Quantitative assessment of rhodamine spectra
2021-05-14WeiZhouXingningFngQinglongQioWenchoJingYueZhngZhochoXu
Wei Zhou,Xingning Fng,Qinglong Qio,Wencho Jing,Yue Zhng,Zhocho Xu,b,*
a CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
b Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian 116012, China
ABSTRACT With the development of single-molecule detection and super-resolution fluorescence imaging,rhodamine dyes gain new life.Through the modification of the N-substituents and the replacement of the oxygen atom in xanthene, the wavelength and brightness can be effectively changed.However, the spectra of rhodamine, especially due to the balance between ring-closed non-fluorescent lactone and ring-opened fluorescent zwitterion/cation, are sensitive to interference from various environmental factors.In this way,the spectral data of various rhodamines reported by different research groups under different test conditions lacked comparability, sometimes even lacked accuracy.In order to meet the requirements for the accuracy and uniformity of spectral data in the research of single molecule imaging and dye structure-fluorescence relationship study,we have tested the spectra of fifteen rhodamine dyes that cover the visible and near-infrared regions under exactly the same conditions.By studying the dependence of the spectra on dye concentrations,it was confirmed that 1 mmol/L was ideal for detection less from the interference of dye molecule aggregation.We provide comprehensive and reliable spectral data of these fifteen dyes,which are expected to be used as references for future research.And the direct comparison of different rhodamine spectra would help to understand the structure-fluorescence relationship of rhodamines.
Keywords:Rhodamine Quantitative assessment Absorption Emission Quantum yield
Fluorescence imaging and sensing reveal the temporal and spatial position,function and interactions of biomolecules,playing an increasingly important role in life science and medical diagnosis and treatment [1–4].Correspondingly, fluorescent dyes have ushered in new and rapid development opportunities [5–7].Rhodamine is one of the dyes that have received the most attention[8–10].This is mainly because rhodamine dyes have high fluorescence intensity and photo-stability, and the spectra can cover the entire visible and near-infrared region.
Rhodamine derivation and structure diversity helped reveal some typical structure-fluorescence relationships.Rhodamine dyes are derivatives of xanthene (Fig.1).The increase in the electron donating ability of amino substituents will cause a red shift of absorption and fluorescence wavelengths.Conversely, the decrease in electron donating ability will cause a blue shift of absorption and fluorescence wavelengths[11].The replacement of the oxygen atom of xanthene by other heteroatoms can effectively increase the wavelength.The most representative one is that the introduction of silicon atom makes the emission wavelength of rhodamine enter the near-infrared region [12].The dialkylamino substituent in the excited state will undergo intramolecular twist to form a non-emissive TICT state.The structural modification to suppress the TICT excited state can effectively improve the quantum yield of rhodamine dyes [13].For example, Lavis et al.changed N,N-dialkylamino substituents to four-membered azetidine ring to suppress TICTand obtain rhodamine fluorophores with high brightness [14].Xiao and Guo et al.introduced electron withdrawing groups to reduce the generation of TICT [15,16].Rhodamine dyes,especially the balance between ring-closed nonfluorescent lactones and ring-opened fluorescent zwitterions/cations, are sensitive to the effects of aggregation at high concentrations and other environmental factors,which can cause spectral changes [17,18].Due to the different test conditions such as concentration (other factors include polarity and pH, etc.), the spectral data of different structures of rhodamines can often not be reasonably compared.Even for rhodamine dyes of the same structure, the spectral properties reported by different research groups will be very different.For example, the quantum yield of rhodamine 6G (Rho6G) was reported by different authors as 0.95 and 0.48, respectively [19,20], while that of Rho560 (Fig.1) was reported to be 0.1 or 0.06 [14,16].Rapid evolution of fluorescence imaging techniques in recent years demands fluorescent dyes with enhanced brightness and photostability.And at the same time,the accurate spectral data, including absorbance, quantum yield,absorption and emission wavelength, etc., are required to ensure the performance of single-molecule positioning, the accuracy of single-molecule level fluorescence sensing,the feasibility of multicolor imaging, the comparability of different rhodamine spectral data, and the study of structure-fluorescence relationship of rhodamine fluorophore [21,22].
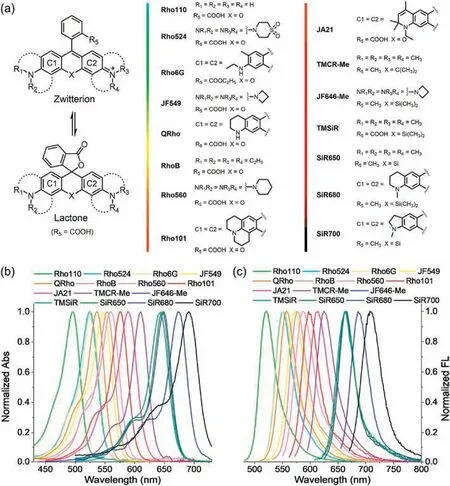
Fig.1.(a) Rhodamine dyes studies in this work.(b) Normalized absorption and (c) emission spectra of these rhodamine dyes.
