CO oxidation on the heterodinuclear tantalum–nickel monoxide carbonyl complex anions
2021-05-14JumeiZhngLiYnBiGngLiDongYngHuijunZhengJinghnZouXingtoKongHongjunFnZhilingLiuLingJingHuXie
Jumei Zhng,Y Li,Yn Bi,Gng Li,Dong Yng,Huijun Zheng,Jinghn Zou,Xingto Kong,Hongjun Fn,Zhiling Liu,*,Ling Jing,*,Hu Xie,*
a State Key Laboratory of Molecular Reaction Dynamics, Collaborative Innovation Center of Chemistry for Energy and Materials (iChEM), Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
b School of Chemical and Material Science, Key Laboratory of Magnetic Molecules & Magnetic Information Materials, Ministry of Education, Shanxi Normal University, Linfen 041004, China
c University of Chinese Academy of Sciences, Beijing 100049, China
ABSTRACT The series of heterodinuclear metal oxide carbonyls in the form of TaNiO(n=5–8)are generated in the pulsed-laser vaporization source and characterized by mass-selected photoelectron velocity-map spectroscopy.During the consecutive CO adsorption, the -O-bent structure initially is the most favorable for TaNiO,and subsequently both-O-bent and -O-linear structures are degenerate for TaNiOthen the -O-linear structure is most preferential for TaNiOand finally the-CO2-tagged structure is the most energetically competitive one for TaNiO,i.e.,the CO oxidation occurs at=8.In contrast to the literature reported CO oxidation on heteronuclear metal oxide complexes generally proceeding via Langmuir–Hinshelwood-like mechanism,complementary theoretical calculations suggest that both Langmuir–Hinshelwood-like and Eley–Rideal-like mechanisms prevail for the CO oxidation reaction on TaNiOcomplex.Our findings provide new insight into the composition-selective mechanism of CO oxidation on heteronuclear metal complexes, of which the composition be tailored to fulfill the desired chemical behaviors.
Keywords:CO oxidation Photoelectron imaging Heteronuclear oxide Density functional theory Transition metal carbonyl
The low-temperature oxidation of CO has received considerable attention from the environmental and material scientists,due to its important role in controlling of vehicle exhaust emissions and purification of gas streams derived from petrochemical industry[1–3].Particularly interesting are the nanoscale catalysts, the composition and structure of which can be tailored to fulfill the desired chemical behaviors [4–6].With the aid of state-of-art in-situ spectrum technologies, two dynamically distinct types of bimolecular surface reactions have been proposed in the heterogeneous catalysis of CO oxidation, which are now denoted as Langmuir–Hinshelwood (LH)- and Eley–Rideal (ER)-type mechanism,respectively[7].In the LH mechanism,both the CO molecule and oxygen species are coadsorpted on the catalyst surface, and subsequently, the CO2is formed by either the rearrangement between chemisorbed CO and oxygen,or direct attachment of the CO ligand to the near-neighbor oxygen centers(intrasystem attack)[8].On the contrary, in the ER mechanism, the CO molecule from the gas phase reacts directly with the chemisorbed oxygen species on the catalyst surface to give rise to CO2(intersystem attack)[8].
Microscopically,the real-life catalytic event usually takes place on a specific active site consisting of only a small number of atoms,of which the particular electronic, geometric, and bonding properties are the root of selectivity of CO oxidation mechanism.The gas-phased cluster reaction researches performed under isolated,size-controllable,and reproducible conditions provide an alternative route to capture clear molecular- and electronic-level mechanisms of the catalytic CO oxidation.The CO oxidation of the binary transition metal oxide (TMO) clusters serves as a welldefined model pertinent to mechanistic understandings of the surface catalysis.Recent researches of particular interest are the heteronuclear TMO clusters and highlight the potential to modulate chemical processes by selective cluster doping [9,10].The different metallic fractions of heteronuclear TMO clusterscan mimic either the individual active sites or their supports of real-life catalysts [11].The well similar behaviors paralleled with the condensed-phase CO oxidation by supported catalysts have been found in the gas-phase catalytic CO oxidation mediated by nanocatalysts doped by noble-metal single-atom and dimer[12–15].The noble-metal-like behavior in many catalytic processes has also been found for a few noble-metal-free heteronuclear TMO clusters[14–16].The high adsorption energy of CO on the atomic or dimeric dopants and dynamic nature of dopants in terms of the electron storage and release are found to be the driving force for the CO oxidation [17].Due to the strong carbonyl binding to the doped TM, especially for the noble TM, the atomic TM dopant usually functions as a preferred trapping site and electron acceptor for CO adsorption and then acts as a deliverer of CO ligand for CO oxidation by the oxygen species on the heteronuclear TMO cluster.In this sense, available gas-phase experiments in the literature indicate that the CO oxidation reaction on heteronuclear TMO clusters preferentially proceeds via LH-like mechanism.
