Synthesis and Characterization of Metal-Organic Framework Based on 2,6-Bis(4-carboxybenzylidene)cyclohexanone
2021-05-16PANWeiMAChiXiaoZHOUChuanJiangZHANGLuZHANGJunYongSHIYanBoXUHaoZHUDunRuXIEJingLi008600000
PAN Wei MA Chi-Xiao ZHOU Chuan-Jiang ZHANG Lu ZHANG Jun-Yong*, SHI Yan-Bo XU Hao ZHU Dun-Ru XIE Jing-Li*,,( 00)( 86)( 000)( 00)
Abstract:By virtue of 2,6-bis(4-carboxybenzylidene)cyclohexanone(H2 L)as the ligand,metal-organic framework[MnL]n has been successfully fabricated.This compound has been characterized by FT-IR,powder X-ray diffraction,cyclic voltammogram,solid UV,X-ray photoelectron spectroscopy and single-crystal X-ray diffraction.It crystalizes in triclinic system with space group P,and the asymmetric unit is composed of one Mnion and one ligand.The carboxyl groups at both ends of the ligand are monodentate coordination,and the oxygen on the carbonyl group in the middle of the ligand coordinate with the central ion as well.Each ligand is coordinated with three Mnions and consequently,achieving a relatively stable triangular coordination configuration.The Mnion forms a sixcoordinated configuration with oxygen atoms from six different ligands,i.e.,those four O atoms in the equatorial plane derived from four different ligands′carboxyl groups,the other two O atoms on the two vertices from the different ligands′carbonyl groups,respectively.As a consequence,the octahedral configuration[MnO6]unit is formed.Based on the topological analysis,its two-dimensional(2D)kgd structural character could be observed.The cyclic voltammogram showed that the half-wave potential,E1/2=(E cp+E ap)/2,was 171 mV when the sweep speed was 30 mV·s-1.The solid UV absorption spectra showed the band gap was~1.76 eV.Photocatalytic activity has been observed in the process of degrading of dye molecules such as methylene blue and methyl orange.CCDC:2045728.
Keywords:2,6-bis(4-carboxybenzylidene)cyclohexanone;manganese compound;crystal structure;topology structure
0 Introduction
As a type of functional crystalline material,metalorganic frameworks(MOFs)has developed rapidly and it could be exploited in the fields of gas adsorption and separation,electrochemistry,catalysis,ion exchange and sensors[1-6].Recently,Zang′s group used highly ordered macro-micropore zeolitic imidazolate framework-8(ZIF-8)to encapsulate enzyme such as horseradish peroxidase(HRP)and notably,the HRP enzyme not only maintained its activity,but also exhibited stability,recyclability,low leakage as well as resistance to chelating compounds.This work demonstrated the great potential of MOFs materials to help to fabricate biocatalysts[7].By using the multifunctional ligand of 3-(3,5-dicarboxylphenyl)-5-(4-carboxylphenyl)-1-H-1,2,4-triazole(H3DBPT)to bridge hexanuclear{Cd6}clusters,a chemical-stable and thermal-stable luminescent metal-organic framework(MOF),[Cd3(DBPT)2(H2O)4]·5H2O,has been reported by Liu et al.The resulting material has shown sensitivity and selectivity toward nitroaromatic compounds and nitroimidazole-based drug molecules[8].Taking advantage of the ligand 2,6-bis(((2-hydroxybenzyl)(2-hydroxyethyl)amino)methyl)-4-methylphenol,a fluorescent tetranuclear[ZnⅡ4(L-3H)2](ClO4)2·3.5H2O could bind to phosphate backbones of DNA covalently.And this complex exhibited its optimal DNA hydrolytic activity,indicating the potential application in the field of biological chemistry[9].
During the process of achieving MOFs materials with aesthetical structural characters,organic ligands that containing versatile coordination sites have important roles.We have embarked on several kinds of MOFs materials by using the 2,6-bis(4-carboxybenzylidene)cyclohexanone(H2L)ligand(Scheme 1)[10-11].The H2L ligand contains one keto and two carboxylic acid groups,which make it a suitable candidate to combine with oxophilic metal ions to achieve MOFs.Various structural characters have been explored in our laboratory:(a)when using lanthanide metals such as La,Ce,Pr,Nd,Sm,Eu and Gd,three-dimensional(3D)metalorganic frameworks with honeycomb cavities have been obtained[10];(b)when using transition metals such as Zn and Cd,two-dimensional(2D)metal-organic frameworks with structural diversity have been observed[11].It is believed that more elegant structural characters could be observed and in any case,experimental conditions such as pH,temperature,solvent type,reaction duration,template,auxiliary ligands and reaction type(batch or flow type)play important role in the process of achieving the final product.

