Trace Nb-doped Na0.7Ni0.3Co0.1Mn0.6O2with suppressed voltage decay and enhanced low temperature performance
2021-05-14RuyunYueFngXiRuijunQiTieShnshnShiZhipingLiYufengZhoJiujunZhng
Ruyun Yue,Fng Xi,Ruijun Qi,D Tie,Shnshn Shi,,Zhiping Li,Yufeng Zho,,*,Jiujun Zhng
a Key Laboratory of Applied Chemistry, Yanshan University, Qinhuangdao 066004, China
b Institute for Sustainable Energy & College of Sciences, Shanghai University, Shanghai 200444, China
c Key Laboratory of Polar Materials and Devices (MOE), Department of Optoelectronics, East China Normal University, Shanghai 200241, China
ABSTRACT The P2-type manganese-based Na0.7MnO2cathode materials attract great interest due to their high theoretical capacity.However,these materials suffer from rapid capacity fading,poor rate performance and severe voltage decay resulting from phase transition and sluggish reaction kinetics.In this work we report a novel Nb-doped Na0.7[Ni0.3Co0.1Mn0.6]1-xNbxO2with significantly suppressed voltage decay and enhanced cycling stability.The strong Nb-O bond can efficiently stabilize the TMO framework,and the as prepared material demonstrates much lower discharge midpoint voltage decay (0.132 V) than that of pristine one(0.319 V)after 200 cycles.Consequently,a remarkably improved cycling performance with a capacity retention of 87.9% after 200 cycle at 0.5 C is achieved, showing a 2.4 fold improvement as compared to the control sample Na0.7Ni0.3Co0.1Mn0.6O2(~37%rotation).Even at 2 C,a capacity retention of 68.4% is retained after 500 cycles.Remarkably, the as prepared material can be applied at low temperature of -20, showing a capacity retention of 81% as compared to that at room temperature.
Keywords:Manganese-based oxides Sodium ion battery Low temperature Voltage decay Cycling stability
Increasing concerns about energy shortages and serious environmental problems in the modern society requires reasonable utilization sustainable energy sources, for which efficient large-scale electric energy storage technology is urgently demanded [1–6].Owing to the low cost and abundant sodium resources in earth,sodium-ion batteries(SIBs)are considered as a promising candidate to substitute lithium-ion batteries (LIBs) for large-scale grid energy storage [7,8].However, the lack of high performance electrode materials, limits the practical application and commercialization of SIBs.In the past decades,various cathode materials have been intensively investigated[9–12].Among them,layered sodium transition metal oxides NaxMO2(M is a transition metal,Co,Mn,Fe,Ni,etc.),have been considered as one of the most potential cathode materials,which can be classified into P2 and O3 phases according to the Na occupation sites and repeated unit cell[13].Especially, the manganese-based P2 layered structure(Na0.7MnO2) have attracted considerable attention due to their low cost,high theoretical capacity and environment friendliness of Mn [14–17].Nevertheless, such structures usually undergo a structural degradation and serious voltage decay upon Na+(de)intercalation, thus cannot fully satisfy the demands of advanced applications.Thus, voltage fade is the pivotal problem that urgently needs to be overcome.
As is generally accepted,the irreversible phase transition,Jahn–Teller lattice distortion of Mn(III) and Na+/vacancy ordering occurred in the electrochemical process are mainly responsible for the SIB fadings [18–20].Various approaches including lattice doping and surface/interface modifications,have been intensively investigated to alleviate the structural change upon cycling.Cationic doping with heteroatoms, such as Li+, Mg2+, Al3+, Ti4+,has been proved as an effective method to promote the performance of cathode materials [21–25].For instance, the incorporation of more Cu ions can suppress the valance change of Fe ions and voltage decay in the Fe-based layered oxides[26].Li as an inert element, is also found can act as the structural support point to stabilize the crystal structure during Na+intercalation/deintercalation [27].Meanwhile, considering the cost of production in practice, trace amount doping with a heteroatom concentration generally two to three orders lower is placed on the agenda.Trace Ti-doping is recently reported to effectively modify the microstructure of LiCoO2and stabilize the surface oxygen at high voltages,resulting in a promoted cycling stability at 4.6 V [28].