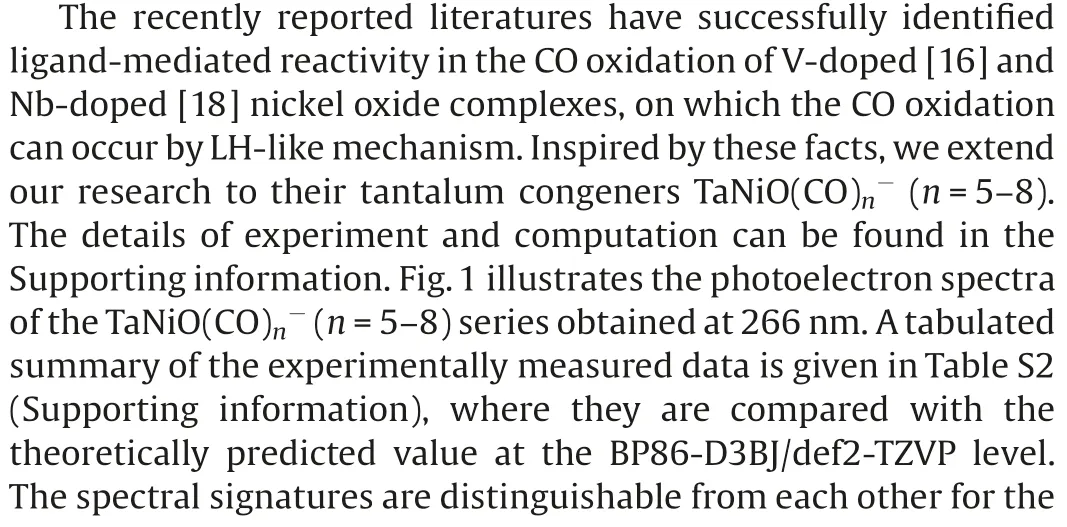
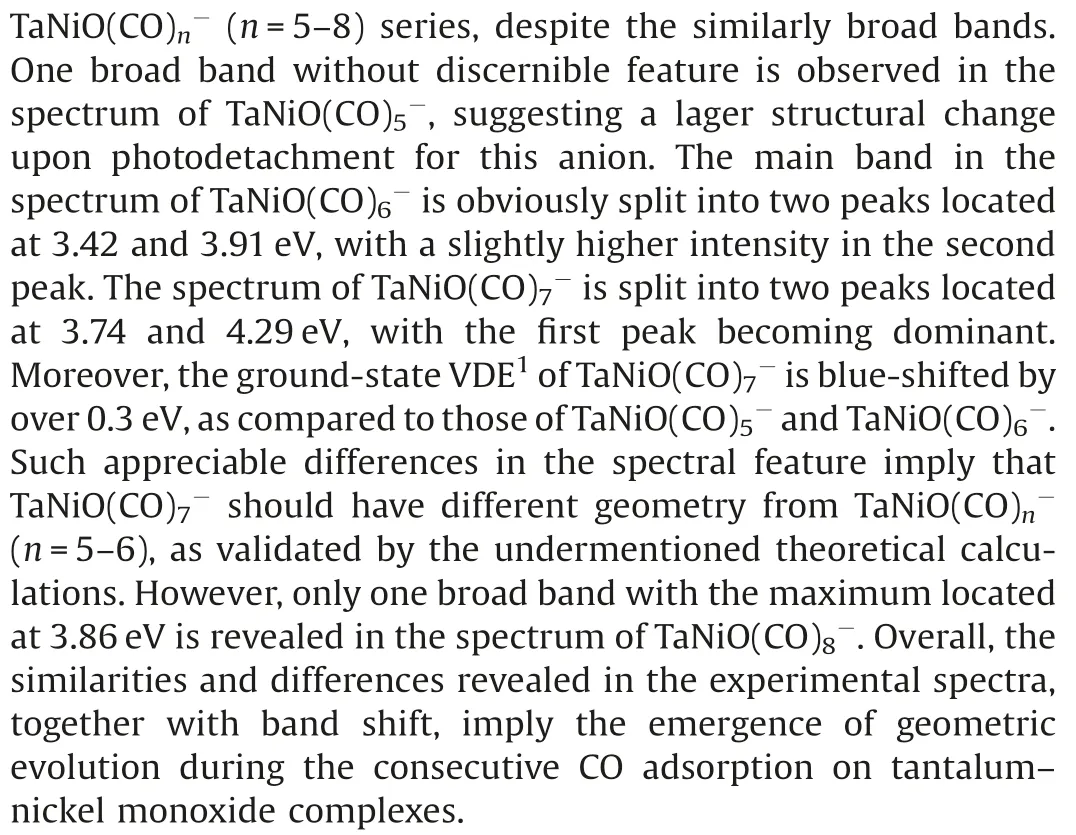
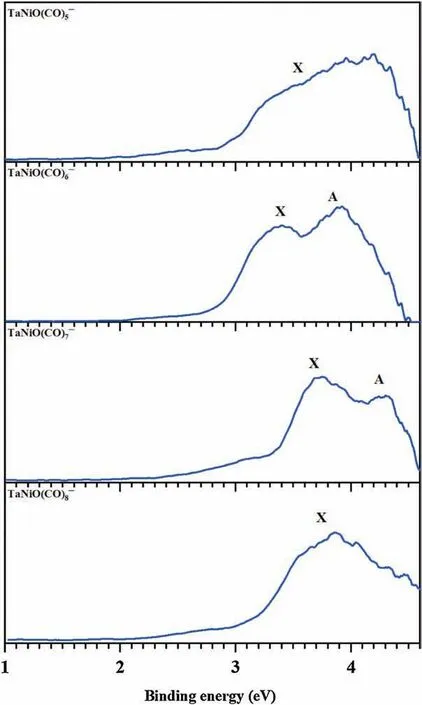
Fig.1.Photoelectron spectra of TaNiO((n=5–8) recorded at 266 nm(4.661 eV).
