One-step straightf oward solid synthesis of high yield white fluorescent carbon dots for white light emitting diodes
2021-05-14BingyanHanJingmeiJiang1QifangYan1ZeXinQinYan
Bingyan Han*,Jingmei Jiang1,Qifang Yan1,Ze XinQin Yan
a State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116023, China
b School of Chemical Engineering, Dalian University of Technology, Panjin 124221, China
ABSTRACT Herein,we directly prepared white fluorescent CDs(W-CDs)using 1,6-dihydroxynaphthalene(1,6-DHN)and L-asparagine (L-Asn) as carbon sources through a simple solvent-free method.As-prepared W-CDs can be obtained in high yield (95%).A relative pure white LEDs (WLEDs) were fabricated with Commission Internationale de L’Eclairage(CIE)coordinates of(0.32,0.31).As-prepared W-CDs will have promising future for a wide range of optoelectronic devices.
Keywords:Fluorescent carbon dots Solid synthesis High yield White LEDs One-step
White light-emitting diodes (WLEDs) are rapidly replacing incandescent and fluorescent light sources because of their long lifetime,small footprint,tunable spectra and high efficiency in the conversion of electricity to light [1–4].There are two popular strategies for fabricating LED-based devices capable of white light generation: (i) a blue LED chip is used as a pumping light source and excites one or two phosphors,which convert part of blue light to yellow or green and red light to yield white light, and (ii)ultraviolet (UV) LED chip are employed to excite three-color(red,green and blue, RGB) phosphors [5].The present blue LED chip used for producing this white light can result in retinal damage.Thus, reducing the harm brought by blue excitation is another important component to optimize the WLEDs devices.UV-excited LED chips are desired to excite a broad white emission to avoid phototoxicity to human eyes from the blue LED chip [6].Conventional multicolor emissive materials have been explored in the past several decades,including not only inorganic materials like semiconductor quantum dots and rare-earth based nanoparticles, but also organic fluorescent dyes and molecular nanomaterials[7–11].However,the existing problems of the multicolor emissive materials encompass expensive resources, high energy consumption, high toxicity and instability due to photobleaching and water-insolubility which have precluded their use for niche applications.As such, this dictates a pressing requirement for a green substitution.
Due to intrinsic merits of high stability, low cost, low toxicity and environmental-friendliness, fluorescent carbon dots (CDs)have emerged as promising materials for optoelectronic applications [12–14].Their excellent optical properties make them an appealing candidate for LED devices,of which multiple colors can be applied for white light-emitting diodes [15–19].At present,most of the preparation methods of CDs are hydrothermal method and solvothermal method, but it is impossible to determine whether the solvent participates in the reaction during the formation of CDs [20], which hinders the exploration of the formation mechanism of CDs.Moreover,CDs in the above methods have a lower yield.Solvent-free preparation of CDs avoids solvent interference and has a high yield.One of the problems with the powders of CDs is the very weak fluorescence due to aggregation induced quenching (AIQ) effect, which greatly hampered the development and applications of CDs in LEDs.To avoid the AIQ effect, many groups have demonstrated that CDs embedded in solid matrices such as borax [21], silicone [22,23], starch [24],cellulose nanofiber [25], resin [26] and polymer film [27] can preserve the fluorescence,renewing the promise of CDs in WLEDs.Wang et al.fabricated yellow CDs derived from N-acetylcysteine precursor, they applied these CDs as yellow phosphor through mixed with epoxy resin A and epoxy resin B coated on blue-LED chips to produce WLEDs [28].Lin group prepared RGB CDs, they obtained full-color emissive PVA films through mixing two or three CDs in the appropriate ratios, and made these CDs potentially useful in LEDs [29].Nevertheless the most WLEDs are usually constructed by mixing different color-emitting CDs.The report about the direct preparation of white fluorescent CDs(W-CDs)by one-step method are rarely.W-CDs can save a lot of money and time,and do not need to spend energy to adjust the ratio between RGB CDs.
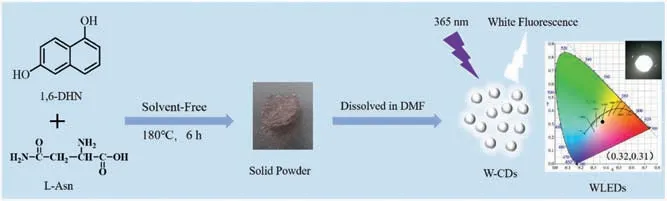
Scheme 1.Schematic illustration of the W-CDs preparation and its application in WLEDs.

Fig.1.(a) TEM image of the W-CDs.(b) FT-IR spectrum of W-CDs.