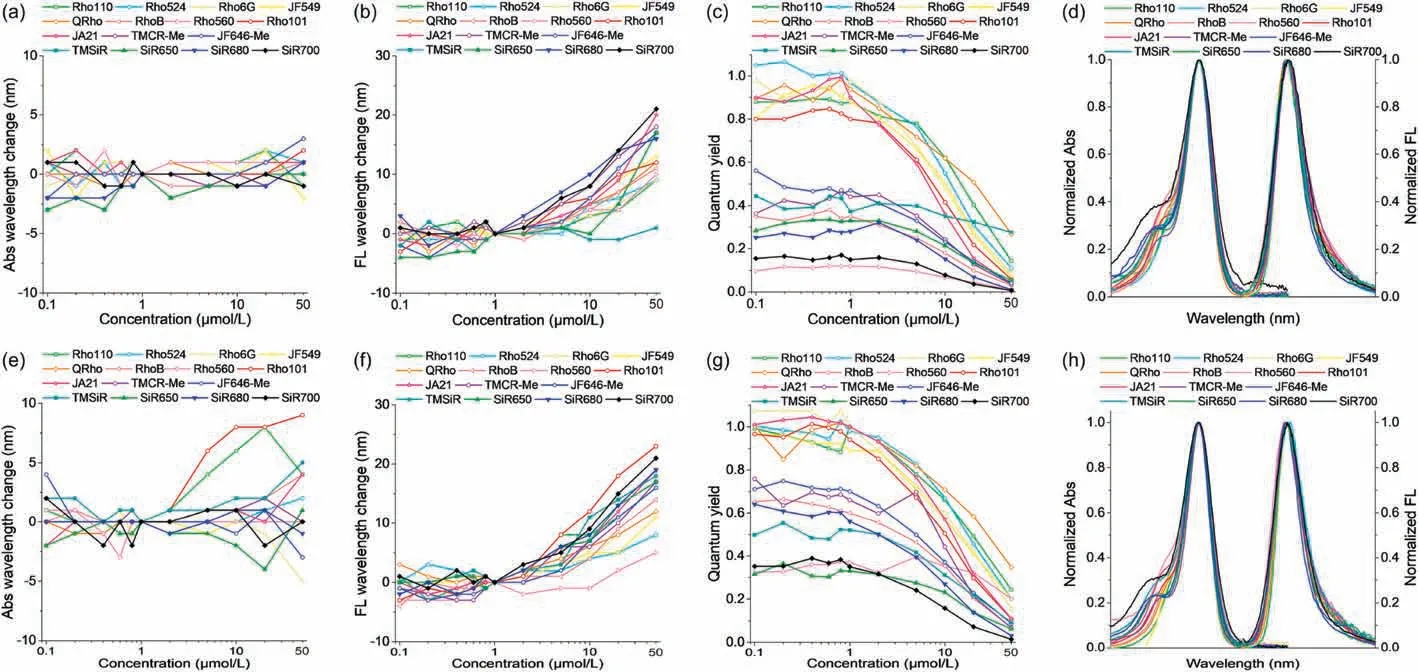
Fig.2.The concentration dependence of the spectra of 15 rhodamine dyes in PBS(a-d)and ethanol solutions(e-h).(a,e)Absorption wavelength changes with different dye concentrations ranging from 0.1to 50.(b, f) Emission wavelength changes with different dye concentrations ranging from 0.1to 50 (c, g)Quantum yield changes with different dye concentrations ranging from 0.1to 50.(d, h) Normalized absorption and emission profile of these 15 dyes.
In order to obtain accurate and comparable data,here we tested the spectra of fifteen rhodamine dyes under the same test conditions as shown in Fig.1.The emission wavelengths of these dyes covered the visible and near-infrared regions and the HPLC purity of these dyes was all above 97%(Figs.S1–S14 in Supporting information).The absorption and emission spectra of 11 different concentrations ranging from 0.1to 50were detected for each dye.Absolute fluorescence quantum yields for all dyes were measured using a Quantaurus-QY spectrometer.Considering the balance between ring-closed non-fluorescent lactones and ring-opened fluorescent zwitterions/cations in rhodamine, we added 1m trifluoroacetic acid (v/v) to the ethanol solution to get zwitterionic rhodamines.In the PBS buffer solutions(pH 7.4), rhodamines mainly exist as zwitterions.
