Ceria supported Ru0-Ruclusters as efficient catalyst for arenes hydrogenation
2021-05-14YanweiCaoHuanZhengGangliZhuHaihongWuLinHe
Yanwei Cao,Huan Zheng,Gangli Zhu*,Haihong Wu*,Lin He*
a State Key Laboratory for Oxo Synthesis and Selective Oxidation (OSSO), Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP),Chinese Academy of Sciences (CAS), Lanzhou 730000, China
b University of Chinese Academy of Sciences, Beijing 100049, China
c Henan Province Key Laboratory of New Opto-Electronic Functional Materials, College of Chemistry and Chemical Engineering, Anyang Normal University,Anyang 455000, China
ABSTRACT Selective hydrogenation of aromatic amines, especially chemicals such as aniline and bis(4-aminocyclohexyl)methane for non-yellowing polyurethane, is of particular interests due to the extensive applications.To conquer the existing difficulties in selective hydrogenation,the Ru0-Rud+/CeO2catalyst with solid frustrated Lewis pairs was developed for aromatic amines hydrogenation with excellent activity and selectivity under relative milder conditions.The morphology, electronic and chemical properties, especially the Ru0-Rud+ clusters and reducible ceria were characterized by X-ray diffraction(XRD),transmission electron microscopy(TEM),scanning electronic microscopy(SEM),X-ray photoelectron spectroscopy(XPS),CO2temperature programmed desorption(CO2-TPD),H2temperature programmed reduction (H2-TPR), H2diffuse reflectance Fourier transform infrared spectroscopy (H2-DRIFT),Raman,etc.The 2%Ru/CeO2catalyst exhibited good conversion of 95%and selectivity greater than 99%toward cyclohexylamine.The volcano curve describing the activity and Ru state was found.Owning to the"acidic site isolation"by surrounding alkaline sites,condensation between the neighboring amine molecules could be effectively suppressed.The catalyst also showed good stability and applicability for other aromatic amines and heteroarenes containing different functional groups.
Keywords:Ceria Ruthenium Clusters Amines Hydrogenation
Catalytic hydrogenation of aromatic compounds is one of the important organic transformations process in chemical industry[1,2].The hydrogenated aromatic amines can be exploited in a variety of applications.For instance, cyclohexylamine (CHA),which is produced by hydrogenation of aniline, can be used in the synthesis of dyestuff, plasticizers, emulsifier and rubber vulcanizing additives.Its derivative,bis(4-aminocyclohexyl)methane (H12MDA) is an important diamine intermediate for synthesizing various high-value added compounds, like non-yellowing polyurethane.Unfortunately, hydrogenation of aromatic amines usually requires expensive catalysts and harsh conditions.For example, Ru/C catalyst with assistance of alkali additives was applied in the methylenedianline (MDA) hydrogenation reaction under conditions of 18 MPa and 190[3].Furthermore, under such conditions,it generates many undesirable by-products,which would deteriorate product quality of polymers, especially in chroma and fiber strength.
To overcome these problems, supported precious metal catalysts have been developed for hydrogenation reactions,such as Ir, Pt, Rh and Ru usually have higher catalytic performance than non-precious metal.Among them, Ru based catalysts have attracted much attention due to their relative low cost (ca.of Pd) and potential excellent catalytic performance by careful modulation of Ru NPs and its interaction between the supports [4,5].Ceria has been recognized recently as suitable supports in various catalytic processes, owning to its peculiar nature [6], such as surface-bound defects (oxygen vacancy), reducible character, strong interaction between the support and metal.In addition, there are solid frustrated-Lewis-pair sites (FLPs) over ceria [7], which possessed the ability ofbond dissociation and expectantly could be advantageous in the hydrogenation reactions [8].To the best of our knowledge, there are only very limited works reported on hydrogenation of aniline and the derivatives like MDA over Ru/CeO2catalysts.
Herein,the Ru0-Rud+/CeO2catalyst with solid frustrated Lewis pairs was developed for aromatic amines hydrogenation with excellent activity and selectivity.The morphology, electronic and chemical properties,especially the Ru0-Rud+clusters and reducible ceria were characterized by X-ray diffraction (XRD), transmission electron microscopy(TEM),scanning electronic microscopy(SEM),X-ray photoelectron spectroscopy (XPS), CO2temperature programmed desorption (CO2-TPD), H2temperature programmed reduction (H2-TPR), H2diffuse reflectance Fourier transform infrared spectroscopy (H2-DRIFT), Raman spectrometry, etc.The volcano curve describing the activity and Ru loading amount was found.And the"acidic site isolation"by surrounding alkaline sites effect was proposed for the high selectivity.