Despite high relevance in structure and electrochemistry,strong oxygen redox accompanied by the oxygen gas release from the Mn-based material was revealed, but none in the high-valent ion doped materials.Inspired by the above work,we report a novel low-concentration Nb doping of Na0.7Ni0.3Co0.1Mn0.6O2as high performance SIB cathode material.Considering the relationship between transition-metal ion migration and voltage decay,the size of doped ions and the bond dissociation between metal and oxygen play an important role in suppressing voltage decay.Herein, 5d metal niobium has been incorporated into manganese-based layered oxides [29–32].Particularly, the ionic radius of Nb5+(0.64 Å) is close to that of Mn3+(0.645 Å) and Co3+(0.61 Å),ensuring the successful doping of Nb element in the layered structure.Besides, the Nb-O bonds generally demonstrate higher metal-oxygen bond energy[33–36],which is expected to enhance structural stability, and alleviate the structural degradation upon cycling.The improved electrochemical performance is resulted from the enhanced oxygen stability induced by local structural variation around doping Nb.Consequently, the as prepared Na0.7[Ni0.3Co0.1Mn0.6]0.98Nb0.02O2demonstrates a high capacity retention of 87.9% after 200 cycle at 0.5 C, showing a 2.4 fold improvement as compared to that of the control sample Na0.7Ni0.3Co0.1Mn0.6O2(37%).It is worth noting that,even in trace amounts,Nb-doping suppresses significantly the voltage decay of Na0.7Ni0.3Co0.1Mn0.6O2.Na0.7[Ni0.3Co0.1Mn0.6]0.98Nb0.02O2shows much lower discharge midpoint voltage decay(0.132 V) than that of pristine one (0.319 V) after 200 cycles.Remarkably, the as prepared material also demonstrates good performance at low temperature (
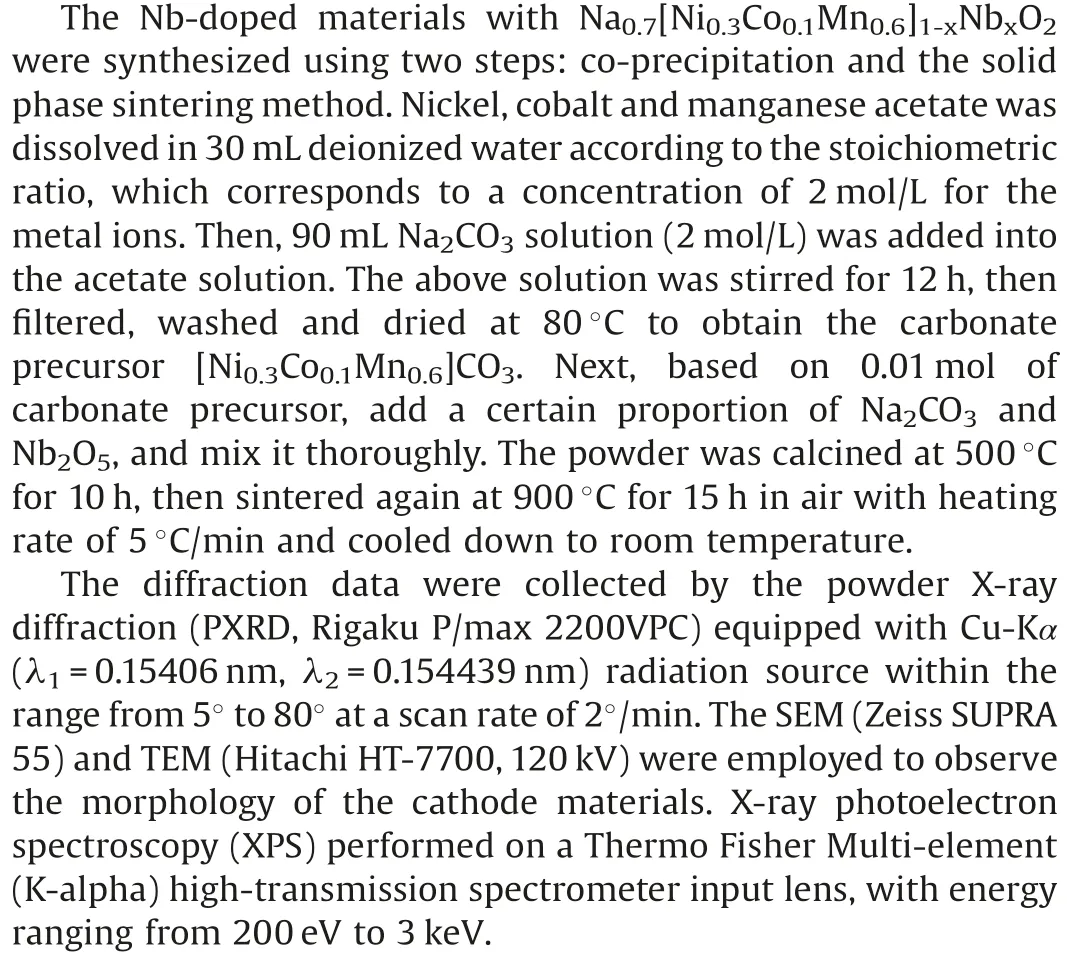
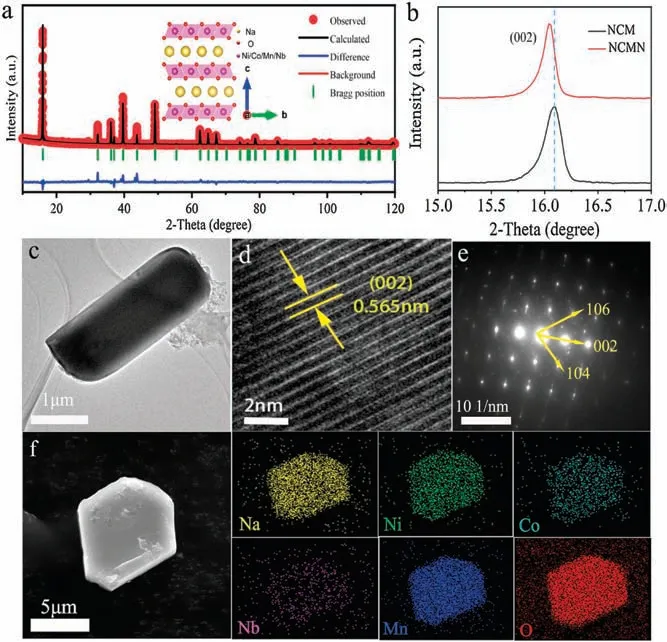
