Hendecanuclear [Cu6Gd5] magnetic cooler with high molecular symmetry of D3h
2021-05-14WeipengChenGuojunZhouZhuolunGouSenWngYunqiZhiTinHnrgenSchnckYnzhenZheng
Weipeng Chen,Guojun Zhou,Zhuolun Gou,Sen Wng,Yunqi Zhi,Tin Hn,J ürgen Schnck*,Ynzhen Zheng,*
a Shenzhen Research School and Frontier Institute of Science and Technology (FIST), State Key Laboratory for Mechanical Behavior of Materials, MOE Key Laboratory for Nonequilibrium Synthesis of Condensed Matter, Xi ’an Key Laboratory of Sustainable Energy and Materials Chemistry and School of Science,Xi ’an Jiaotong University, Xi ’an 710054, China
b School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China
c Faculty of Physics, Bielefeld University, D-33501 Bielefeld, PO Box 100131, Germany
ABSTRACT A new family of isostructural 3d-4f polymetallic complexes,formulated as[Cu6Ln5( m3·OH)9(C4H8O2N)6(C5H4ON)6(H2O)9]·(ClO4)6·(H2O)22(Ln=Pr,1;Nd,2;Sm,3;Eu,4;Gd,5),was successfully isolated through the simple hydrolysis reaction of 2-aminoisobutyric acid, 2-hydroxypyridine, Cu(CH3COO)2·H2O, andNotably,the[Cu6Ln5]clusters with high molecular symmetry of D3hare rare examples of 2-aminoisobutyric acid-based 3d-4f clusters.The successful theoretical modeling of 5 yielded that the Gd-Gd exchange is of order 0.2 K, whereas the Gd-Cu exchange is an order of magnitude larger.Magnetizationdatacollectedforcomplex5yieldamagneticentropychange ()of19.6Jat3K and 7 T,which may be attributed to the weak magnetic interactions between the component metal ions.
Keywords:Polymetallic complexes Lanthanide elements Hydrothermal reaction Magnetic interactions Magnetic refrigeration
A hendecanuclear 3d-4f cluster [Cu6Gd5] with high molecular symmetry of D3hdisplays potential application in magnetic cooling,which thanks to the weak magnetic interactions between the component metal ions.
Magnetic refrigeration, which works on the magneto-caloric effect(MCE),was among others studied by Warburg in 1881[8,9].In recent years, due to the environmental pollution issues becoming more prominent, this environment friendly cooling technology has attracted much attention.To prepare magnetic coolers with large MCE,in addition to the factors mentioned above,several other ingredients also need to be considered such as the large metal/ligand mass ratio and the low spin excited state [2].Furthermore, previous findings have shown that the ferromagnetic exchange interactions can lead to large spin ground states and the weak exchange coupling constant can also ensure close-lying excited states, and subsequently help to enhance the MCE [2].Controlling the sign of magnetic exchange is usually difficult, but we noticed themagnetic interaction within 3d-4f clusters is frequently found to be weak ferromagnetic,owing to the orthogonality of the d- and f-orbitals and thus the electron transfer from the occupied 3d-orbital of Cu(II) ion to the empty 5d-orbital of Gd(III)ion[10].To date,although there are several Cu-Gd magnetic refrigerants been reported,e.g.,[Cu5Gd4],[Cu6Gd6], [Cu8Gd4], [Cu6Gd12], [Cu24Gd6] and [Cu36Gd24], in contrast to the reported Ni(II)and Co(II/III)-contained 3d-4f ones,the design of Cu-Gd clusters still remains a challenge [11].The challenge stems from the different coordination behaviours of Cu(II)and Gd(III)ions[12].One powerful strategy to construct such clusters relies on the use of amphipathic ligands, since 4f metal ions are more attracted to hard atoms like O, while 3d ions are likely to bind soft atoms such as N and S.Hence, we proposed a “mixed-ligand”strategy in our previous work,which exhibits great potential in shaping high-nuclearity 3d-4f clusters [3a,4,7].