Herein, we prepared the W-CDs using 1,6-dihydroxynaphthalene(1,6-DHN)and L-asparagine(L-Asn)as carbon sources through a simple in situ solvent-free carbonization method.The yield of W-CDs reached up to 95%.As-prepared W-CDs had dual-emissive peak at 451 and 518 nm under 365 nm excitation.By exploring the functional groups and hydroxyl positions of the carbon source,we speculated a possible reaction mechanism.In order to conquer solid-state fluorescence quenching of W-CDs, we used polyvinyl butyral(PVB)to disperse W-CDs.Due to the well-dissolved CDs in PVB, we had fabricated CDs/PVB composites for LEDs, as well as WLEDs with Commission Internationale de L’Eclairage (CIE)coordinates of (0.32, 0.31) under UV excitation of 365 nm in Scheme 1.
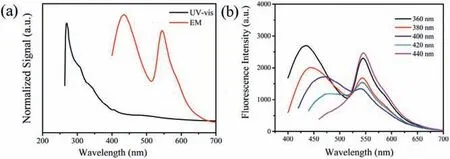
Fig.3.(a) UV–vis spectra (black) and the maximum emisssion spectra (red) of W-CDs(images under daylight(left)and UV light(right).(b)The emission spectra with different excitation wavelength of W-CDs.
The solid CDs were prepared by solvent-free method of 1,6-DHN and L-Asn(see detailed synthesis in Supporting information).Then the solid CDs were gently into powder and the yield of CDs can reach as high as 95%.This means that the CDs can be prepared on a large scale and at low cost.The CDs powder were dissolved in N,N-dimethylformamid(DMF)to obtain W-CDs solution.In Fig.1a,the transmission electron microscope (TEM) image revealed that the W-CDs were homogeneous and well-dispersed in DMF,with an average diameter of about 2 nm.In Fig.1b, the broad absorption peak at 3197was attributed to the stretching vibration intensity of —OH.The absorption peaks at 1578and 1385can be assigned to the C¼N andstretching vibrations,respectively.The peak at 1182was related to thestretching vibrations.In Fig.2a,the full X-ray photoelectron spectroscopy(XPS)spectra displayed three typical peaks at 284.4,399.8 and 531.9 eV for C 1s,N 1s,and O 1s,respectively.In the highresolution XPS spectra, the C 1s band can be deconvoluted into three binding energy peaks, which wereand CO,as shown in Fig.2b.The N 1s spectra displayed two peaks at 398.5 eV and 399.2 eV in Fig.2c, attributed to pyrrolic N and pyridinic N,respectively.From Fig.2d,the O 1s spectra contained two peaks at 531.7 eV and 532.8 eV forand
As shown in Fig.3a, the obvious absorption peaks in UV–vis absorption spectrum at 270 nm was attributed to thetransition of aromaticThe weak absorption band in the range of 320-400 nm belonged to n-p*transition ofbond on the surface of W-CDs.In Fig.3b, as-prepared W-CDs had dualemission spectra at 451 and 518 nm under the optimal excitation of 365 nm.As-prepared W-CDs emitted white fluorescence under 365 nm.The two peaks represented blue and yellow fluorescence,which were complementary light.This emission feature was believed to be responsible for the observed white-light emission of W-CDs solution.As shown in Fig.3b, the emission peaks showed excitation-independent when excitation wavelength changed from 360 nm to 440 nm.
Explore the reaction mechanism of W-CDs.First, L-Asn and 1,6-DHN were used as carbon sources to prepared CDs under the same conditions,respectively.It can be seen in Fig.S1a(Supportinginformation)that the obtained production just only emitted bluegreen and green fluorescence at 365 nm,respectively.From Fig.4a,the emission wavelength of the CDs prepared by L-Asn and 1,6-DHN were 471 and 490 nm.On the contrary, W-CDs showed dual-emission peak.In Fig.4b, the peaks ofandon L-Asn disappeared after the reaction,and onlypresented on the surface of W-CDs.It can be seen from the FT-IR spectra thaton L-Asn reacted with 1,6-DHN.Second, by changing the position of theon the DHN, it was found that the emission wavelength and color of the CDs were greatly different.From Fig.4c,the CDs prepared by the reaction of 1,5-DHN,2,3-DHN and 2,7-DHN with L-Asn had the strongest emission peaks centered at 460, 440 and 540 nm, respectively, with appropriate excitation wavelengths.The fluorescence photographs were showed in Fig.S1b (Supporting information).Only 1,6-DHN reacted with L-Asn produced W-CDs with dual emission peak at under 365 nm UV light.We speculated that 1,6-DHN had appropriate reactive sites and steric effect for cross-linking polymerization, dehydration and carbonization process.Last,the effect of functional groups on L-Asn molecules was further explored.If -NH2at the end of L-Asn were replaced byCOOH,OH andCH3.It can be seen from Fig.4d that the maximum emission peaks of the CDs prepared by the reaction of L-Asp, L-Ser and L-Thr with 1,6-DHN were 446,537 and 506 nm, respectively.As-prepared CDs emitted blue,yellow-green and blue-green fluorescence,respectively in Fig.S1c.We concluded that the -NH2at the end of L-Asn reacted with 1,6-DHN, forming large conjugate structure.