Scheme 1 Ligand H2 L with potential coordination sites
The overarching aim of this research is to broaden MOFs chemistry and in this contribution,[MnL]nhas been achieved in a feasible way.The described(3,6)-connectedkgdtopological character with schläfli sym-bol(43)2(46·66·83)adds value to the research field.
1 Experimental
1.1 Materials and measurements
All the chemicals were received as reagent grade and used directly without further purification.The ligand H2L was synthesized via Claisen-Schmidt condensation reaction.IR spectrum was recorded on a Varian 640 FT/IR spectrometer with KBr powder in a range of 4 000~500 cm-1.Powder X-ray diffraction(PXRD)pattern was collected on a DX-2600 spectrometer with MoKαradiation(λ=0.071 073 nm)at 293 K(Voltage:40 kV,Current:30 mA,Scan range:0°~60°).Thermal gravimetric analyses(TGA)were carried out under N2flow on an SDT 2960 differential thermal analyzer at a heating rate of 10℃·min-1.Cyclic voltammograms(CVs)were obtained with a CHI 440A electrochemical workstation at room temperature.Platinum wire was used as a counter electrode and Ag/AgCl electrode was used as a reference electrode.Chemically bulk-modified carbon paste electrode(CPE)was used as the working electrode.The diffuse reflectance spectra(DRS)of the crystalline solid was recorded by the UV-Vis-NIR spectrophotometer(Cary 5000).The UVVis measurements of solution were carried out on a Thermo EV 201CP.X-ray photoelectron spectroscopy(XPS)analyses was carried out with a Kratos Axis Ultra spectrometer using a monochromatic AlKαsource(15 mA,14 kV).
1.2 Syntheses of Mn-MOF[MnL]n
A mixture of MnCl2·4H2O(9.89 mg,0.05 mmol),H2L(18.1 mg,0.05 mmol),DMF(4 mL),C2H5OH(2 mL)and H2O(1 mL)was placed in a 10 m L glass bottle and then under ultrasonic for 30 minutes.The mixture was heated in an oven at 80℃for 20 hours.After slowly cooling to room temperature,dark brown block crystals were obtained by filtering and washing(Yield:68%,based on Mn).IR(KBr,cm-1):3 425(m),2 925(s),1 624(vs),1 405(s),1 266(m),1 139(m),779(s).Elemental analysis Calcd.for C22H16MnO5(%):C,63.63;H,3.88.Found(%):C,62.76;H,3.91.
1.3 X-ray crystallography
The suitable crystal of Mn-MOF was selected and characterized on an Oxford Diffraction Gemini R Ultra diffractometer with graphite-monochromated MoKα(λ=0.071 073 nm)at 293(2)K.The structure was solved by direct methods and refined onF2by full-matrix least-squares methods using the SHELXTL package[12-13].The hydrogen atoms attached to carbon atoms were placed at geometrically estimated positions.A summary of the crystal data and structure refinements of the compound is provided in Table 1 and the selected bond lengths and angles are given in Table 2.

Table 1 Crystal data and structure refinements for Mn-MOF

Table 2 Selected bond distances(nm)and bond angles(°)for Mn-MOF

Continued Table 2
CCDC:2045728.
1.4 Photocatalytic degradation of organic pollutants by Mn-MOF
In this contribution,methylene blue(MB)and methyl orange(MO)were selected as model pollutants to explore their degradation process in the presence of Mn-MOF.Mn-MOF(20 mg)was added into 50 mL aqueous solution of MB or MO,then the obtained suspension was magnetically stirred in the dark for 15 minutes to ensure the equilibrium.The solution was exposed to UV irradiation from a 100 W Hg lamp(λ=365 nm)and it was kept stirring.At given time intervals,3 mL of sample was taken out for UV-Vis measurements.
2 Results and discussion
2.1 Structural analysis of Mn-MOF
Crystallographic analysis reveals that Mn-MOF crystalizes in the triclinic crystal system with space groupP.The two carboxyl groups in the L2-ligand are deprotonated,and the coordination mode is shown in Fig.1.The carboxyl groups at both ends of the ligand are monodentate coordination,and the oxygen on the carbonyl group in the middle of the ligand participates the coordination as well.Each ligand is coordinated with three Mnions,thus forming a relatively stable triangular coordination configuration.The Mnion is coordinated with six O atoms from different ligands to form a octahedral[MnO6]unit(Fig.1c).The four O atoms in the equatorial plane are derived from four different L2-ligands′carboxyl groups(O2,O2v,O3iv,O3iii),and the other two O atoms on the two vertices from the two ligands′carbonyl groups(O1i,O1ii),respectively.

