研究中心,纳米催化与能量转化研究部,合肥230026)
In terms of the current environmental crisis and increasing energy demands,the development of renewable sources to replace traditional fossil energy is in an inevitable trend[1,2]. Large-scale energy storage(LSES)devices such as rechargeable batteries have been considered as an integration of renewable yet intermittent energy sources of wind and solar power due to their high efficiency and reliable storage of electricity[3,4]. Among various rechargeable batteries,lithium-ion batteries(LIBs)are considered as one of promising candidates for LSES owing to their high energy density and good cycling stability[5]. However,they have to overcome many challenges such as high cost and safety issues,which limit their further application for LSES[6]. As alternatives to LIBs,multivalent ion(Zn2+,Mg2+,Al3+,etc.)batteries have attracted much attention due to their abundant natural resources and intrinsic safety[7—10]. However,their relatively low power densities,poor lifetime and high-cost electrode materials hindered their applications for LSES[11,12]. Therefore,much effort has been devoted to explore new battery systems with high safety,low cost,and long cycling stability.
Hydrogen(H2)gas,which widely exists in the universe,serves as a prospective sustainable energy due to its environmental benignity and high energy density[13]. Thanks to the high reversibility and low redox potential(0 Vvs. standard hydrogen electrode)of the redox couple of hydrogen evolution and oxidation reactions(HER/HOR),it is desirable to develop rechargeable battery chemistry by using the hydrogen gas as anode,thus creating a new battery system of rechargeable H2gas batteries[14]. Traditional H2gas batteries in the Ni-H2chemistry showed remarkably long-term cycle lifetime of over 50000 times and superb service life of over 30 years in aerospace applications[15]. However,the anodes of the traditional H2gas batteries were driven by the high-cost Pt catalysts,which strictly limited their widespread applications. Nevertheless,the outstanding cycling performance of the H2gas batteries has attracted much interest for researchers to develop new H2gas batteries with low cost and high stability,targeting for LSES applications. Recently,we have successfully developed new Ni-H2and Mn-H2gas batteries by pairing Ni(OH)2/NiOOH and Mn2+/MnO2cathode couples respectively with the H2gas anodes,demonstrating excellent electrochemical performance and reasonable cost for LSES applications[16,17]. It is believed that a vast number of rechargeable H2gas batteries with different charge storage mechanisms and materials selections are expected to be fully explored.
Lithium intercalation compounds such as LiMn2O4(LMO),LiCoO2(LCO),and LiFePO4(LFP)serve as excellent cathode materials for LIBs and different aqueous batteries[18—20]. For example,Chenet al.[21]reported an LMO/Zn hybrid aqueous battery in a mild acidic electrolyte,which showed high Coulombic efficiency and long cycling life over 1000 cycles. Bakenovet al.[22]designed an LFP/Zn battery that operated in an optimized Li+/Zn2+binary electrolyte,achieving lifetime of over 400 cycles. However,the corrosion and dendrite problems of the Zn metal anode in aqueous electrolyte have hindered their further development for LSES applications. Recently,our group reported a LMO-H2battery by applying the superior hydrogen gas anode and LMO cathode,showing a good rate of up to 50C and cycling performance of 600 cycles[23]. Thanks to the large number of the lithium intercalation compounds,it is important to explore more lithium intercalation compounds-H2gas batteries with different storage systems to enrich the H2gas chemistry and to achieve the targeting performance for LSES applications.
In this work,we further develop two lithium intercalation compounds-H2gas batteries,naming LCO-H2and LFP-H2batteries,which are constructed by a Pt/C-catalysts-based hydrogen gas anode and LCO or LFP cathodes in aqueous Li2SO4electrolyte. The LCO-H2and LFP-H2batteries show good charge/discharge behaviors and high specific capacity at a low rate current. In addition,the LCO-H2and LFP-H2batteries exhibit a high rate of 10C,demonstrating the robustness of the rechargeable hydrogen gas battery chemistry.