Fig.1.(a) Rietveld refinement plot of NCMN and inset of crystal structures of P2-phase.(b) Shift of the (002) peak of both materials.(c) TEM image.(d) HRTEM image.(e) The SAED pattern of NCMN.(f) SEM image and the corresponding elemental EDX mapping images of NCMN.
The Nb-doped Na0.7[Ni0.3Co0.1Mn0.6]1-xNbxO2(x=0, 0.02,denoted as NCM, NCMN) samples were successfully synthesized through a classical solid-state reaction.The inductively couple plasma(ICP)results(Table S1 in Supporting information)of NCMN confirm the ratio of 0.723:0.290:0.099:0.568:0.013 for Na:Ni:Co:Mn:Nb,which is very close to the expected stoichiometry.The XRD results of the obtained samples show all the diffraction peaks are well indexed to a typical P2-type structure with the P63/mmc space group (Fig.S1a in Supporting information).As shown in Fig.1a and Table S2 (Supporting information), the Rietveld refinement of the NCMN gives the lattice parameters a=b=2.8771(6) Å, c=11.1332(5) Å and V=79.8143 Å3with a reliability index (Rwp) of 4.53%.The molecular model structure diagram for the P2-NCMN is shown in inset of Fig.1a, which is composed of alternating MnO6slabs and Na layers.Besides,niobium ions occupy the transition metal sites in metal oxide octahedral.The refined XRD patterns of NCM sample is also displayed in Fig.S1b(Supporting information)and corresponding crystallographic parameters are listed in Table S3.After Nbreplacement,the(002)peak shifts to lower degree(Fig.1b),which reveals that Nb has been successfully doped into Na0.7Ni0.3-Co0.1Mn0.6O2without affecting the hexagonal structure.The crystallographic lattice parameters after Rietveld refinement are summarized in Table S4 (Supporting information).The d-spacing of Na+layers is decreased by 0.181 Å,whereas the slab thickness of TMO2is increased 0.135 Å after Nb substitution.Thereby c parameter is generally decreased from 11.225 Å to 11.133 Å.The possible reason is the comprehensive effect of larger size and high valence state of Nb, which leads to the shrinkage of Na layer in c axis and expands TMO6octahedron [37].
The transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images further confirm that the as-prepared sample are highly crystalline(Figs.1c and d).The morphologies of NCM is shown in Fig.S2 (Supporting information).It is clear that there is no obvious change in morphology before and after doping.From the HRTEM image in Fig.1d,the interplanar distance between neighboring lattice fringes is 0.565 nm,corresponding to the(002)planes of the layered structure.Furthermore, the selected area electron diffraction(SAED)patterns(Fig.1e)are indexed to the P2-type crystalline structure viewed from the [001] direction, in accordance with the results of HRTEM images.The scanning electron microscopy (SEM) image shows that the morphology of the NCMN,which is an irregular layered structure in general with size ranging from 2 mm to 5 mm.The results of EDS mapping on the crystallites reveal that Na, Ni, Co, Mn, Nb and O elements are homogenously distributed in the materials (Fig.1f).
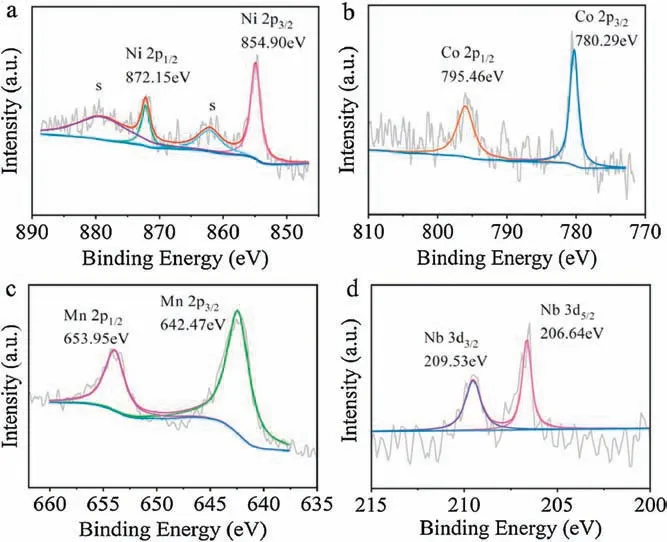
Fig.2.XPS spectra of the NCMN samples:(a)Ni 2p,(b)Co 2p,(c)Mn 2p and(d)Nb 3d.
X-ray photoelectron spectroscopy (XPS) was employed to investigate the oxidation states of elements in NCMN electrode(Fig.2).Dominant peaks located at 854.90 and 872.15 eV are attributed to the peak of the Ni 2p line,indicating that the valence of nickel ion is +2 [38].Two peaks of Co 2p3/2and Co 2p1/2respectively correspond to 780.29 and 795.46 eV, demonstrating the presence of Co3+.In addition,Mn 2p3/2and Mn 