Fig.2 and Figs.S15–S46 (Supporting information) showed the concentration dependence of the spectra of 15 rhodamine dyes in PBS and ethanol solutions.The emission wavelength of all rhodamine dyes gradually red-shifted as the concentration increased (Figs.2b and f), while the absorption wavelength remained unchanged (Figs.2a and e).As shown in Figs.2b and f,the better the planarity of the fluorophore, the more obvious the red shift of the emission wavelength,such as Rho101,JA21,SiR680 and SiR700.The fluorescence quantum yields were most affected by the dye concentration (Figs.2c and g).As the concentration increased,the quantum yield of each dye dropped sharply.All the above results should be ascribed to the inner filter effect(IFE)and excimer formation of rhodamine with the increase of concentration, which both red-shifted and quenched the emission.We changed the light absorption path length form 1 cm to 0.25 cm,and found that the rate of decrease in quantum yield had slowed which proved the existence of IFE (Fig.S47 in Supporting information).However, the shortening of the light path did not change the tendency of wavelength redshift (Fig.S48 in Supporting information),and there was still a strong decrease in quantum yield even at a concentration as low as 2Considering that there was no change in the absorption wavelength of rhodamine at different concentrations, we guessed that rhodamine in the excited state,due to the increase in charge separation, would interact with rhodamine molecules in the ground state.That may be the generation of excimer.Many crystal structures had proved that rhodamine molecules can interact as a dimer through theinteraction between xanthene planes, while the two mesosubstituted benzoic acids were oriented in the opposite direction.Most importantly,we found that these 15 rhodamine dyes all have relatively large changes in their spectra when the concentration was greater than 1 mmol/L, while the spectra remained the same when the concentration was less than 1Therefore, the formation of rhodamine excimer at 1was considered negligible, so 1was recommended to be a reasonable concentration for detecting rhodamine optical spectra.It deserved to mention that the absorbance of 0.05 to 0.10 was the guideline to avoid IFE.And it happens that 1was a good choice for rhodamines.For other compounds with different molar extinction coefficients, other concentrations might be a better choice.In addition, it should be pointed out that the sensitivity of fluorescence detection is higher than that of absorption detection.In order to reduce the interaction between fluorescent molecules and IFE,while ensuring that absorption can follow Beer Lambert’s law,fluorescence detection can be performed at a relatively lower concentration, while the absorption detection can be sought at higher concentrations.For rhodamines, fluorescence detection is recommended to be carried out at 1 mmol/L or less,and absorption can be performed at higher concentrations.
Table 1 showed the spectral data of these 15 rhodamine dyes at a concentration of 1in PBS(pH 7.4)and ethanol solutions.Spectral data in the literature was also shown[14,16,19,20,23–28].Since the detection conditions were exactly the same, the comparison between these data would have better reliability,which could help to further understand the structure-fluorescence relationship of rhodamine dyes.By comparison, it can be clearly seen that TICT would strongly quench the fluorescence.RhoB,Rho560,TMCR-Me and TMSiR that can form an excited TICT state have a yield of less than 0.35.For near-infrared dyes, even if the formation of TICT was suppressed,the quantum yield can only be improved appropriately.For example,the quantum yield of JF646-Me was 0.47,but the quantum yield of short-wavelength dyes will not increase to about 0.9 (0.88 for JF549).The longer the fluorescence wavelength of the dye,the lower the quantum yield.These dyes have higher absorbance and fluorescence quantum yield in ethanol than in water, which is probably due to the solubility of the dye in different solvents and the hydrogen bonding between water and dye.