1 Experimental
1.1 Reagents and Instruments
Pt/C powder(20%Pt on Vulcan XC-72,Premetek,USA),glass fiber separator(GF-8,Whatman,Germany),polyvinylidene fluoride(PVDF,MTI),N-methyl-2-pyrrolidone(NMP,Aladdin,A.R. grade),gas diffusion layer(GDL,Fuel Cell Store,USA),Li2SO4(Sinopharm Chemical Reagent Co.,Ltd.,A.R.grade),LiCoO2powder(LCO,MTI),LiFePO4powder(LFP,MTI),acetylene black(AB,MTI),and titanium foil(50 μm,Ti,MTI)were received and used without further treatment.
X’Pert PRO SUPER powder X-ray diffractometer equipped with graphite monochromatized CuKαradiation(Smartlab,Rigaku,Japan),HT-7700 transmission electron microscope(Hitachi,Japan),JEOL-6700F scanning electron microscope(JEOL,Japan),battery test system(LandHe,Wuhan),and CHI760E electrochemical work station(Chenhua Instrument,Shanghai)were used as characterization and battery electrochemical performance measurement instruments.
1.2 Preparation of Pt/C Anode,LCO and LFP Cathodes
To prepare the Pt/C anode,Pt/C powder and PVDF with a mass ratio of 9∶1 were dispersed in NMP solution. The slurry was uniformly coated onto the one side of GDLviathe suitable doctor blading with a mass loading ofca.1 mg/cm2. Then the prepared Pt/C electrodes were dried at 70 ℃.
To prepare the LCO cathodes,LCO powder,AB,and PVDF with a mass ratio of 8∶1∶1 were dispersed in NMP solution. The slurry was uniformly coated onto the one side of Ti foilviathe appropriate doctor blading with a mass loading ofca. 2 mg/cm2. Afterwards,LCO cathode was dried at 70 ℃. The preparation of LFP electrode was the same as the LCO electrode except that LFP was used in the replacement of LCO.
1.3 Fabrication of LCO-H2 and LFP-H2 Batteries
To fabricate LCO-H2and LFP-H2batteries,the electrode materials and GF-8 separator were assembled into the Swagelok cell,which was consistent with our previous reports[16,17,23]. The Swagelok cell was composed of Klein Flange to Swagelok adapters in a polytetrafluoroethylene-centered O-ring by a clamp and stainlesssteel inlet and outlet valves.
1.4 Testing of LCO-H2 and LFP-H2 Batteries
To test the half-cell cyclic voltammetry and galvanostatic charge/discharge of the LCO and LFP electrodes,three-electrode cells in beaker were performed using LCO or LFP as working electrode,Pt foil as counter electrode and Ag/AgCl(0.205 Vvs. NHE)as reference electrode. The LCO-H2and LFP-H2batteries tests were conducted on the assembled Swagelok cells. The cycling and rate performances of the cells were tested on LandHe test system. The cyclic voltammetry was collected using a CHI760E electrochemical work station.
2 Results and Discussion
2.1 Working Mechanisms of LCO-H2 and LFP-H2 Batteries
The working mechanisms of the LCO-H2and LFP-H2batteries are shown in Scheme 1,which are the hybrid of the cathode lithium intercalation mechanism with the anode HER/HOR catalytic redox reaction mechanism. During the state of charge,the Li+ions are extracted from the cathode and the hydrogen gas is generated from electrolyte on the anode. When the battery is discharging,the Li+ions are intercalated into the cathode materials and the hydrogen gas is oxidized on the anode. The electrode reactions of the LCO-H2or LFP-H2batteries can be written in the followings:

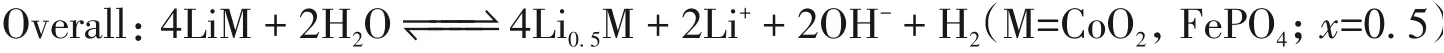

Scheme 1 Working mechanisms of LCO-H2 and LFP-H2 batteries in the charge/discharge states
2.2 Characterization of Electrode Materials
The morphology of the commercial LCO and LFP electrode materials were investigated by scanning electron microscopy(SEM)and transmission electron microscopy(TEM). As shown in Fig.1(A),the shape of LCO material is micro-particular. The size of particle is around 6—10 μm[Fig.1(B)]. Powder X-ray diffraction(PXRD)patterns of the LCO sample exhibit that it has a good crystallization nature,which is consistent with the standard pattern(PDF#50-0653)[Fig.1(C)]. Meanwhile,Fig.1(D)shows that the commercial LFP sample has a nano-particular morphology with particle size in the range of 200—600 nm. Fig.1(E)further demonstrates the irregular morphology feature of the LFP nanoparticles. The PXRD analysis confirms that the LFP sample has a high purity,whose pattern matched well with the standard LFP(PDF#40-1499)[Fig.1(F)].