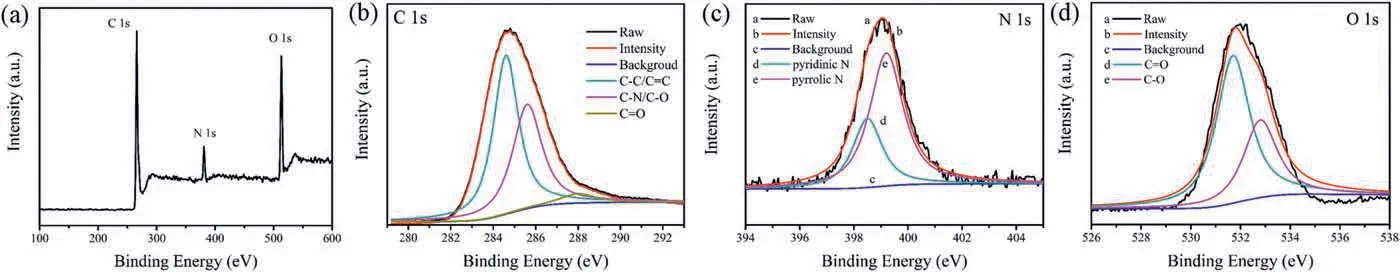
Fig.2.(a) Survey of XPS spectra and high-resolution (b) C 1s, (c) N 1s, and (d) O 1s spectra of the W-CDs.

Fig.4.(a)The fluorescence spectra and(b)FT-IR spectra of the CDs prepared by 1,6-DHN,L-Asn,1,6-DHN and L-Asn,respectively.(c)The CDs obtained by changing the position of —OH on DHN.(d) The CDs prepared by changing the functional group at the end of L-Asn.
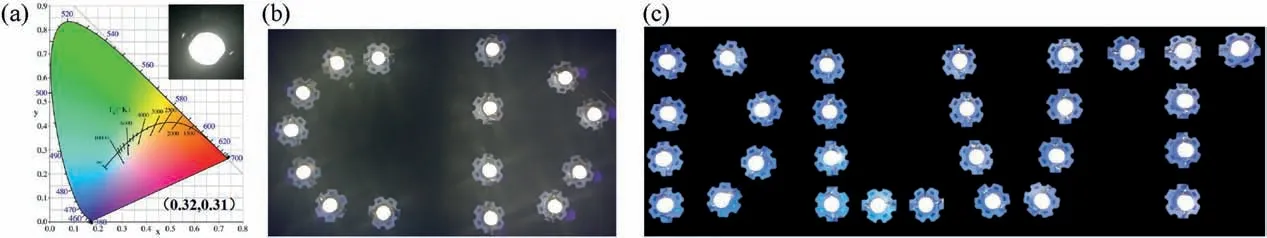
Fig.5.(a) CIE color coordinate of WLEDs (Inset: photograph of single WLEDs).Image of (b) CDs and (c) Dalian University of Technology (DLUT) with multiple WLEDs.
WLEDs are popular illumination device due to their advantages such as long lifetime, small footprint, tunable spectra and high efficiency.As a new type of fluorescent nanomaterials, CDs have excellent fluorescence properties comparable to other.We prepared W-CDs through solvent-free method for WLEDs.To meet the need of W-CDs applied in WLEDs devices, solid-state luminescent W-CD/PVB composite materials were prepared and combined with UV-LED to fabricate WLEDs.It emitted bright white light at the operating voltage of 4.0 V and the CIE color coordinates of LED shown in Fig.5 is(0.32,0.31).When multiple WLEDs were connected in series and in parallel,they were combined into DLUT for Dalian University of Technology and CDs logo design.
In summary, the W-CDs using 1,6-DHN and L-Asn as carbon source through solvent-free method were prepared by one-step straightfoward synthesis.As-prepared W-CDs can be obtained in high yield (95%) and had dual-emissive peak at 451 and 518 nm under 365 nm excitation that emitted bright white fluorescent.The W-CDs/PVB composites were further used for the design of LEDs with CIE coordinates of(0.32,0.31)under a single UV excitation.By exploring its luminescence mechanism, as-prepared W-CDs will have promising future for a wide range of optoelectronic devices.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.11.042.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- Copper-cobalt-nickel oxide nanowire arrays on copper foams as self-standing anode materials for lithium ion batteries
- Design of activatable red-emissive assay for cysteine detection in aqueous medium with aggregation induced emission characteristics
- An aqueous zinc-ion hybrid super-capacitor for achieving ultrahigh-volumetric energy density
- Assembly and packing models of [Ti6Co12] ring based on the titanium-capped cobalt clathrochelates
- A stable Co(II)-based metal-organic framework with dual-functional pyrazolate-carboxylate ligand: Construction and CO2selective adsorption and fixation