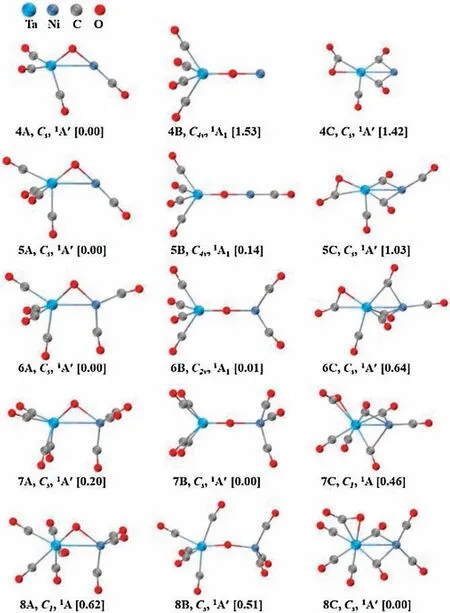
Fig.2.Ground-state structures and selected low-lying isomers of the TaNiO(CO)n-(n=4–8)anions calculated at the BP86-D3BJ/def2-TZVP level.Relative energies are given in square brackets (eV).
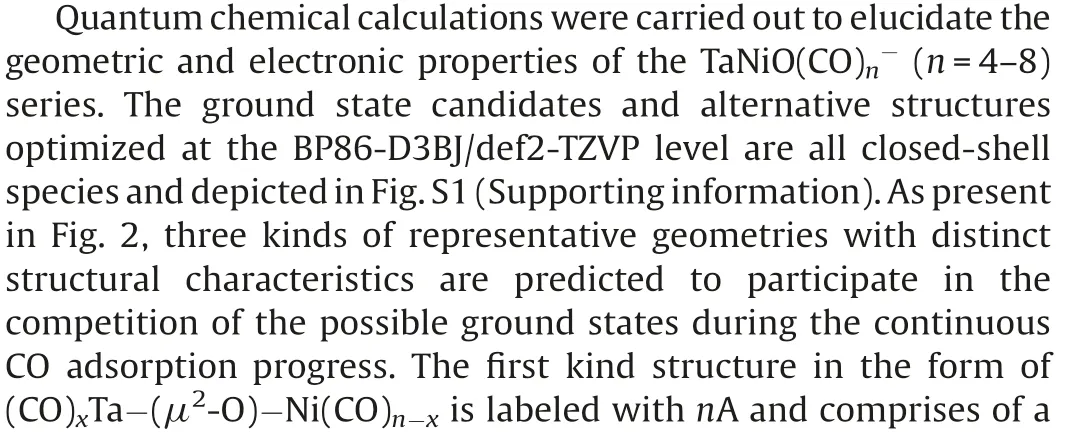
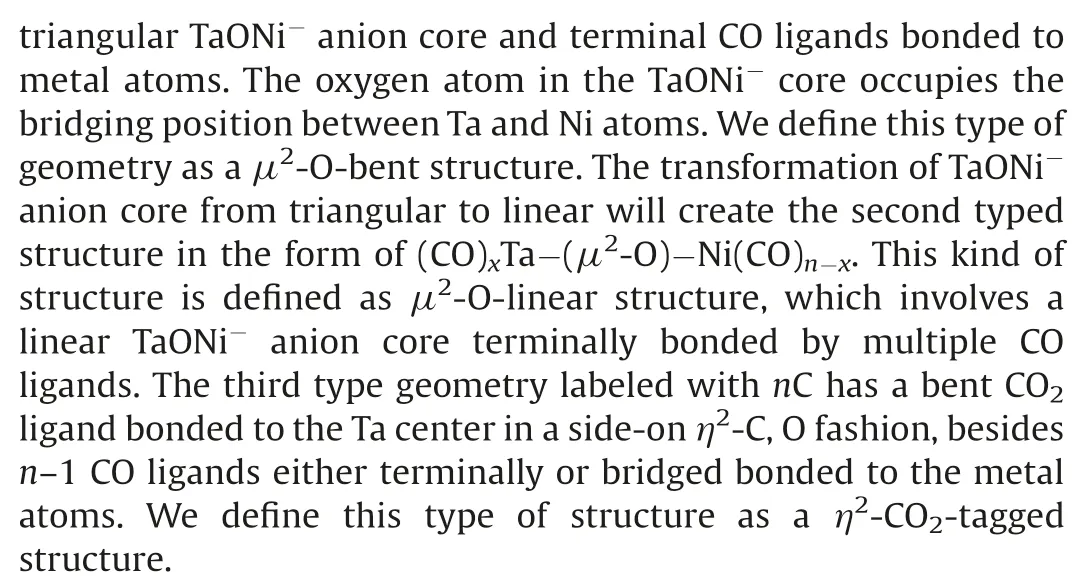
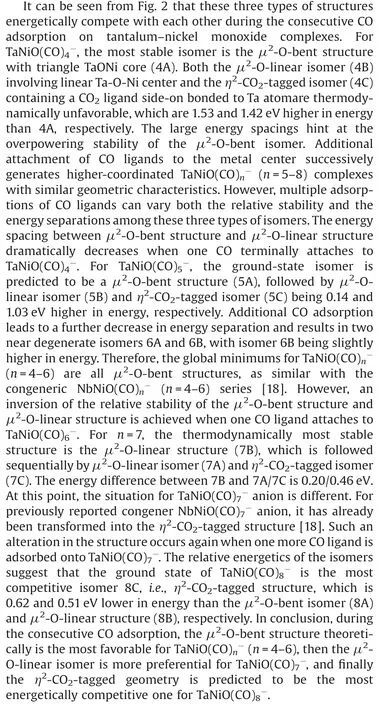
Table S2 provides a comparison of the theoretical calculated VDEs and ADEs at the BP86-D3BJ/def2-TZVP level and the experimental data.Considerable reorganization energies (ROEs)are predicted for all of the complexes, correlating well with the experimentally observed broad band in the PES.The ROE is defined as the energy difference between the ground-state ADE and VDE,the amount of which roughly characterizes the anion-to-neutral structural relaxation upon electron detachment.As an aside, it is interesting to note that the predicted VDEs for the nA isomers with a triangular TaONi-anion core and nC isomers involving a bent CO2ligand are approximately located at around 3.4 and 3.7 eV,respectively.While for the-O-linear structure,the VDE roughly increases with the multipleadsorptions of CO ligands.