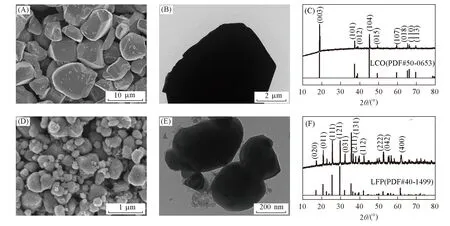
Fig.1 SEM images(A,D),TEM images(B,E),and PXRD patterns(C,F)of LCO(A—C)and LFP(D—F)electrode materials
2.3 Electrochemical Performance
Prior to the fabrication of the LCO-H2and LFP-H2full cells,the electrochemical performance of the commercial LCO and LFP electrodes were explored by cyclic voltammogram(CV)and galvanostatic charge/discharge measurements. The CV curves of the LCO and LFP electrodes in 2.5 mol/L Li2SO4aqueous solution were tested by a three-electrode setup at a scan rate of 1 mV/s,where the LCO or LFP served as working electrode,Pt foil as counter electrode,and Ag/AgCl as reference electrode. A couple of redox peaks with potentials of 0.83/1.05 V(vs. NHE)can be clearly seen from Fig.2(A),which correspond to Li+ions intercalation/extraction from LCO in aqueous electrolyte. After two cycles,the peak currents show a gradual decrease tendency,indicating the gradual capacity decay of the cathode in aqueous electrolyte. Fig.2(B)shows the charge/discharge curves of the LCO electrode at a current rate of 0.5C(1C=140 mA/g). The LCO electrode shows good charge/discharge voltage plateaus which agree well with the CV result and delivers a high capacity of 95 mA·h·g-1at a current rate of 0.5C. In addition,the LFP electrode exhibits a couple of redox peaks with potentials of 0.31/0.64 Vvs. NHE[Fig.2(C)],which correspond to Li insertion/extraction into/out of LFP.After two cycles,the reduction peak potentials keep the same,but the oxidation peak potentials and currents show gradual decay,indicating slow capacity degradation of the LFP electrode,which is consistent with the reported results about the untreated LFP electrode elsewhere[24]. As shown in Fig.2(D),the LFP electrode shows pronounced charge/discharge plateaus with a high capacity of 149 mA·h·g-1(1C=170 mA/g),which is close to its theoretical capacity. Therefore,the commercial LCO and LFP electrode materials are good cathodes for aqueous LCO-H2and LFP-H2batteries.
