In-Situ Grow th of NiCo-Layered Double Hydroxides Nanosheets Deposited on Carbon Fiber Cloth for High-Performance Asymmetric Supercapacitors
2021-05-16GUORongGeLIYingLiHUZeYuanSHIMeiYuMIAOYiDongSUIYanWeiQIJiQiuWEIFuXiangRENYaoJianZHANZhenZhenLIUJinLongSUNZhiZHOUMeiHuaMENGDongMei
GUO Rong-Ge LI Ying-Li HU Ze-Yuan SHI Mei-Yu MIAO Yi-Dong SUI Yan-Wei*,, QI Ji-Qiu WEI Fu-Xiang REN Yao-Jian ZHAN Zhen-Zhen LIU Jin-Long SUN Zhi*, ZHOU Mei-Hua MENG Dong-Mei( 6)( 6)
Abstract:The NiCo-layered double hydroxide(LDH)was prepared directly on different carbon fiber substrates by potentiostatic deposition.This method did not need to add any binder,which can prevent the phenomenon of conductivity reduction due to the resistance of the binder.In the layered crystal structure of NiCo-LDH,the positively charged host layer and the interlayer charge compensating anion can promote ion diffusion between electrode materials,so that the active sites can be efficiently utilized.In this paper,an electrode material with a wrinkled structure was synthesized by a potentiostatic deposition technique.Benefiting from the folded lamellar structure features,this composite material(NiCo-LDH/carbon fiber cloth)had sterling specific capacitance(1 387.5 F·g-1 at 1 A·g-1).In addition,with the composite as the positive electrode and the rGO coated on the surface of nickel foam(NF)as negative material,the assembled asymmetric supercapacitor(ASC)presented superb electrochemical performance.ASC had an energy density of 26.6 Wh·kg-1 and a power density of 850.4 W·kg-1 at 1 A·g-1.The energy density remained 14.9 Wh·kg-1 as the maximum power density(8 500.3 W·kg-1).
Keywords:layer double hydroxides;electrodeposition;current collector;asymmetric supercapacitor;nanostructures;energy conversion
0 Introduction
With the sustainable development idea striking root in the hearts of the people gradually,the demand for environment-friendly power conversion and storage devices is increasing[1-4].In the past decades,supercapacitors as a new type of power reservoir have drawn great interest because of their improved performance compared with traditional capacitors and batteries[5-10].Specifically,they have greater capacitance,broader range of working temperatures than traditional capacitors[11-13],and larger power density(1~2 orders of magnitude higher),longer cycle lifetime(2~3 orders of magnitude longer)than conventional batteries[14].The conductivity of electrode materials is the key to the supercapacitor function[15-16].Currently,the most commercial supercapacitor electrodes are pure carbon-based materials[17],which however have a low specific capacitance or energy density(the energy density is generally only 3~5 Wh·kg-1)[18].Hydroxide as a kind of electrode for assembling supercapacitors is the current research hotspot,especially for nickel hydroxide and cobalt hydroxide[17],which has fine electrochemical properties.Nevertheless,the capacitance of single metal hydroxides cannot meet the requirements of high energy density and specific capacitance of supercapacitors[18].Some researchers consider layer double hydroxide(LDH)as an ideal electrode material.The large theoretical specific surface area of LDH provides certain double-layer capacitance,and the transition metal elements on the lamellae can be used as active sites for electrochemical reactions which can provide greater false capacitance[19-20].For example,Feng et al.prepared NiCo-LDH as electrodes,which exhibited a specific capacitance up to 1 551.1 F·g-1at 1 A·g-1[21].
Nowadays,many methods have been developed to prepare LDH,such as hydrothermal method[22],coprecipitation method[23]and electro-deposition method[24].Electro-deposition has become a common preparation method because of its uncomplicated process,safety,environmental protection and short periodicity,requiring to apply no binder or conductive devices as needed in the ordinary preparation process[25-27].The direct growth of LDH on collectors by electrochemical deposition has been widely studied.Li et al.deposited NiCo-LDH to the nickel foam(NF)board via an electrodeposition process.The composite exhibits high specific capacitance(1 540 F·g-1at 1 A·g-1)[28].At present,LDH grown on different collectors(an important component of electrode materials),is rarely studied.The selection of collectors can directly affect the morphology and electrochemical properties of active substances grown on the collector′s surface[29-30].