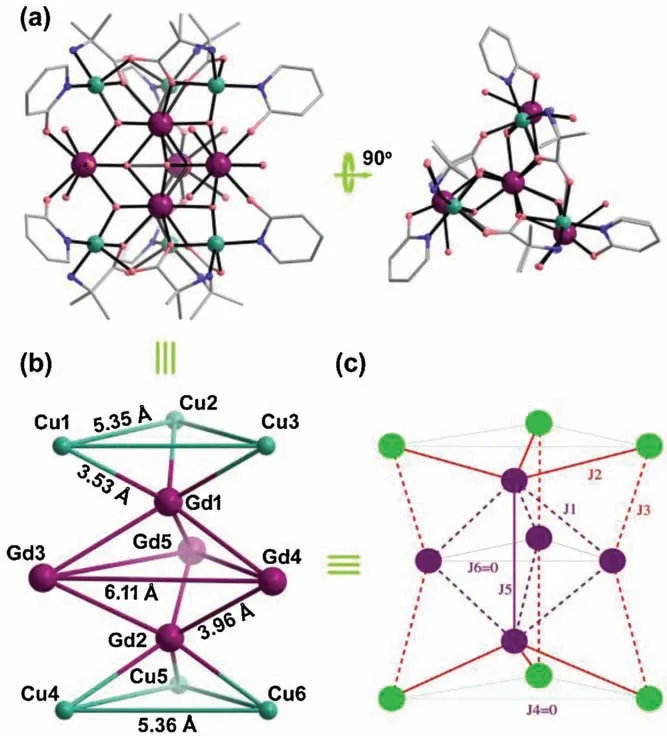
Fig.1.(a) Ball-and-stick view of 5 with H atoms removed for clarity.(b) The arrangement of eleven metal ions in [Cu6Gd5] core.(c) The magnetic coupling schemes of the metal centers in[Cu6Gd5]core.Color codes:Gd,purple;Ni,green;N,blue; O, red; C, grey.