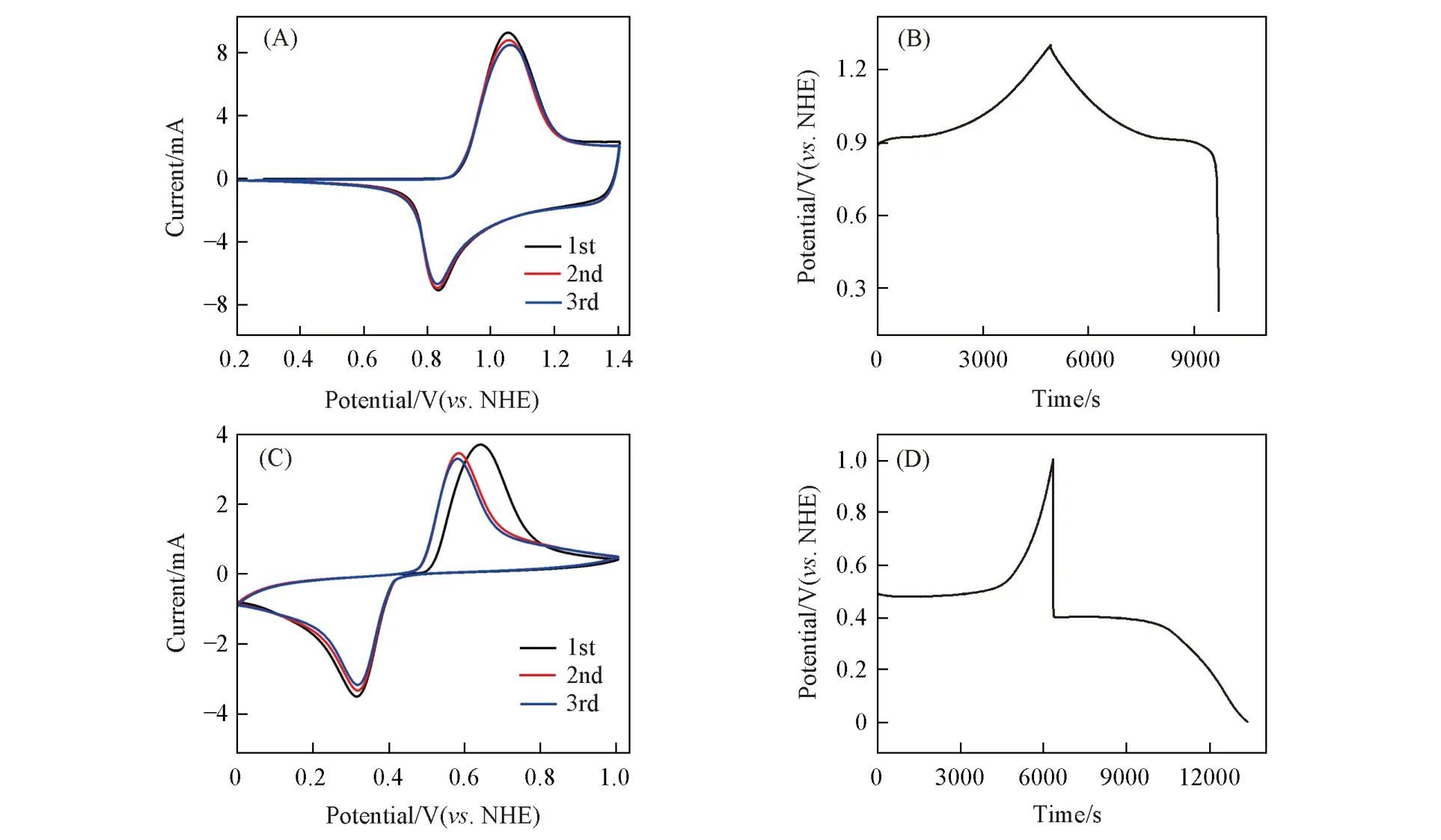
Fig.2 CV curves at a scan rate of 1 mV/s in the 1st,2nd,and 3rd cycles(A,C),and charge/discharge curves at a current rate of 0.5C[(1C=140 mA/g)(B)and(1C=170 mA/g)(D)]of LCO(A,B)and LFP(C,D)
The LCO and LFP electrodes were deployed as cathodes for the fabrication of the LCO-H2and LFP-H2batteries. Their electrochemical performances were evaluated by CV and galvanostatic charge/discharge experiments. As shown in Fig.3(A),an obvious reduction peak at about 1.1 V was observed from the CV curve of the LCO-H2battery,suggesting Li+ions intercalation into LCO on the cathode and HOR on the anode.Fig.3(B)depicts the charge/discharge properties of the LCO-H2battery at a constant current charge/discharge of 0.5C(1C=140 mA/g),which delivers charge/discharge plateaus of 1.58 V/1.27 V and a high initial capacity of 96.9 mA·h·g-1with Coulombic efficiency(CE)of 85.3%. The rate performance of the LCO-H2battery was shown in Fig.3(C)and(D). The LCO-H2battery was charged at 2C to a cutoff voltage of 1.7 V and then switched to charge under the voltage of 1.7 V with a cutoff current of 0.5C. The discharge of the battery was conducted by galvanostatic currents to a cutoff voltage of 0.8 V. As shown in Fig.3(C),the LCO-H2battery exhibits a high discharge voltage plateau of 1.22 V at 1C,and retains around 1.08 V at a high discharge rate of 10C. The rate capability tests show that the LCO-H2battery can deliver discharge specific capacities of 74,69,and 64 mA·h·g-1at discharge rates of 1C,2C,and 3C,respectively[Fig.3(D)]. Even at high rates of 5C and 10C,the battery can still retain discharge specific capacities of 59 and 49 mA·h·g-1,respectively.However,the capacity of the LCO-H2battery declined quickly to 30 mA·h·g-1at 2C after 50 cycles,as shown in Fig.3(E)and(F). The poor cycling performance is ascribed to the instability of the LCO cathode,which is a common behavior for the LCO materials in aqueous solution[25]. To prolong the cycling lifetime of the LCO-H2battery,we replaced the cycled LCO cathode with a new one while keeping using the cycled Pt/C anode to form a recycled battery named LCO-H2-recycled battery. The LCO-H2-recycled battery was continuously tested to reveal its electrochemical performance. As shown in Fig.3(E)and(F),the LCO-H2-recycled battery is capable of delivering the similar charge/discharge behaviors,capacities and cycling stability as compared to the pristine LCO-H2battery,demonstrating the robustness of the H2anode for the rechargeable H2gas battery chemistry.