Carbon fiber paper(CFP)is a kind of paper material made of many fine three-dimensional carbon fibers as the main raw material,referred to as carbon paper.CFP retains the excellent physical and chemical properties of carbon fiber,and it is composed of random ly arranged short fibers,which lead to be isotropic.At the same time,it has good electrical conductivity,high porosity and better tolerance,and can be used as a current collector for energy storage electrode materials[31].Carbon fiber cloth(CFC)is made from oxidized polyacrylonitrile(PAN)fiber fabric after carbonization or from carbon fiber after textile,referred to as carbon cloth[32].CFC has good flexibility when used in lithium ion battery or supercapacitor electrode current collectors,which plays a key role in future flexible electronic products[33].
In this work,two kinds of composite electrodes were obtained via the deposition of NiCo-LDH on CFC and CFP in order to compare the morphology,electrochemical properties and find a collector with the best performance for NiCo-LDH.The composite electrode prepared by carbon cloth(NiCo-LDH/CPC)as collectors showed a striking specific capacitance(1 387.5 F·g-1at 1 A·g-1),which was much higher than the specific capacitance(989.7 F·g-1)of NiCo-LDH/CFP,and the specific capacitance was kept at 82.3% after 2 000 cycles at 10 A·g-1.In addition,an asymmetric supercapacitor(ASC)was assembled with the NiCo-LDH as the positive electrode and the reduced graphene oxide/NF(rGO/NF)as the negative one.The maximum energy density(1 A·g-1)of ASC was 26.6 Wh·kg-1,and the energy density of ASC was still 14.9 Wh·kg-1at the maximum power density(8 500.3 W·kg-1).
1 Experimental
CFP was purchased from Toray Co.Ltd.,and CFC was purchased from Carbon Energy Co.Ltd.All reagents(AR)were purchased from Aladdin Co.Ltd.,with no further purification.
1.1 NiCo-LDH/carbon fiber matrix composites prepared by potentiostatic deposition
Firstly,0.5 mol Co(NO3)2·6H2O and 1 mol Ni(NO3)2·6H2O were put into a beaker with 50 mL deionized water which was into an ultrasonic cleaner and then dissolved evenly for 10 min.Subsequently,the electrodeposition was carried out in the solution by a potentiostatic deposition method.The working electrode was CFC(1 cm×2 cm),and the opposite electrode was platinum.The saturated calomel electrode(SCE)acted as the reference electrode.Finally,the electrodeposition was applied electrochemical workstation,in which the deposition time and voltage were 600 s and-1.0 V,respectively.When the deposition was finished,the prepared electrode materials were cleaned three times by deionization water and alcohol respectively.Then the product was dried in a vacuum drying box of 70℃for 12 h to get NiCo-LDH/CFC composites.The NiCo-LDH/CFP composites were obtained in a similar procedure.The active mass loading was found to(2.5±0.2)mg.
1.2 Preparation of rGO/NF negative electrode
Firstly,the whole piece of NF was divided into 1 cm×2 cm and put in mass fraction of 5% diluted hydrochloric acid.After that,it was washed with ultrasound for 30 min,then rinsed three times with deionized water and anhydrous ethanol respectively,30 min each time.The cleaning method was to remove the impurities and oxide layer on the surface of NF.Finally,the mass of cleaned NF wasm1,after being dried in the vacuum drying box of 70℃for 12 h.The rGO used in the experiment was prepared by a one-step hydrothermal method.Firstly,the 30 m L GO dispersion solution purchased was added to the inner lining of the reactor.The inner lining was sealed and put into the stainless steel reactor.Then,the rGO was heated to 120℃in the electrothermal constant temperature blast drying box and kept for 12 h.The reactor was taken out,and cooled naturally to the room tmperature.Then the dispersion was dried by a freeze dryer for 48 h,and rGO xerogel was obtained.The as-prepared rGO was smeared on the pretreated NF,weighingm2.The weight of rGO active substance coated on the NF was(m2-m1)×80%,which was also the quality value used in electrochemical testing.
1.3 Assembly of asymmetric supercapacitors