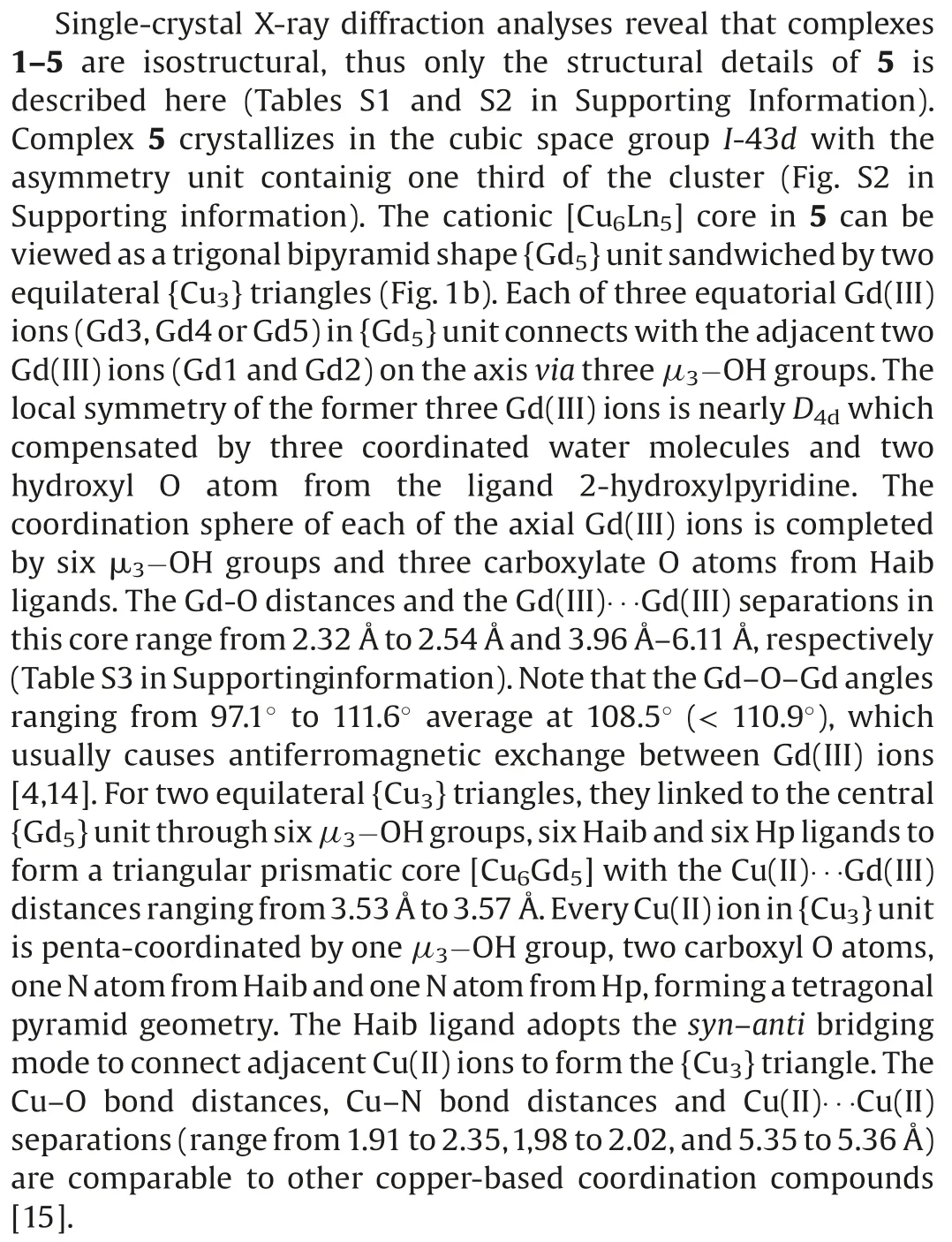
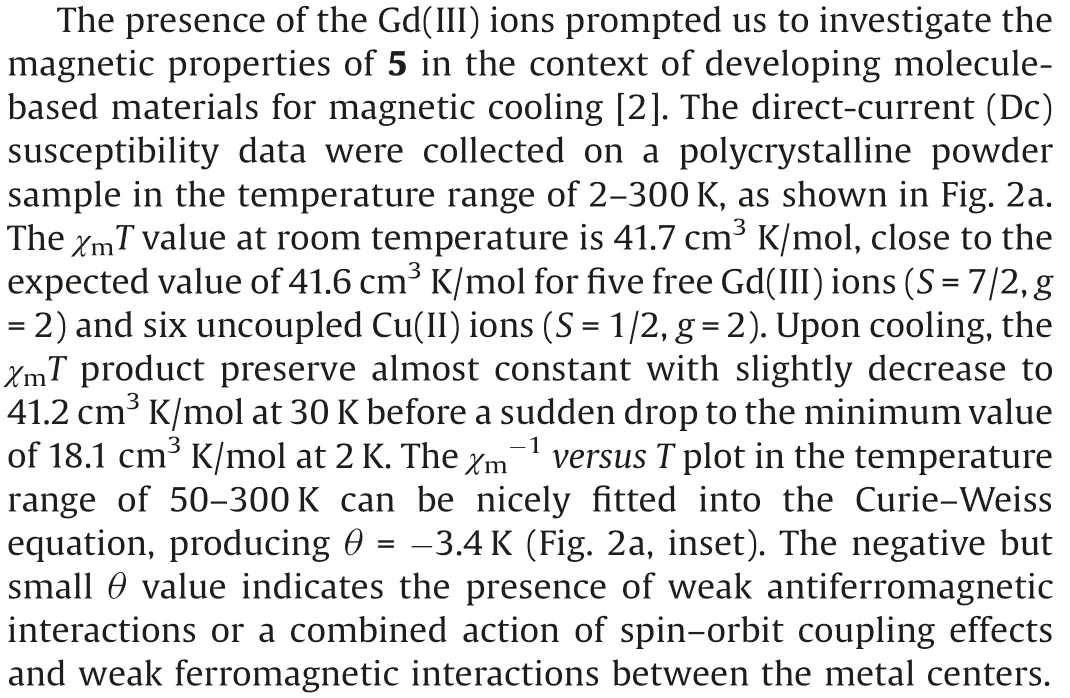
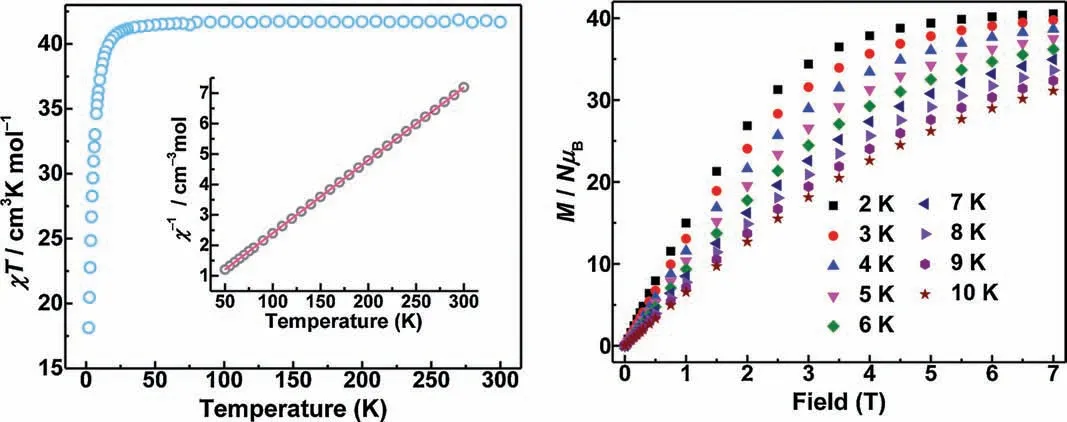
Fig.2.(a) Temperature dependence plots of χmT for 5; Inset: The plots of χ–1vs.T for 5.(b) Field-dependent magnetization plots for 5.