Fig.3 CV curve at a scan rate of 0.5 mV/s(A), charge/discharge curves at a current density of 0.5C(B),charge/discharge curves at different discharge rates(C),and rate performance(D)of LCOH2 battery, charge/discharge curves(E), and cycling performance(F) of LCO-H2 and recycled LCO-H2 batteries at a discharge current density of 2C
Fig.4 shows the electrochemical performance of the LFP-H2battery. The CV profile of the LFP-H2battery in 2.5 mol/L Li2SO4solution with a sweep rate of 0.5 mV/s is displayed in Fig.4(A). A pair of redox peaks in the potential ranges of 1.28—0.55 V were observed during the oxidation and reduction processes. The charge/discharge profiles at a current rate of 0.2C(1C=170 mA/g)were displayed in Fig.4(B). The plateau with potential around 0.66 V should be related to the Li+ions intercalation into LFP at cathode and HOR at anode during the discharge process,whereas the plateau around 1.18 V in the charge curve corresponds to the Li+ions extraction from LFP at cathode and HER at anode. Moreover,the LFP-H2battery shows a high initial discharge specific capacity ofca. 125 mA·h·g-1at 0.2C[Fig.4(B)]. The LFP-H2battery also exhibits excellent rate capability,showing discharge capacities of 83,65,and 49 mA·h·g-1at 0.5C,1C,and 2C,respectively[Fig.4(C)]. Even at high rates of 3C,5C,and 10C,the battery can still deliver discharge capacities of 40,33,and 26 mA·h·g-1,respectively. To evaluate rechargeability of the LFP-H2battery,we cycled the LFP-H2battery at a high rate of 10C over 50 cycles,which shows gradual capacity decay owing to the capacity fading of the LFP cathode in aqueous electrolyte. In this case,we also replaced the cycled LFP cathode by a new electrode and continued to test the cycling performance of the named LFP-H2-recycled battery. Although the LFP-H2battery shows capacity decline at 10C after 50 cycles,the LFP-H2-recycled battery can still reach the initial capacity level,the similar charge/discharge behaviors[Fig.4(E)]and cycling performance[Fig.4(F)]as compared to the pristine LFP-H2battery. Such cycling instability can be attributed to the unstable LFP electrode in aqueous solution rather than the H2gas electrode[24,26].