In order to further study the practical application ability of positive and negative materials,NiCo-LDH/CFC was used as positive electrode and rGO/NF as negative electrode to assemble ASC.The assembled ASC was then tested on two electrodes in a 1 mol·L-1KOH electrolyte.It should be noted that when assembling ASC,the positive and negative charge should be conserved,and the appropriate mass of positive and negative active substances should be selected according to the specific capacitance.According toQ+=Q-(Q+is positive charge andQ-is negative charge),the mass ratio of positive and negative active substances can be deduced[34]:
Among them,m+andm-represent the mass(g),C+andC-represent the specific capacitance(F·g-1),ΔV+and ΔV-represent the potential windows(V)corresponding to positive and negative materials.Combining Equation 1 with theC+andC-,them+/m-should be 0.23.
All the cathode materials in this experiment were directly deposited on the collector,and no additional conductive agent and binder were added,so they could be directly used as working electrodes.The negative material in the powder state needed to be treated.The specific operation was as follows:firstly,the negative powder material,conductive agent(acetylene black)and binder(polyvinylidene fluoride)were weighed at the mass ratio of 8∶1∶1,and mixed evenly in an agate mortar,followed by adding an appropriate amount of N-methyl pyrrolidone(NMP).The viscous slurry was obtained after grinding for about half an hour.Finally,the slurry was evenly coated on the pretreated collector,dried in a 70℃vacuum drying chamber for 12 h,and then pressed under 10 MPa so as to be the electrode.The quality of the electrode after drying was weighed by an electronic balance and multiplied by 80% of the mass of the collector before applying the slurry,which was the quality of the active material of the negative electrode.Synthesis of polyvinyl alcohol(PVA)and KOH organic gel electrolyte:3 g PVA was dissolved in 30 mL deionized water,heated and stirred for 1 h at 80℃until the solution became thicker.Meanwhile,3 g KOH was dissolved in 10 m L deionized water,and then slowly dripped into a viscous solution.As the temperature gradually decreased,a homogeneous gel,that is,the solid electrolyte was formed.The positive and negative electrode materials were coated with gel electrolyte respectively;and then the diaphragm was placed between positive and negative electrodes,pressed and fixed with polytetrafluoroethylene(PTFE)sheets and tape,and dried for several hours at room temperature.
1.4 Phase and characterization analysis
X-ray diffraction(Bruker,XRD,D8 Advance)was employed to analyze the crystal structure and composition.The test parameters were as follows:the radiation source was theKαray of the Cu target,and the wavelength was 0.154 16 nm,the current and voltage were 40 mA and 40 kV respectively,the scanning rate was 2(°)·min-1,and the angle range was 10°~80°.Fourier transform infrared spectrometry(FTIR,Bruker,VERTEX 80v)was used to identify the molecular structure and chemical bond types.X-ray photoelectron spectroscopy(Thermo Fisher,XPS,ESCALAB 250Xi)was selected to analyze the elemental composition and chemical valence states of the samples.Among them,the X-ray excitation source was AlKα.Wide scan and narrow scan were performed,with the scanning range from 0 to 1 200 eV.The surface morphology of the samples was observed by the scanning electron microscopy(SEM,SU8200,10 kV)while the composition and structure was analyzed by transmission electron microscope(TEM,Tecnai G2 F20,200 kV).The system was also equipped with high-resolution transmission electron microscopy(HRTEM),energy dispersive spectrometer(EDS)and selective electron diffraction(SAED),which could further quantitatively and qualitatively determine the elemental composition of the material surface.
1.5 Electrochemical measurement
The electrochemical performances were tested by the three-electrode test equipment with the prepared electrode material as the working electrode,the stable platinum plate electrode(1 cm×1 cm)as the opposite electrode and the SCE as the reference electrode.The electrochemical performance was tested by using a twoelectrode system.All samples were tested with alkaline KOH solution(1 mol·L-1)as an electrolyte.The electrochemical paraments were calculated by the flowing equations:

WhereCexpresses the specific capacitance(mF·g-2);Δtis the discharge time(s);mis the mass of active substance(g);ΔVis the potential window(V);Eis the energy density(mWh·cm-3)andPis the power density(mW·cm-3).
2 Results and discussion
The NiCo-LDH/carbon fiber matrix electrode material was prepared by one-step potentiostatic deposition.The specific preparation process of NiCo-LDH/CFC composite is shown in Fig.1.The reaction equation is shown below[35]:


Fig.1 Preparation of NiCo-LDH/CFC composite by electrodeposition

The XRD patterns of NiCo-LDH/CFP and NiCo-LDH/CFC are shown in Fig.2a.CFP had better crystallinity,so the peaks of hydroxide were more obvious.The characteristic diffraction peaks correspond to the(101)and(110)crystal planes of Ni(OH)2(PDF No.14-0117)and Co(OH)2(PDF No.30-0443).
The FTIR spectra of NiCo-LDH/CFC is shown in Fig.2b,where the absorption peak at 2 926 nm corresponds to the stretching vibration absorption peak of hydroxyl O—H.The bending vibration absorption peak at 1 634 cm-1is the absorption vibration absorption peak of water molecules.The absorption peak at 1 385 cm-1corresponds to the vibration absorption peak of NO3-which is an interlayer anion of LDH and increases the interlayer spacing of the structure.The absorption peak near 1 105 cm-1is the bending vibration absorption peak of water molecule absorbed by C—H,and the absorption peak around 1 036 cm-1coincides with the C—N stretching vibration.The absorption peaks at 649 and 557 cm-1are the tensile bending vibration peaks of M—O,which can prove the existence of NiCo-LDH[36].In conclusion,NiCo-LDH/CFC composites have been successfully prepared.

Fig.2 (a)XRD patterns of pure NiCo-LDH/CFP and NiCo-LDH/CFC composites;(b)FTIR spectrum of NiCo-LDH/CFC composite
The chemical elements and valence states of the materials were further characterized by XPS spectra,which is shown in Fig.3.No other element was found except the main Ni,Co,O and C elements(Fig.3a),indicating no impurities in the prepared composites and C elements coming from the carbon cloth matrix.The high resolution spectra were performed on the remaining elements,and then the peak was fitted by Gauss fitting method.The XPS spectrum of Ni2pis shown in Fig.3b.At the binding energy of 855.4 and 872.7 eV,attributed to Ni2+[37],there were two spinorbit split peaks,Ni2p3/2and Ni2p1/2,both of which are accompanied by satellite peaks.Fig.3c is the XPS spectrum of Co2p,in which satellite peaks exist at the peaks of 781 and 796.6 eV,attributed to Co2+[38],corresponding to Co2p3/2and Co2p1/2spin-orbit splitting peaks.The XPS spectrum of O1sis shown in Fig.3d.The peak at 530.8 and 531.6 eV correspond to the binding energy of M—O—H and water molecule,respectively.The results indicate that NiCo-LDH composites have been successfully prepared.

Fig.3 XPS spectra of NiCo-LDH/CFC composite:(a)full survey,(b)Ni2p,(c)Co2p and(d)O1s
SEM was applied to the analysis of the morphology and characteristics of NiCo-LDH/CFP and NiCo-LDH/CFC,as shown in Fig.4a~4f.The different sizes of spherical structures can be seen from the low magnification SEM image of NiCo-LDH/CFP(Fig.4a,4c).When magnified at high magnification,many spherical structures were found to be stacked together,and many small pieces were around each sphere.From the lowrate SEM images(Fig.4b,4d),it can be found that a layer of active substances grew uniformly around the carbon cloth for NiCo-LDH/CFC.The active substance NiCo-LDH was found to be a folded lamellar structure when high-power SEM images were observed.This unique structure can provide a larger specific surface area and more active sites,which are conducive to ion spread and faster transport,thus accelerating the redox reaction of NiCo-LDH[39].