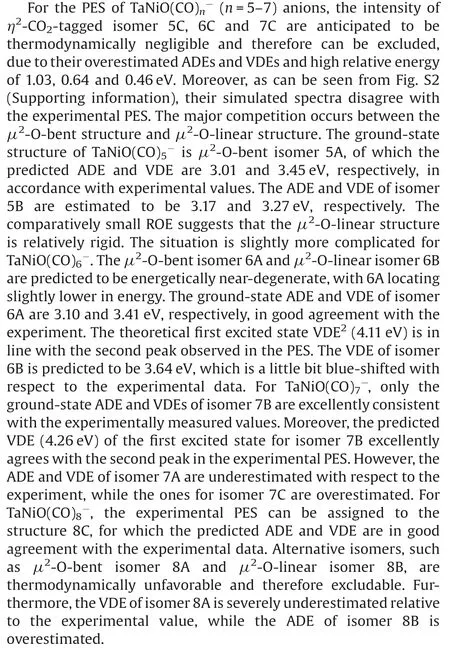
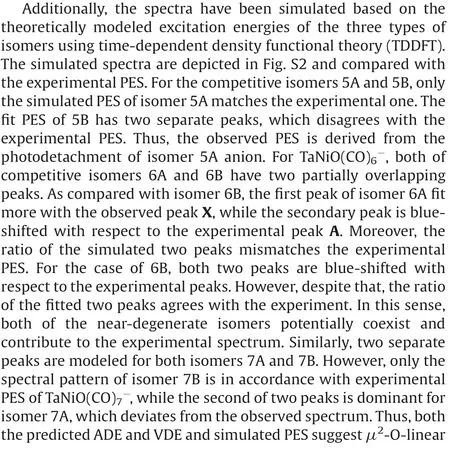
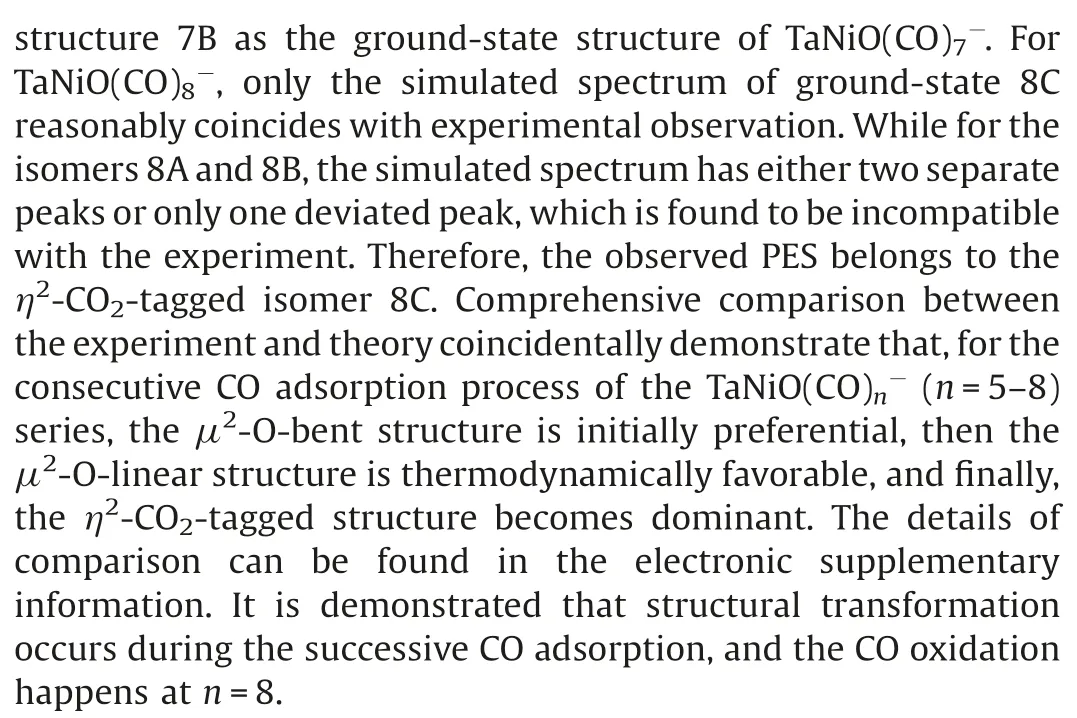
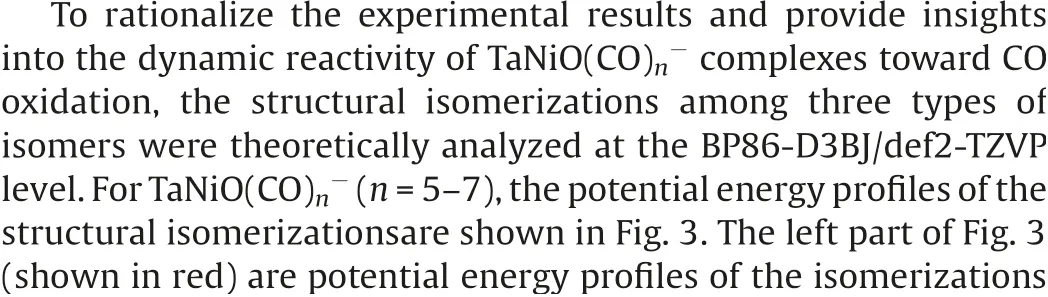
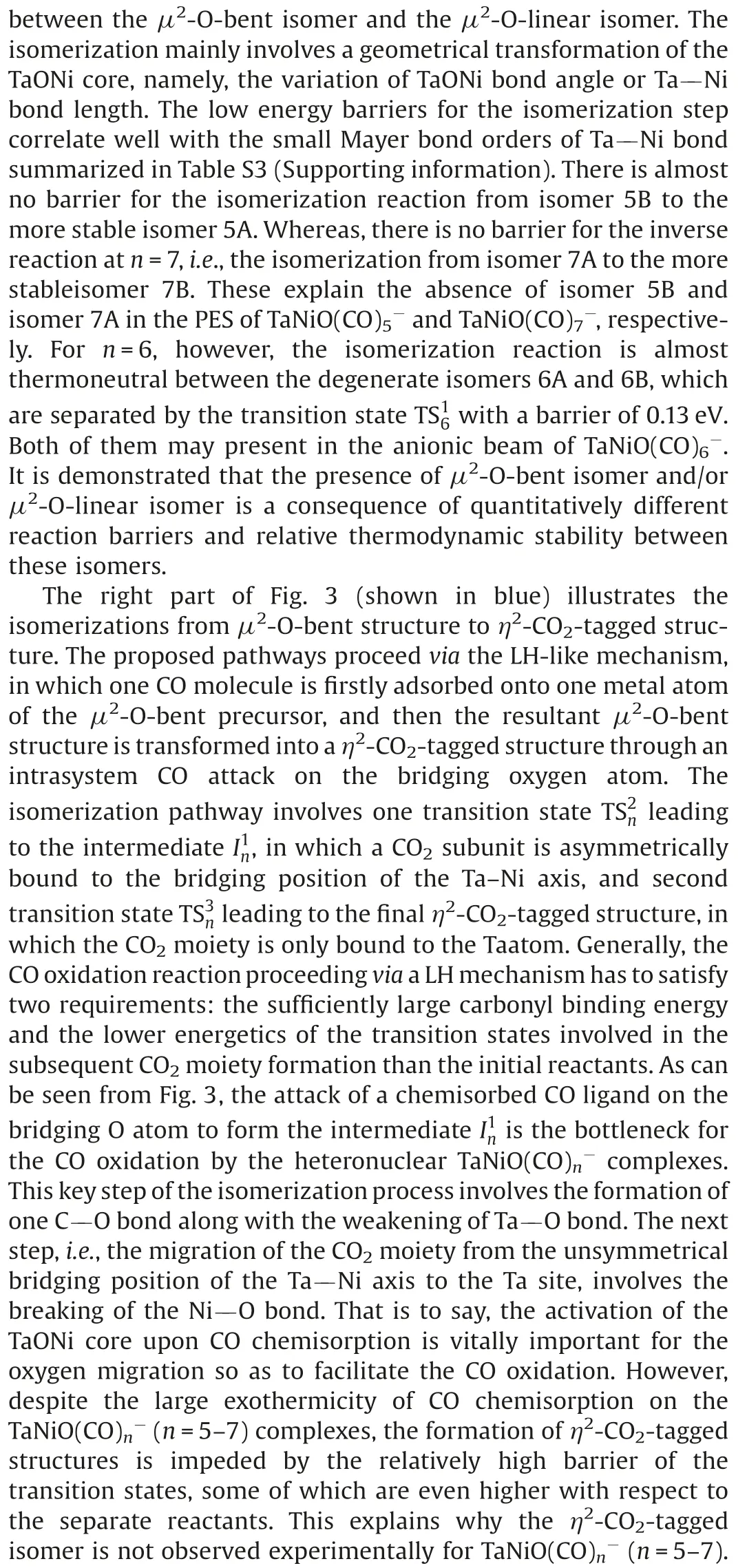
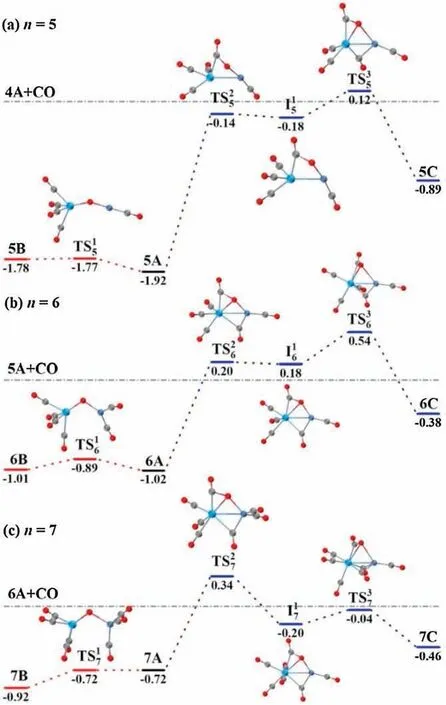
Fig.3.DFT calculated potential energy profiles for the isomerization reactions(nB →nA →nC)of TaNiO((n=5–7).The zero-point vibration corrected energies(eV) with respect to the separated reactants (n-1)A and CO are given.The gray dotdashed lines indicate the energetic baselines ((n-1)A+CO).