2p1/2peaks are located at 642.47 and 653.95 eV,respectively,which clearly reveals that the Mn3+and Mn4+coexist in the material[39].The XPS peaks at 209.53 and 206.64 eV provide direct evidence that valence state of Nb is +5, in accord with previous report [40].
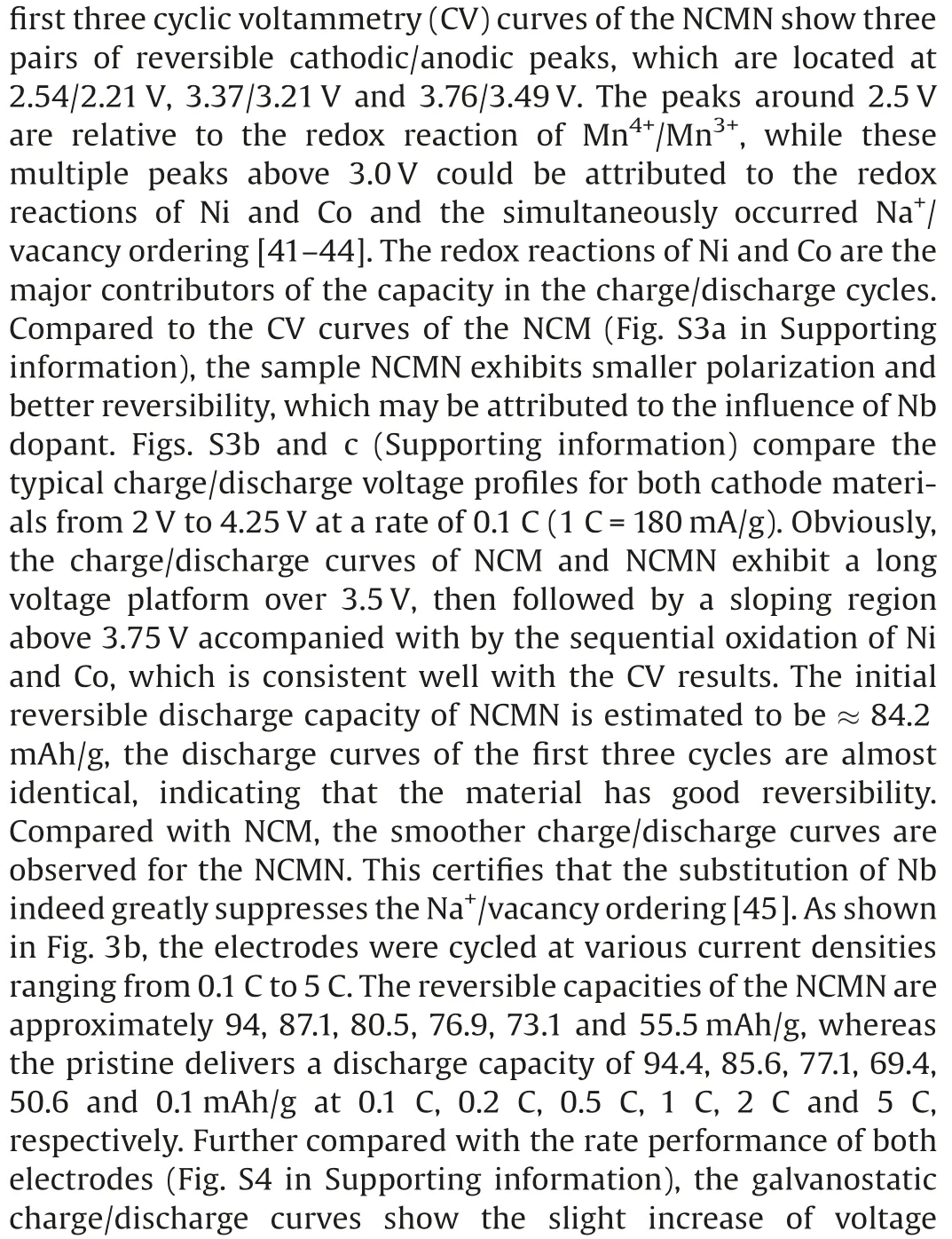
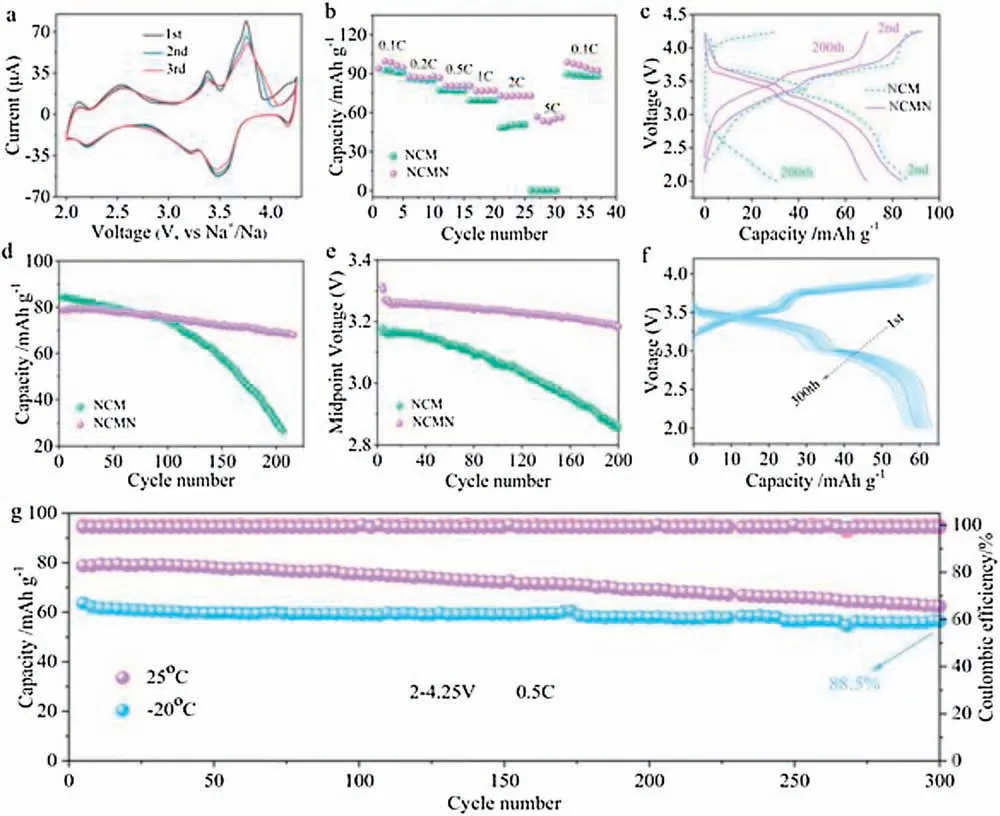
Fig.3.(a)The cyclic voltammograms of NCMN for the first three cycles at a scan rate of 0.1 mV/s.(b)The rate capabilities of both electrodes.(c)Galvanostatic charge/discharge profiles of NCM and NCMN material in 2nd and 200th cycles at 0.5 C.(d)Cycling performance and(e)the discharge midpoint voltage of the pristine and Nb-doped electrodes at 0.5 C for 200 cycles.The low-temperature performance of NCMN:(f)galvanostatic charge/discharge profiles of NCMN cycled at 0.5 C between 2.0 V and 4.25 V at -20.(g)Long-term cycling performance of NCMN at 0.5 C for 300 cycles at 25and -20.
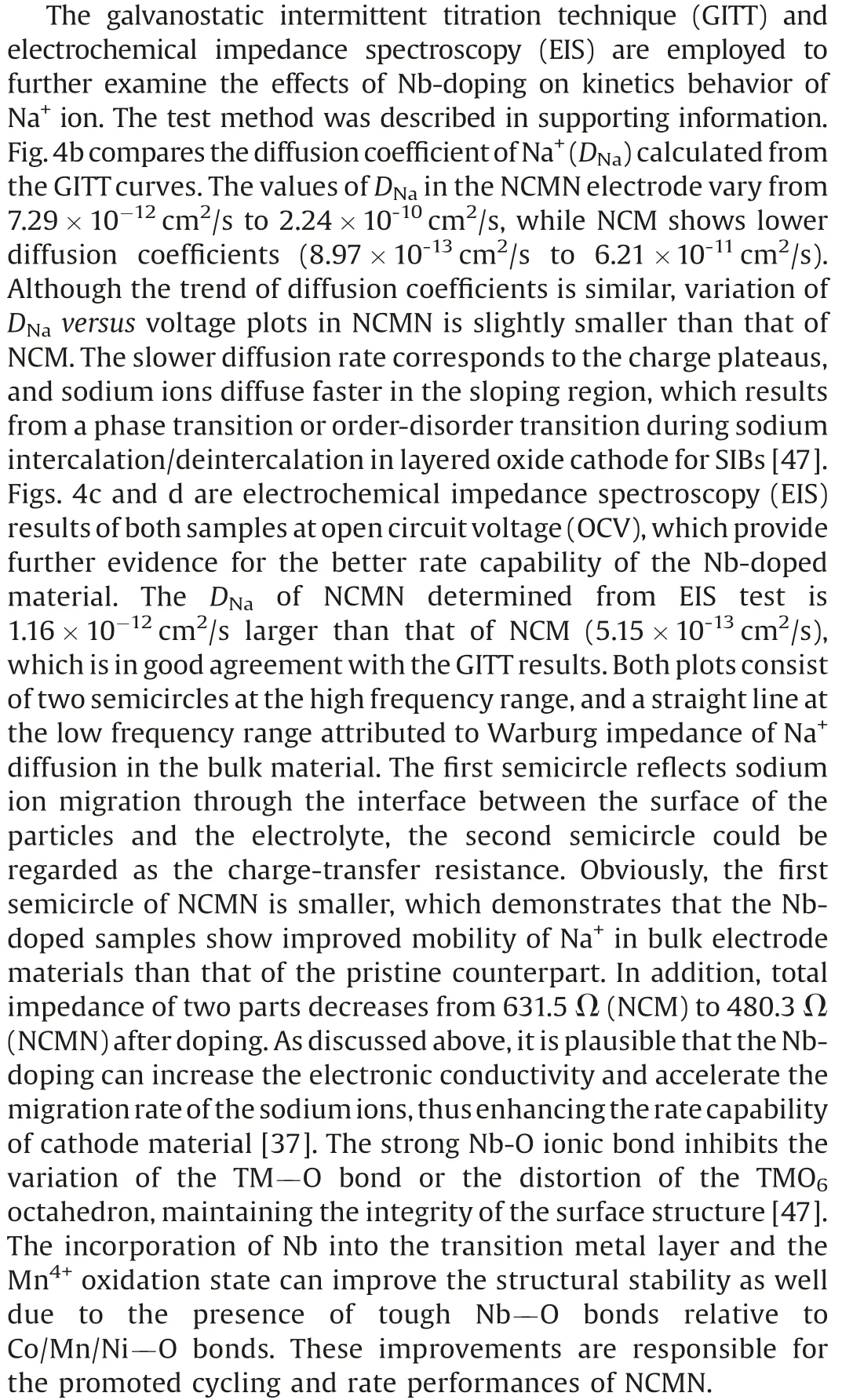
Fig.4.(a)GITT curves of NCMN cathode material in the first cycle.(b)The chemical diffusion coefficient of Na+ions as a function of voltage calculated from the GITT profile.(c)The EIS of the NCM and NCMN electrodes before cycle.(d)Relationship between real impedance with the low frequencies of the pristine (NCM) and Nbdoped (NCMN) electrodes.