Fig.1 (a)Coordination mode of L2-ligand in Mn-MOF;(b)Coordination environment of Mn ion;(c)Coordination mode of Mn ion
The[MnO6]unit bridged each other through the O atoms on the carboxyl groups and four Mnions,gen-erating a one-dimensional(1D)chain alongcaxis direction with the Mn…Mn distance of 1.990 17(36)nm.Based on the one-dimensional(1D)chain(Fig.2a),the oxygen atoms and Mnions inbaxis direction connect the one-dimensional chain with each other,forming a two-dimensional(2D)layered structure(Fig.2b).In addition,the two-dimensional(2D)plane has a hexagonal channel incaxis direction and a rectangular-like window perpendicular toaaxis.

Fig.2 (a)One-dimensional chain structure and(b)2D layer structure of Mn-MOF
Regarding its structural character,the topological structure is described in Fig.3.Each ligand is coordinated with three Mn atoms,which can be simplified as a three-connected node,namely node 1(Fig.3a),and each Mn atom is connected to six ligands,which can be simplified as a six-connected node,namely node 2(Fig.3b).The Mn-MOF can be simplified to the classic 2D(3,6)-connectedkgdtopology by TOPOS(Fig.3c)and its Schläfli symbol is(43)2(46·66·83).
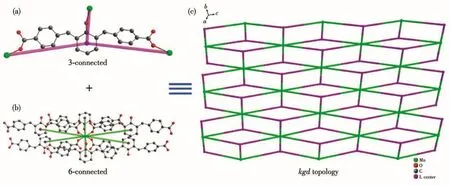
Fig.3 Topological simplification diagram of Mn-MOF
2.2 IR spectrum and PXRD of Mn-MOF
As shown in Fig.4a,the weak absorption peak at 2 944 cm-1is assigned to the C—H stretching vibration of cyclohexanone ring.A strong band at 1 635 cm-1can be attributed to the C=O stretching vibration of the carbonyl group.Two absorption peaks at 1 599 and 1 404 cm-1are assigned to the asymmetric and symmetric stretching vibration of carboxyl group,respectively.The peak at 1 280 cm-1can be assigned to the C—O stretching vibration.In addition,the peak at 975 cm-1is the characteristic absorption peak of C=C,while a peak at 785 cm-1arises from the para-substituted characteristic peak of phenyl ring.
To test the phase purity of Mn-MOF,PXRD experiment was carried out(Fig.4b).The diffraction peaks of both simulated and experimental patterns matched well in the key positions,indicating that the powders of the compound are single phase.The intensity differences may arise from the different orientation of the samples.

Fig.4 (a)IR spectrum and(b)PXRD patterns of Mn-MOF
2.3 TGA analysis and electrochemical property of Mn-MOF
The thermal stability of Mn-MOF has been investigated.The TGA curve showed one step of weight loss(Fig.5a).The whole skeleton began to collapse at about 375℃with the weight loss being 62.37%,indicating that this MOF has relatively high thermal stability.The structure became stable after 700℃,and the final residue was MnO(37.63%).
The electrochemical property of Mn-MOF has been investigated in detail in 0.5 mol·L-1Na2SO4+0.1 mol·L-1H2SO4aqueous solution.In a potential range of-200~500 mV,there was a pair of reversible redox peak.The half-wave potentialE1/2was 171 mV(Scan rate:30 mV·s-1)(Fig.5b).
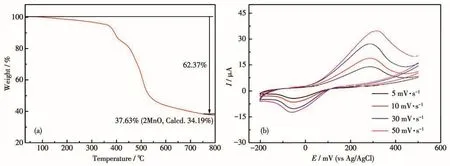
Fig.5 (a)TGA curve and(b)CV curves of Mn-MOF
2.4 DRS of Mn-MOF
The absorption data of H2L and Mn-MOF were calculated from reflectance data by using the Kubelka-Munk function.BaSO4powder was used as reference(100% reflectance)[14].Compared with H2L ligand,the red shift of Mn-MOF has been observed.The peak around 450 nm may arise from the metal-to-ligand charge transfer(MLCT)[15],while those two absorptions at 235 and 296 nm might originate from theπ→π*absorption of the phenyl ring and double bond unit(Fig.6a).The estimated band gap of H2L and Mn-MOF sampleswere~2.50and~1.96eV,respectively(Fig.6b).