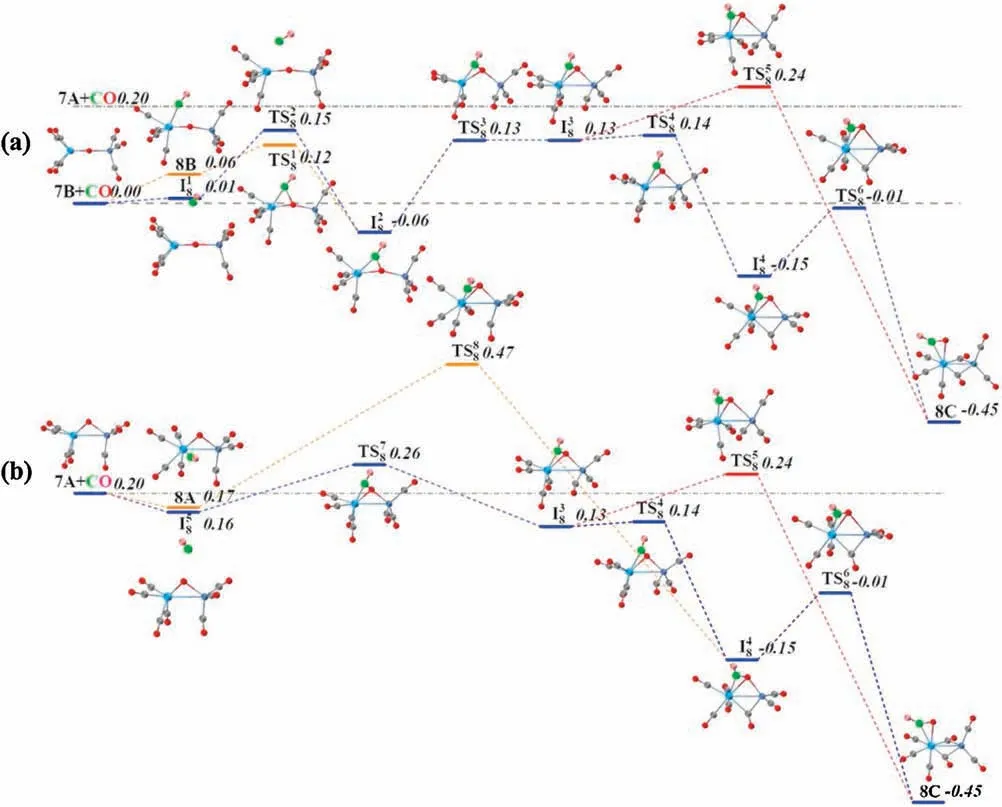
Fig.4.DFT calculated potential energy profiles for the CO oxidation reactions starting from 7B+CO(a)and 7A+CO(b).The zero-point vibration corrected energies(eV)with respect to the separated reactants are given.The dot-dashed line indicates the energetics of reactants 7A and CO,while the dashed line stands for the energetics of the isomer 7B and CO.For the sake of distinction, the C and O atoms of the freshly adsorbed CO ligand are depicted in different colors from the rest of CO ligands.
The pathways of CO oxidation on the TaNiOcomplex are also evaluated to understand the evolution from the CO association to the CO oxidation at n=8.Fig.4a shows the potential energy profiles of the CO oxidation reaction on the isomer 7B.Two distinct reaction channels are identified for the formation of the CO2unit.The very first reaction step is the formation of the weakly bound complex, in which the fresh CO molecule can be either chemisorbed onto the Ta atom to form isomer 8B or physisorbed onto 7B to generate intermediate.As opposed to the strongly exothermic association of CO onto TaNiO(n=4–6) complexes, the CO adsorption on isomer 7B is almost thermoneutral,suggesting that the adsorption of CO on TaONi core presumably reaches saturation.These CO association complexes are separated by transition statesandrespectively, from the same intermediatein which the freshly adsorbed CO is obliquely attached to the bridging oxygen atom to form a bent CO2unit.The freshly chemisorbed CO ligand to the Ta atom bends forwards to the bridging oxygen atom,leading to the formation of CO bond in the transition stateThe transition statehas a structure related to the initial complex with a physisorbed CO ligand much closer to counterpart.Apparently,the pathway for CO chemisorption leading to the formation of intermediate.involves an internal CO attack and belongs to the LH-like mechanism,while the other pathway for CO physisorption involves an intersystem CO attack and can be attributed to the ER-like mechanism.Note that the CO2subunit has already been formed at this critical juncture of intermediateAs compared with those of TaNiO((n=5–7),the reaction energy barriers for the attack of CO ligand on the bridging O atom are dramatically reduced, suggesting that the TaONi core becomes significantly reactive towards CO oxidation upon additional CO chemisorption.