Fig.4 SEM images at different magnifications:(a,c,e)NiCo-LDH/CFP and(b,d,f)NiCo-LDH/CFC
In order to further analyze the microstructure of NiCo-LDH/CFC,TEM,HRTEM,SAED and EDS were obtained,as shown in Fig.5.It can be seen that NiCo-LDH/CFC(Fig.5a)was formed by interlacing sheets of folds,which matched well with Fig.4b,4d and 4f.Fig.5b is the HRTEM image of an area in Fig.4f,which presented the lattice fringes.By further measurement,the crystal plane spacing was 0.23 nm,which corresponds to(015)crystal plane of the LDH phase of hydrotalcite-like.Fig.5c is the SAED pattern of NiCo-LDH/CFC composite.It can be seen that the structure of NiCo-LDH/CFC composite was polycrystalline and had two rings corresponding to(110)and(101)planes of LDH(PDF No.38-0715).The EDS spectra of NiCo-LDH/CFC composites is shown in Fig.5d.Diffraction peaks of Ni,Co,O and C could be clearly detected,in which C came from CFC,and Ni,Co and O came from the prepared NiCo-LDH.As shown in Fig.5d,the molar ratio of Co to Ni was close to 1∶2,which coincided with the proportion of drugs added.It indicates that the NiCo-LDH composite has been successfully prepared.

Fig.5 (a)TEM image,(b)HRTEM image,(c)SAED pattern and(d)EDS spectra of NiCo-LDH/CFC composite
In order to further examine the influence of different carbon fibers on the electrochemical properties of NiCo-LDH composites,the three-electrode system was used.The electrochemical performance comparison of NiCo-LDH/CFP and NiCo-LDH/CFC electrode materials is shown in Fig.6a.The specific capacitance of NiCo-LDH/CFC electrode was larger than that of NiCo-LDH/CFP.Fig.6b shows the galvanostatic charge-discharge(GCD)curves of NiCo-LDH/CFP and NiCo-LDH/CFC at a current density of 1 A·g-1.The discharge time of NiCo-LDH/CFC composites was longer than that of NiCo-LDH/CFP.And the capacitance of NiCo-LDH/CFC was larger than that of NiCo-LDH/CFP,which is consistent with the results of cyclic voltammetry(CV)curves.The specific capacitance of NiCo-LDH/CFC composites(1 384.5 F·g-1)was 394.8 F·g-1higher than that of NiCo-LDH/CFP composites(989.7 F·g-1).The results show that the morphological characteristics of NiCo-LDH/CFC composites are more conducive to improve the electrochemical properties.The equations for the chemical mechanism of NiCo-LDH in the alkaline environment are as follows:
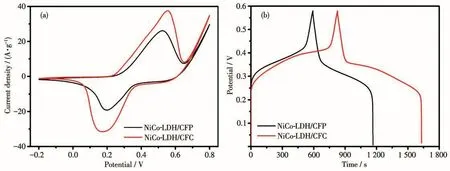
Fig.6 (a)CV curves at 10 mV·s-1 and(b)GCD curves at 1 A·g-1 of NiCo-LDH/CFP and NiCo-LDH/CFC composites