Fig.6 (a)UV-Vis DRS spectra and(b)band gaps of H2 L and Mn-MOF
Apparently,compared with the ligand,Mn-MOF exhibited absorbance around 450 nm.It may act as photocatalyst and its activity has been explored[16-17].
2.5 XPS of Mn-MOF
To further clarify the valence of Mn ion in Mn-MOF,the Mn2pXPS spectrum was recorded and fitted(Fig.7).Analysis of Mn2pXPS spectrum show that the binding energies of Mn2p3/2(640.9 eV)and Mn2p1/2(653.5 eV)and their spin energy difference(12.6 eV)are consistent with the Mn.Moreover,the unique satellite peak could further confirm that Mn ion is divalent[18-20].
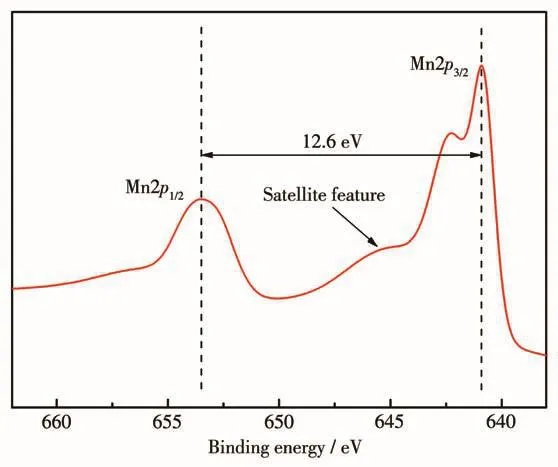
Fig.7 XPS spectrum of Mn2p in Mn-MOF
2.6 Photocatalytic activities
MOFs materials could be exploited as green photocatalysts for the removal of organic pollutants from water[11].So,the degradation of MB and MO is used to evaluate the photocatalytic activity of Mn-MOF.Fig.8 shows the photocatalytic effect of Mn-MOF and its degradation efficiency for MB is better than MO.The calculation results show that the conversion of MB and MO under irradiation after 5 h was 23% and 14%,respectively(Fig.8c).

Fig.8 UV-Vis spectra varying with light time of(a)MB and(b)MO dye in the presence of Mn-MOF;(c)Degradation rates of MB/MO in the absence and presence of Mn-MOF
3 Conclusions
Using 2,6-bis(4-carboxybenzylidene)cyclohexanone(H2L)as organic ligand,one 2D Mn-MOF withkgdtopology has been fabricated and characterized by IR,PXRD,TGA,CV,solid UV,XPS and X-ray crystal structure analysis.Those O atoms of L2-and Mnions connect the one-dimensional chain with each other,forming the 2D layered structure.Currently,we are actively pursuring along this research line as following:(1)apart from the main ligand,we aim to construct diverse types of structures by introducing various auxiliary ligands;(2)we are using the emergent technique,flow chemistry,and taking advantage of its impressive abilities such as high effective mass-and heat-transfer to synthesize MOFs and hopefully,we could finely control reaction rate,speed up the synthetic process and obtain target molecules with high yields[21-24].
杂志排行
无机化学学报的其它文章
- Two Metal-Organic Frameworks Built from 2,2′-Dimethyl-4,4′-biphenyldicarboxylic Acid
- Three Photochromic Co-crystals Based on Viologen Moiety
- Effect of Mass Ratio of Ni and Co in Initial Solution on Oxygen Evolution Reaction Performance of Ni-Co-S-O/NF Catalyst in Alkaline Water Electrolysis
- Structure and Fluorescence Properties of Three 1D/2D/3D Zn/Cocomplexes Based on Flexible Tetracarboxylic Acid
- Two Nitronyl Nitroxide Biradical-Bridged Lanthanide One-Dimensional Chains:Crystal Structure,Magnetic Properties and Luminescent Behavior
- Synthesis and Visible-Light-Driven Photocatalytic Properties of Floating BiFeO3/Expanding Perlite Photocatalysts