To further explain the structural stability and internal mechanism, the morphology and electrochemical behavior of NCMN were examined before and after cycling, and the results are displayed in Fig.S6 (Supporting information).In terms of morphology, the main layered structure was still remained even after 200 cycles at 0.5 C as shown in Figs.S6a and b,suggesting the good structure stability for NCMN.Figs.S6c and d show the major oxidation/reduction peaks located at about 3.5 V is still sharp after 200 cycles,which further confirms that NCMN has high structure reversibility upon Na+(de) intercalation.This is probably a result from reducing migration of Ni2+and dissolution of Mn3+in the transition metal layers, and to some extent, restraining the phase transition after Nb substitution [26,28,45,46].These results indicate that substitution of Nb can effectively stabilize the structure of the crystal and suppress voltage fading during the charging/discharging process,further improving the electrochemical properties of the materials.
In summary,we have successfully synthesized a novel trace NbdopedcathodematerialsandexploredtheinfluenceofNbsubstitution on the electrochemical performance of P2-Na0.7Ni0.3Co0.1Mn0.6O2composites.The NCMNelectrodes deliveran initial discharge capacity of78.mAh/gat0.5C(testedat),withretentionof87.9%and negligible voltage decay of 0.132 V after 200 cycles.Besides, it also keeps initial capacity of 63.mAh/g and retention of 88.5% at 0.5 C underafter 300 cycles.EIS and GITT tests demonstrate that the pronounced rate performance is attributed to high sodium diffusivity during the intercalation/deintercalation.The results indicate that Nbdoping has a positive effect on the improvement cycling stability and the sodium ion diffusion coefficient.In particular,it can dramatically suppress the discharge voltage decay during the cycling process.Nbdoping is a possible strategy to maintain structural stability for suppressing the detrimental voltage fade to improve energy density for practical applications.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
We thank the financial supports from the National Natural Science Foundation of China(No.51774251),Hebei Natural Science Foundation for Distinguished Young Scholars (No.B2017203313),Hundred Excellent Innovative Talents Support Program in Hebei Province(No.SLRC2017057),Talent Engineering Training Funds of Hebei Province(No.A201802001), and the Opening Project of the State Key Laboratory of Advanced Chemical Power Sources (No.SKL-ACPS-C-11).
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.05.025.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- One-step straightf oward solid synthesis of high yield white fluorescent carbon dots for white light emitting diodes
- Free-standing nitrogen doped graphene/Co(OH)2composite films with superior catalytic activity for aprotic lithium-oxygen batteries
- Amorphous silicon from low-temperature reduction of silica in the molten salts and its lithium-storage performance
- Two 2D uranyl coordination complexes showing effective photocatalytic degradation of Rhodamine B and mechanism study
- Recent advances in electrochemical sensors for antibiotics and their applications