Meanwhile,the field-dependent molar magnetization of 5 was also measured at 2–10 K in the range of 0–7 T (Fig.2b), which reveals that M increases upon increasing the magnetic field,almost saturation until 7 T(Mexp=40.5 N β,Msat=41 Nβ for five Gd(III)ions and six Cu(II)ions,where N is the Avogadro constant).The detailed investigation of inter-ion exchange coupling in polymetallic 3d –4f complexes has always been a challenge due to the large number of magnetic states [4,16].To further gain the magneto-structural correlation of 5,the magnetic interactions were modeled with selfwritten software [17], by using the Hamiltonian equation below:

which uses the sign convention where J > 0 represents a ferromagnetic interaction,and we neglect the single ion magnetic anisotropy on the Gd(III) sites.


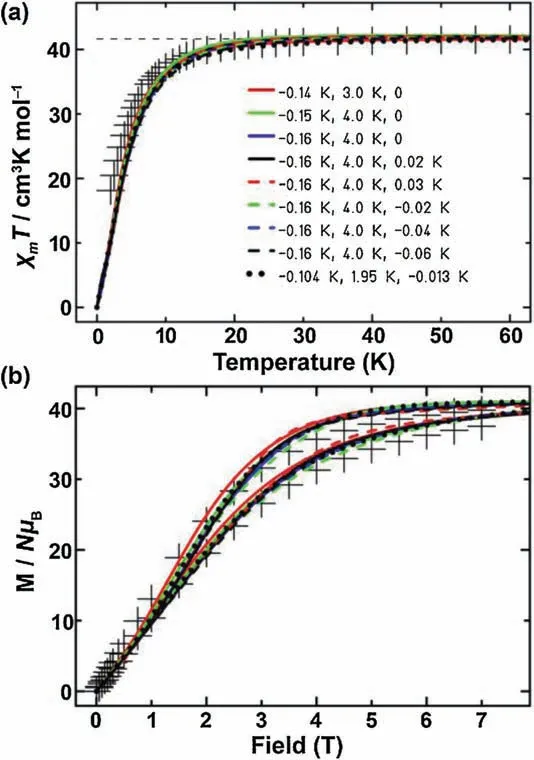
Fig.3.(a)Dc susceptibility data of 5 under 0.1 T(+measured data)and calculation results.The numbers represent the respective values of J1,J2and J5(J3,J4and J6are omitted).(b) Field dependent magnetization data (+ measured data) of 5 and calculation results at 2 and 4 K.
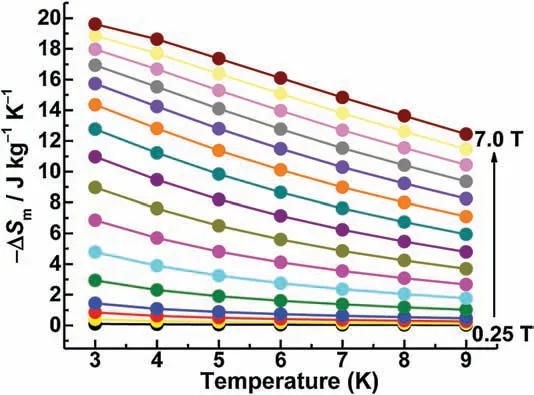
Fig.4.Experimental values obtained from the magnetization data of 5.
In conclusion,a series of hendecanuclear isostructural[Cu6Ln5]clusters with high molecular symmetry of D3hwas successfully synthesized via the simple hydrolysis reaction.Magnetic studies indicate that the [Cu6Gd5] displays significant magnetic entropy change at low temperature.The theoretical calculation confirmed the coexistence of both antiferromagnetic and ferromagnetic interactions between the metal centers.Further work on the Haib-based 3d-4f clusters is currently under investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Shenzhen Science and Technology Program (No.JCYJ20180306170859634), National Natural Science Foundation of China (Nos.21773130, 21801202, 21871219,21971203 and 21620102002),Shaanxi National Science Foundation(No.2019JQ-016), China Postdoctoral Science Foundation (Nos.2019T120891 and 2018M643615), Key Laboratory Construction Program of Xi'an Municipal Bureau of Science and Technology(No.201805056ZD7CG40), Cyrus Chung Ying Tang Foundation and Fundamental Research Funds for Central Universities.This work was also supported by the Deutsche Forschungsgemeinschaft DFG(Nos.314331397, SCHN 615/23-1).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2020.05.018.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- One-step straightf oward solid synthesis of high yield white fluorescent carbon dots for white light emitting diodes
- Free-standing nitrogen doped graphene/Co(OH)2composite films with superior catalytic activity for aprotic lithium-oxygen batteries
- Amorphous silicon from low-temperature reduction of silica in the molten salts and its lithium-storage performance
- Two 2D uranyl coordination complexes showing effective photocatalytic degradation of Rhodamine B and mechanism study
- Recent advances in electrochemical sensors for antibiotics and their applications