The CV curves of NiCo-LDH/CFC at a potential window of-0.2~0.8 V with different scanning rates(5~30 mV·s-1)are shown in Fig.7a.Each curve had a pair of intense redox peaks,and with the increase of the scanning rate,the redox peaks were more obvious.At the same time,the shape of the CV curves did not change much,which shows that the redox reaction of electrode materials had good reversibility.The GCD curves of NiCo-LDH/CFC in different current densities are shown in Fig.7b,where the charge-discharge potential window was 0~0.58 V.There was a platform for the GCD curve in the charge-discharge conversion process,and the corresponding redox peaks in the CV curve also revealed a reversible charging and discharging behavior.
According to Equation 2,as the current densities were 1,2,5,8,10 and 15 A·g-1,the specific capacitances of NiCo-LDH/CFC were corresponding to 1 387.5,1 348.3,1 284.5,1 251.0,1 224.1 and 1 194.8 F·g-1(Fig.7c).with the increase of current density,the specific capacitance decreased.This is because the high current density limited the speed of proton migration in the active substance.The proton cannot migrate to the inside of the active substance in a short time,and it results in the redox reaction of only a few active substances on the surface and inside,thus reducing the specific capacitance.When the current density of NiCo-LDH/CFP composites increased from 1 to 15 A·g-1,the specific capacitance retention of NiCo-LDH/CFP composites was only 62.2%,lower than that of NiCo-LDH/CFC composites(86.3%).Therefore,the ratio of NiCo-LDH/CFC was better than that of NiCo-LDH/CFP electrode materials.Fig.7d depicts EIS(electrochemical impedance spectroscopy)spectra of LDH composites with two different matrices in a certain fre-quency range(0.01 Hz~10 kHz).The curve was composed of a semi-circular arc at a high-frequency area and a straight line at a low-frequency area[40].The intercept between the high-frequency area and the real axis is expressed as an equivalent series resistor(Rs),which includes the intrinsic resistance and contact resistance between the active substance and the collector,and the self-resistance of the ionic electrolyte.Obviously,the EIS curve of NiCo-LDH/CFC and had a smaller intercept with the real axis,which indicated that the equivalent series resistance of LDH/CFC was smaller.The size of semicircle in the high-frequency area reflects the size of charge transfer resistance(Rct).TheRctof NiCo-LDH/CFC was smaller than that of NiCo-LDH/CFP.The straight line of slope in the low-frequency area reflects Warburg impedance(Zw),which refers to the diffusion resistance of ions between active substances and electrolytes.The smaller the resistance is,the stronger the diffusion ability is,which leads to the larger specific capacitance of the electrode material.It can be seen from Fig.7d that the slope of the curve of NiCo-LDH/CFC was larger than that of NiCo-LDH/CFP,which indicates that theZwof NiCo-LDH/CFC was smaller.In general,the total resistance of NiCo-LDH/CFC was smaller than that of NiCo-LDH/CFP,which is conducive to having a larger specific capacitance.

Fig.7 NiCo-LDH/CFC composite:(a)CV curve at different scanning rates,(b)GCD curves with different current densities;(c)Comparison of specific capacitance at different current densities and(d)EIS spectra of NiCo-LDH/CFP and NiCo-LDH/CFC composites
In summary,NiCo-LDH on CFC composites had higher specific capacitance,better ratio and lower electrochemical impedance.For the sake of further studying the cycle performance of NiCo-LDH/CFP and NiCo-LDH/CFC,the LAND battery test system was used to test the cycle performance at a current density of 10 A·g-1.Fig.8 shows the cyclic stability of the two electrode materials after 2 000 GCD cycles.It can be seen that the capacitance of NiCo-LDH/CFP composite decreased rapidly before 500 cycles,and then kept a gentle decline,which shows that its cycling performance was poor.The specific capacitance retention of NiCo-LDH/CFC maintained 82.3% of the initial one after 2 000 cycles.The results show that the cyclic stability of NiCo-LDH/CFC was better than that of NiCo-LDH/CFP,but it still needs to be further improved.

Fig.8 Cyclic stability of NiCo-LDH/CFP and NiCo-LDH/CFC composites
Fig.9 shows the results of CV,GCD and EIS performance of ASC tested under a two-electrode system.Fig.9a was a device diagram of an asymmetric supercapacitor under a two-electrode test system.Fig.9b shows the CV curves of NiCo-LDH/CFC and rGO/NF cathode at 10 mV·s-1.The positive potential window was-0.2~0.8 V and the negative potential window was-1.0~0 V.Theoretically,the potential window of ASC can reach 1.8 V.Such a voltage window had a wide range,which provided an excellent precondition for ASC to have high energy density.Fig.9c is the CV curves of ASC at 10 mV·s-1.The potential window increased from 1.3 to 1.8 V.The CV curve was rectangular in shape,accompanied by a certain oxidation-reduction peak,which shows that ASC stores energy through physical adsorption and oxidation-reduction reaction.Because the polarization of the CV curve occurred when the potential window was 0~1.8 V,the most suitable potential window in the ASC test should be 0~1.7 V.