Then the reaction proceeds further to form the more stable CO2-tagged structure through the CO2migration.By the rotation of CO out of the TaONi core plane and shortening of Ta–Ni distance, the structure.can be further isomerized to important intermediate., which requires surmounting a low barrier of 0.19 eV ().Thereafter, the reaction proceeds following two different channels, which both involve the migration of CO2and the conversion of two CO ligands from the terminal to the bridging coordination.The coordination pattern evolutions of two CO ligands can happen step by step in one reaction channel, or simultaneously in the other reaction channel.The energetically more favorable pathway involves firstly an almost no barrier stepto form CO singly bridging stereochemistry, followed by a deviation of the CO2unit away from the Ni atom and a transition from terminal to bridging coordination for another CO ligand,which is connected with the barrier of 0.14 eV (.The second reaction pathway involves the breaking of the Ni-O bond and the simultaneous formation of the doubly CO bridging stereochemistry.The corresponding transition stateis 0.24 eV higher in energy than the initial reactants 7B and CO.At first glance, the oxidation reaction might be prevented due to the transition states with barriers slightly higher than the separate reactants 7B and CO.However,the heights of the involved activation barriers are much smaller with respect to the cases of TaNiO(n=5–7), which can be readily surmounted by thermal collision in the supersonic molecular beam[19].In addition,the whole CO oxidation reaction is thermodynamically exothermic by 0.45 eV.Therefore, the CO oxidation onTaNiOvia both ER-and LH-like mechanisms are facile.
For comparison, the CO oxidation reactions starting from 7A and CO are also calculated, provided that 7A exists, and the corresponding potential energy profiles are present in Fig.4b.Firstly, the CO molecule can be physisorbed or chemisorbed to generate complexor 8A, respectively.For complex 8A, the CO oxidation reaction proceeds further following the similar pathways as TaNiO(n=5–7),i.e.,firstly the attack of a chemisorbed CO ligand on the bridging O atom to form CO2subunit and secondly the change of coordination patterns of CO2moiety from a bridgetype coordination to a side-on coordination on only Ta atom.While the further approaching of freshly physisorbed CO in the complexto the bridging oxygen atom will give rise to the same intermediatewhich can react following the same pathways as the abovementioned (Fig.4a).It is demonstrated that the important intermediatecan be generated either from the 7A and CO via an ER-like mechanism or from 7B and CO via both an ERlike mechanism and a LH-like mechanism.Obviously,the reactions occur through an ER-like mechanism is more favorable, whereas the reactions via a LH-like mechanism may still not easily proceed,as the CO molecule will experience quite a higher reaction barrierwhen the adsorbed CO approaches to the bridging oxygen atom.
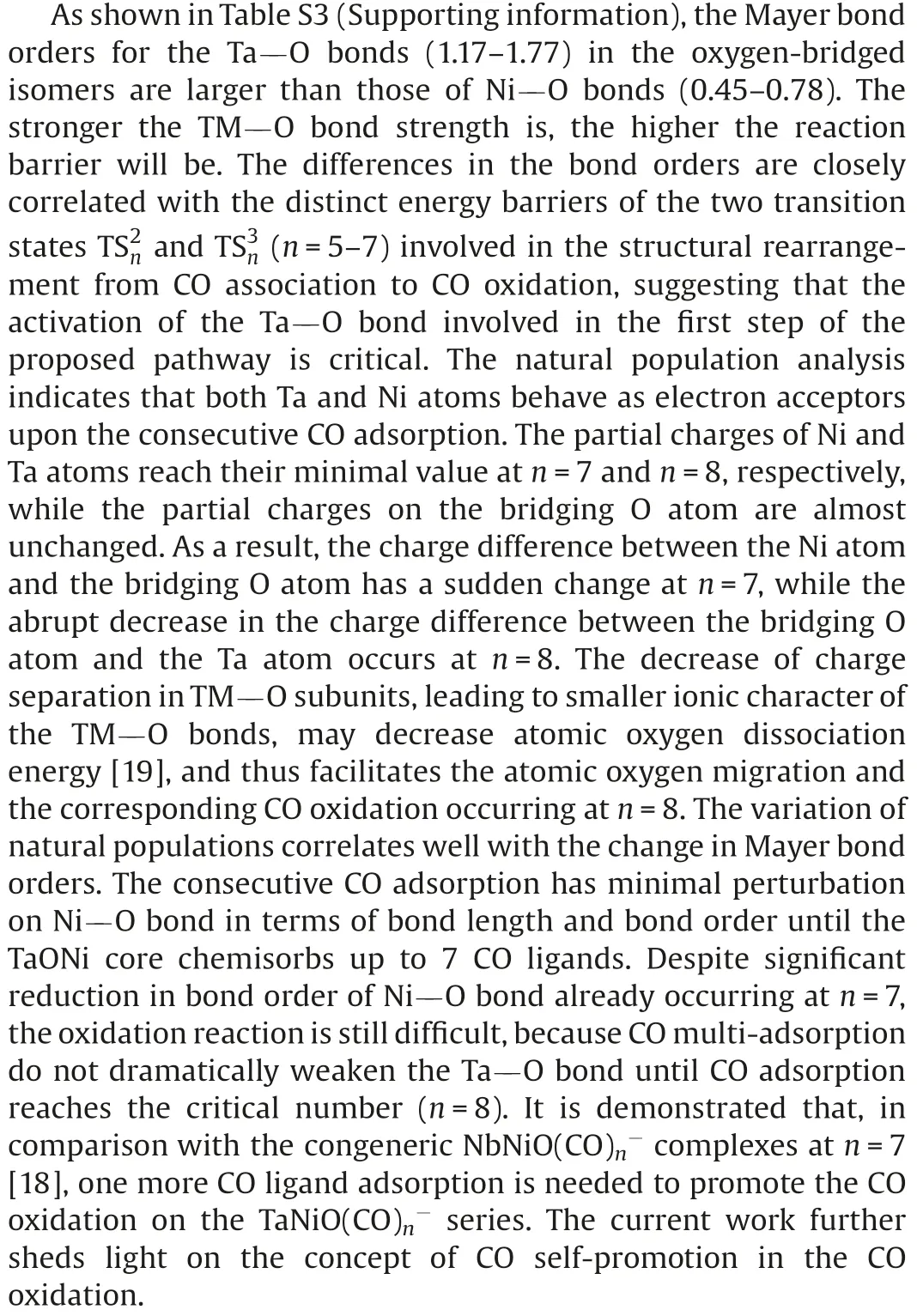