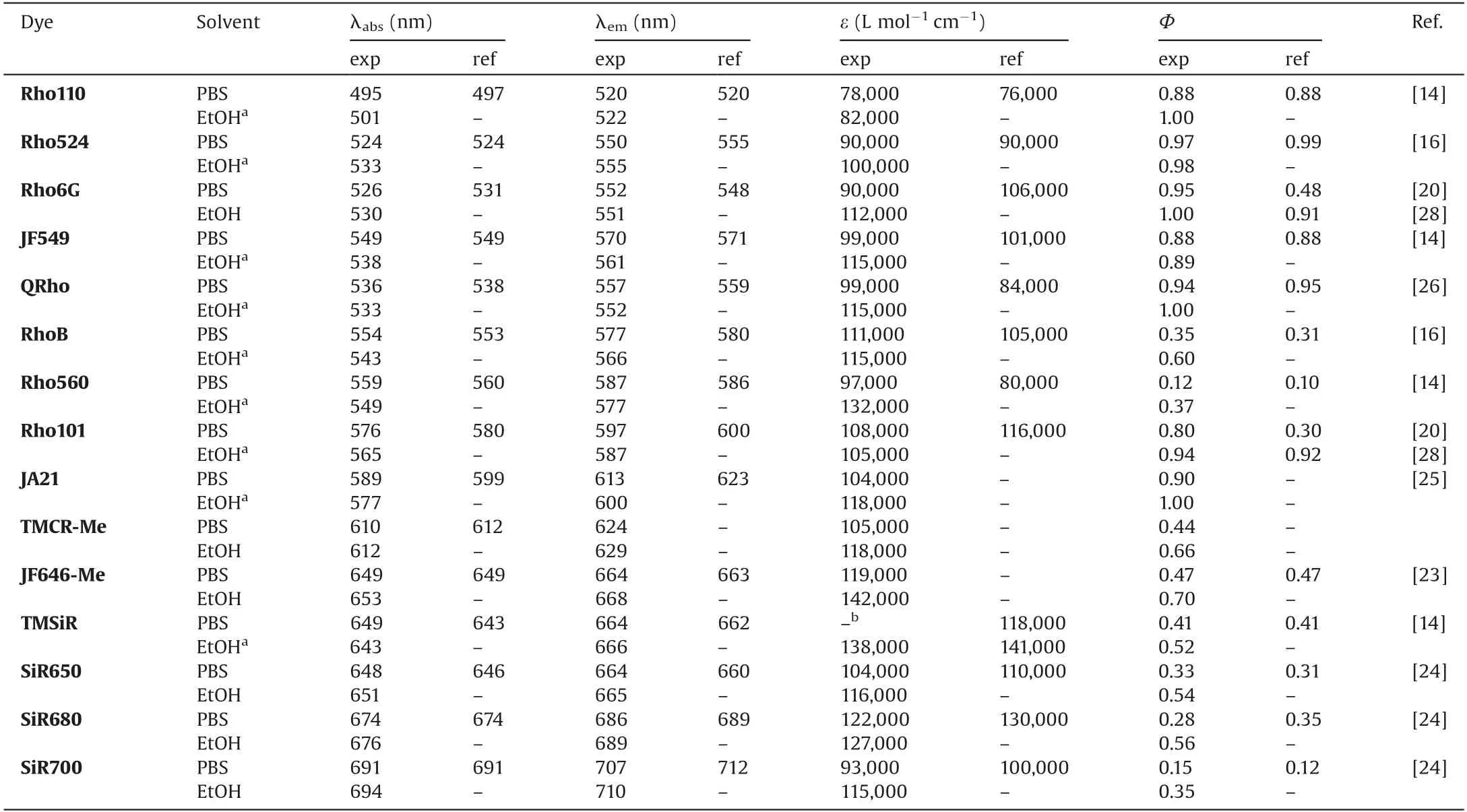
Table 1 Optical properties of these 15 rhodamine dyes.
We further normalized the absorption and emission spectra of these 15 rhodamine dyes, and found that the absorption and emission profiles of these dyes completely overlap, except that RhoB and SiR700 have higher shoulder absorption(Figs.2d and h).This peak absorption was previously thought to be caused by H-aggregation of the dye.However, through the comparison and analysis of the spectra of different dyes above, we suspected that this peak absorption was likely to be caused by the influence of different substituents in the conjugated system.
In conclusion, the absorption and fluorescence spectra of 15 rhodamine dyes covering the visible to near-infrared regions were tested under exactly the same conditions, and the concentrationdependent spectral changes were obtained.It was further determined that 1was reasonable concentration for detecting rhodamine spectroscopy to exclude molecular aggregation interference.We hope that the data obtained in this paper can be used as reference for rhodamine spectra (especially quantum yield), and provide a reference for understanding the structurefluorescence relationship of rhodamine and developing rhodamine dyes with better performance.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China (Nos.22078314, 21878286,21908216) and Dalian Institute of Chemical Physics (Nos.DICPI201938, DICPZZBS201805).
Appendix A.Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.02.003.
杂志排行
Chinese Chemical Letters的其它文章
- Copper-cobalt-nickel oxide nanowire arrays on copper foams as self-standing anode materials for lithium ion batteries
- Design of activatable red-emissive assay for cysteine detection in aqueous medium with aggregation induced emission characteristics
- An aqueous zinc-ion hybrid super-capacitor for achieving ultrahigh-volumetric energy density
- Assembly and packing models of [Ti6Co12] ring based on the titanium-capped cobalt clathrochelates
- A stable Co(II)-based metal-organic framework with dual-functional pyrazolate-carboxylate ligand: Construction and CO2selective adsorption and fixation
- Nitrogen-doped holey graphene nanoscrolls for high-energy and high-power supercapacitors