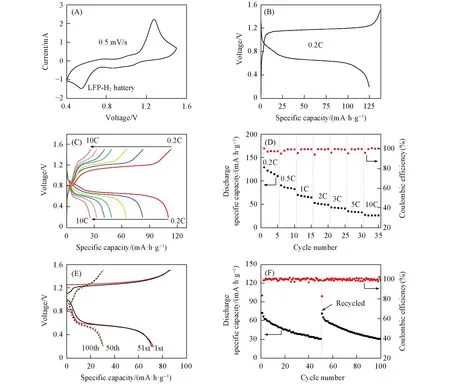
Fig.4 CV curve at a scan rate of 0.5 mV/s(A), charge/discharge curves at a current rate of 0.2C(B),charge/discharge curves at different rates(C), rate performance(D) of LFP-H2 battery; charge/discharge curves(E)and cycling performance(F)of LFP-H2 and recycled LFP-H2 batteries at a current rate of 10C
2.4 Characterization of Electrode Materials After Cycling
To further reveal the unstable cycling performance of the LCO-H2and LFP-H2batteries,the SEM characterization technique was applied to study the morphology and composition evolution of the hydrogen gas anode and the LCO and LFP cathodes after 50 cycles(Fig.5). Fig.5(A)shows morphology of pristine Pt/C electrode that is made up of many Pt/C nanoparticles. After 50 cycles,the Pt/C electrode shows little change in both LCO-H2and LFP-H2batteries[Fig.5(B)and(C)],demonstrating its excellent stability in rechargeable H2gas batteries. In contrast,the LCO and LFP cathodes have encountered significant changes upon the battery cycling. For example,some crackings were found on the surfaces of the LCO particles after 50 cycles,as shown in Fig.5(D),which leads to the capacity decay of the LCO-H2battery. Fig.5(E)shows that the smooth surfaces of the LFP nanoparticles became rough by creating numerous secondary nanoparticles after 50 cycles[Fig.5(F)]. Similar to the result of the LCO-H2battery,the structural damage of the LFP cathode causes the cycling instability of the LFP-H2battery. These observations indicate that the capacity fading in the LCO-H2and LFP-H2batteries is mainly attributed from the LCO and LFP cathodes,rather than the H2gas anode,demonstrating the versatility and robustness of the H2gas anode for rechargeable H2gas batteries. Future work is suggested to focus on the exploration of stable lithium intercalation materials for the lithium intercalation coumpounds-H2batteries with optimized performance for LSES applications.
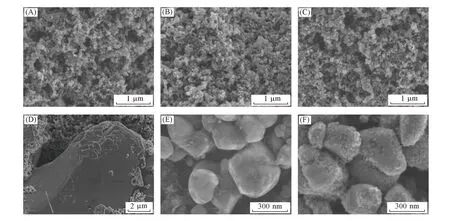
Fig.5 SEM images of pristine Pt/C electrode(A),Pt/C electrode after 50 cycles in LCO-H2 battery(B),Pt/C electrode after 50 cycles in LFP-H2 battery(C),LCO electrode after 50 cycles in LCO-H2 battery(D),pristine LFP electrode(E)and LFP electrode after 50 cycles in LFP-H2 battery(F)
3 Conclusions
Two new battery systems of LCO-H2and LFP-H2batteries were developed using two different lithium intercalation compounds of LCO and LFP as the cathodes and Pt/C catalyst as the H2anode. It was found that the LCO-H2battery has a discharge potential of 1.27 V,a high capacity of 96.9 mA·h·g-1at a low rate of 0.5C and an excellent rate capability. The LFP-H2battery showed a high capacity of 125 mA·h·g-1at a low rate of 0.2C and a good rate capability with a discharge potential of 0.66 V. SEM analysis on the battery electrodes after cycling suggested that the capacity fading of the LCO-H2and LFP-H2batteries was due to unstable cathode materials in aqueous electrolyte. However,the H2gas anode retained good electrocatalytic activity as a robust electrode for rechargeable H2gas batteries. Considering the large number of lithium ion battery cathodes and the unoptimized materials in the current study,it is expected and highly desirable to develop a new category of rechargeable H2gas batteries based on the lithium ion battery cathodes with improved properties,achieving high performance for LSES applications in the future.
This paper was supported by the Startup Funds from USTC,China(No.KY2060000150).