The CO adsorption energy retained by the TMO clusters is deem to be the driving force for CO oxidation [20].The gold oxide clusters with different charge states, bearing great diversity in CO binding energy, express a preference for an ER-like or a LH-like mechanism for CO oxidation on them [20].The LH-like mechanism is generally considered to be preferential for the CO oxidation in the gas phase [21], because the gained energy from the initial CO chemisorption in the LH-like mechanism usually is larger than that from the initial CO physisorption in ER-like mechanism.For the initial process of consecutive CO adsorption, the CO physisorption energies on TaNiOcomplexes are so small that the ER-like mechanism becomes uncompetitive relative to the LH-like mechanism.However, as shown in Fig.S3 (Supporting information), the step binding energies of CO to the O-bridged isomers monotonically decrease with the consecutive CO adsorption,and finally converge to the values close to the physisorption energies.Consequently,the chemisorption loses its competitive edge at n=8,and both ER-and LH-like mechanisms prevail for the CO oxidation reaction on TaNiOcomplex.
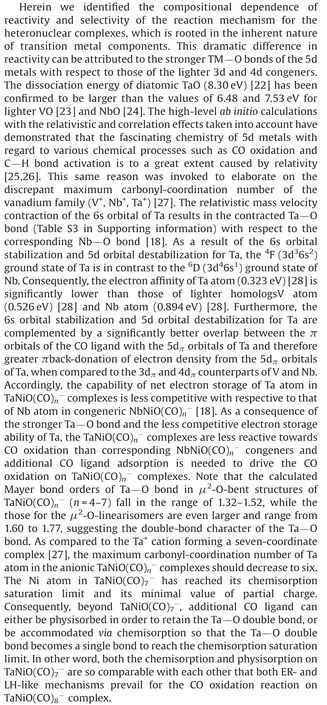
In summary, the structure evolution of the TaNiO(n=5–8)series is characterized by the photoelectron velocity-map spectroscopy combined with DFT calculations.Three different types of structures participate in the competition of ground state,with the-O-bent structure firstly being most favor, then the-O-linear structure being most preferential, and finally, theCO2-tagged structure being dominant.In contrast to the CO oxidation on congeneric V-doped [15] and Nb-doped [22] nickel oxide complexes, which proceed via an LH-like mechanism as a result of the multi-adsorption of CO ligands, complementary theoretical calculations reveal that both ER- and LH-like mechanisms generally become favorable and lead to the self-promoted CO oxidation on TaNiOOur findings shed new light on the role of the composition of heteronuclear metal complexes in the regulation of the reactivity and selectivity of the CO oxidation mechanism, which potentially benefits the rational design and development of the high-efficiency catalysts.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21603130, 21673231, 21688102 and 21873097); the Key Research Program (No.KGZD-EW-T05), the Strategic Priority Research Program (No.XDB17000000) of the Chinese Academy of Science.Zhiling Liu also gratefully acknowledges the Shanxi Province Science Foundation for Youths (No.201901D211395), the 1331 Engineering of Shanxi Province and the Start-up Fund from Shanxi Normal University for support.
Appendix A.Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2020.05.029.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- One-step straightf oward solid synthesis of high yield white fluorescent carbon dots for white light emitting diodes
- Free-standing nitrogen doped graphene/Co(OH)2composite films with superior catalytic activity for aprotic lithium-oxygen batteries
- Amorphous silicon from low-temperature reduction of silica in the molten salts and its lithium-storage performance
- Two 2D uranyl coordination complexes showing effective photocatalytic degradation of Rhodamine B and mechanism study
- Recent advances in electrochemical sensors for antibiotics and their applications