Fig.9 (a)Schematic diagram of a two-electrode test system of ASC;(b)CV curves of positive and negative electrodes;(c)CV curves under different voltage windows,(d)CV curves under different scanning rates,(e)GCD curves under different current densities,and(f)specific capacitances changed with different current densities of ASC
Fig.9d presents the CV curves of ASC with the scanning speed from 10 to 100 mV·s-1at a potential window of 0~1.7 V.The shape of ASC did not change with the increase of the scanning speed,which indicates that ASC can also provide stable power output at high current density.For studying the properties of ASC,the GCD curves at current densities of 1 to 10 A·g-1were measured.As can be seen from Fig.9e,the charge-discharge curve was almost symmetrical,indicating that the ASC had good charge-discharge efficiency.Calculated by Equation 1[34],the specific capacitances at 1,2,5,8 and 10 A·g-1were 66.2,58.2,49.1,41.4 and 37.1 F·g-1,respectively(Fig.9f).
The electrochemical cycle stability of ASC was tested at 5 A·g-1(Fig.10a).After 5 000 GCD cycles,the specific capacitance retention ratio remained 79.9%,suggesting that ASC had good cycle stability.From the inset of Fig.10a,we can see that the slope of the EIS spectrum after the cycle in the low-frequency area became larger.It indicates theZWof the electrode became smaller,hence the capacitance was subject to a smaller influence.According to Equation 8 and 9,Ragone curves representing the relationship between the density of energy and the density of power are acquired(Fig.10b).When the minimum power density of ASC was 850.4 W·kg-1,the maximum energy density was 26.6 Wh·kg-1.And as the maximum power density of ASC was 8 500.3 W·kg-1,the minimum energy density was 14.9 Wh·kg-1.ASC has higher energy density and power density than the reported water-based asymmetric supercapacitors,such as Ni3S2//carbon nanotubes(energy density of 19.8 Wh·kg-1,power density of 798 W·kg-1)[41],Ni0.28Co0.72(OH)2//AC(energy density of 19.4 Wh·kg-1,power density of 80.5 W·kg-1)[42].At the same time,ASC can light up 0.1 W light-emitting diodes.The results demonstrate that ASC is provided with fine practical value.

Fig.10 (a)Cyclic stability and EIS spectra(inset),and(b)Ragone curve and assembly picture of ASC

Table 1 Comparison of the performance of the NiCo LDH electrode
3 Conclusions
In summary,NiCo-LDH nanosheets with different morphologies were successfully prepared on different carbon fibers by a potentiostatic electrodeposition method.The morphology of NiCo-LDH on CFP was spherical,and that electrode prepared on CFC was lamellar.The optimum NiCo-LDH/CFC electrode material had excellent specific capacitance(1 A·g-1,1 387.5 F·g-1),which was much higher than that of NiCo-LDH/CFP(1 A·g-1,989.7 F·g-1).It also had good cycle stability since the special electric capacity can keep 82.3%(at 10 A·g-1)after 2 000 cycle tests.Moreover,the ASC set up with the NiCo-LDH/CFC and the rGO/NF was assembled and showed good performance.At 1 A·g-1,the maximum energy density was 26.6 Wh·kg-1and the power density was 850.4 W·kg-1.In addition,the energy density can still maintain 14.9 Wh·kg-1at the maximum power density(8 500.3 W·kg-1).Therefore,the LDH electrode prepared on CFC has good performance and the potential as a new electrochemical power storage material.
Acknowledgments:This research was financially supported by the National Natural Science Foundation of China(Grants No.51671214 and 51871238),Xuzhou Achievements Transformation Project(Grant No.KC19235)and China innovation project of college students.
杂志排行
无机化学学报的其它文章
- Synthesis and Characterization of Metal-Organic Framework Based on 2,6-Bis(4-carboxybenzylidene)cyclohexanone
- Two Metal-Organic Frameworks Built from 2,2′-Dimethyl-4,4′-biphenyldicarboxylic Acid
- Three Photochromic Co-crystals Based on Viologen Moiety
- Effect of Mass Ratio of Ni and Co in Initial Solution on Oxygen Evolution Reaction Performance of Ni-Co-S-O/NF Catalyst in Alkaline Water Electrolysis
- Structure and Fluorescence Properties of Three 1D/2D/3D Zn/Cocomplexes Based on Flexible Tetracarboxylic Acid
- Two Nitronyl Nitroxide Biradical-Bridged Lanthanide One-Dimensional Chains:Crystal Structure,Magnetic Properties and Luminescent Behavior