The experimental section, including chemicals, catalysts preparation (synthesis of Ru/CeO2), characterization, catalytic hydrogenation experiments can be found in Supporting information
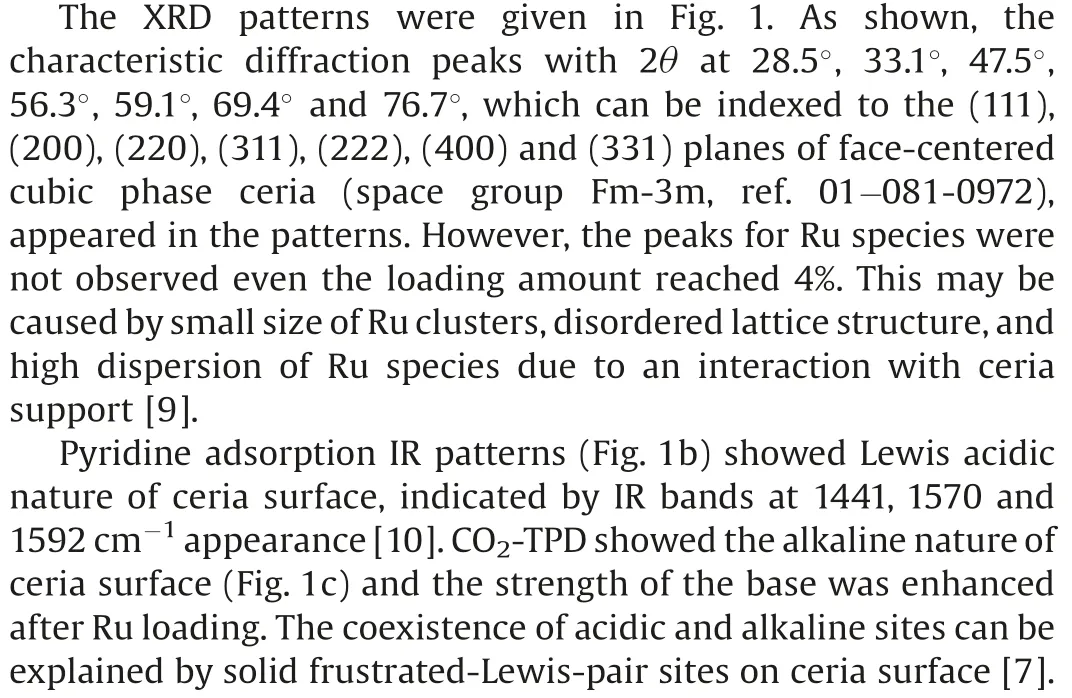
SEM,HAADF-TEM microscopy and EDX spectroscopy were used to study the morphologies and elemental distribution of Ru/CeO2catalyst.As shown in Fig.2, the catalyst was comprised of nanoparticles staking together, and the elemental mapping showed that Ce, O and Ru species were in good dispersion throughout the catalyst, possibly due to interfacial anchoring by immobilization effect of surface oxygen vacancies[10].Ru clusters mainly deposited on the (111) surface of ceria.Some Ru clusters with diameter of 23 nm could be observed, exposing their hcp(101) planes with lattice spacing of 0.21 nm.Ru loading amount would influence the size of Ru clusters.4% Ru loading leading to formation of relative large nanoparticles (ca.58 nm) and there were hardly countable Ru nanoparticles were found in 0.5% Ru/CeO2and 1% Ru/CeO2TEM images (Fig.S4 in Supporting information).Some crystal plane structures of the Ru clusters were disordered (Fig.2c), indicating part of Ru clusters were oxidized to form disorderedlayers due to interfacial Ru-O-Ce anchoring.Some oxygen in ceria with high mobility may also transfer to Ru clusters, while simultaneously, ceria layers at the perimeter of the particles would be reduced,[11,12]which turned out to be an interaction effect.The chemical nature change of Ru clusters, oxidation of the Ru clusters accompanying reduction of Ce4+to Ce3+cations,would consequently influence the activity as it will be showed in the catalytic performance results.
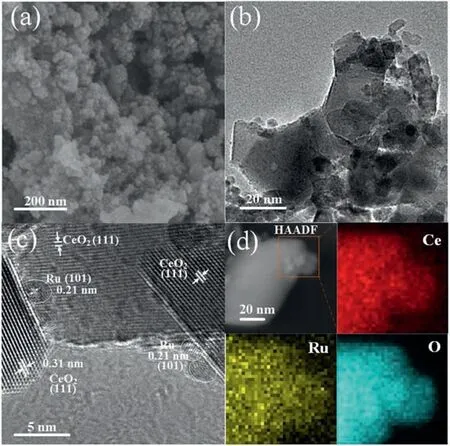
Fig.2.Morphological and elemental analysis of 2%Ru/CeO2:(a)SEM image;(b)TEM image; (c) HRTEM image; (d) elemental mapping.
The interaction strength can be reflected in the reducibility.H2-TPR was performed and the results are shown in Fig.3a.There are two peak regions in the TPR profiles, with region I at low temperatures and region II at high temperatures,attributed to the reduction of Ru species with different interaction strength with support [10,13].In the 2% Ru/SiO2, the reduction peaks at 133and 246for both two regions were higher than 2%Ru/CeO2.The reducibility was not only affected by support types but also the Ru loading amount.In the ceria supported catalysts,region I could be ascribed to reduction of partially oxidized Ru particles that had weak interaction with support.This peak region I shift from 160to 105when the Ru loading amount increased from 1% to 2%.Further raising Ru amount to 4% did not result a lower reduction temperature but led to region I splitting into 3 consecutive peaks,corresponding to the stepwise reduction of supported ruthenium oxides.With regards to the 2%Ru/CeO2,the region I initiated from 55and ended at 172.The high reducibility indicated the interaction between Ru clusters and ceria was relative weak with this loading amount,which was also verified by Raman characterization.
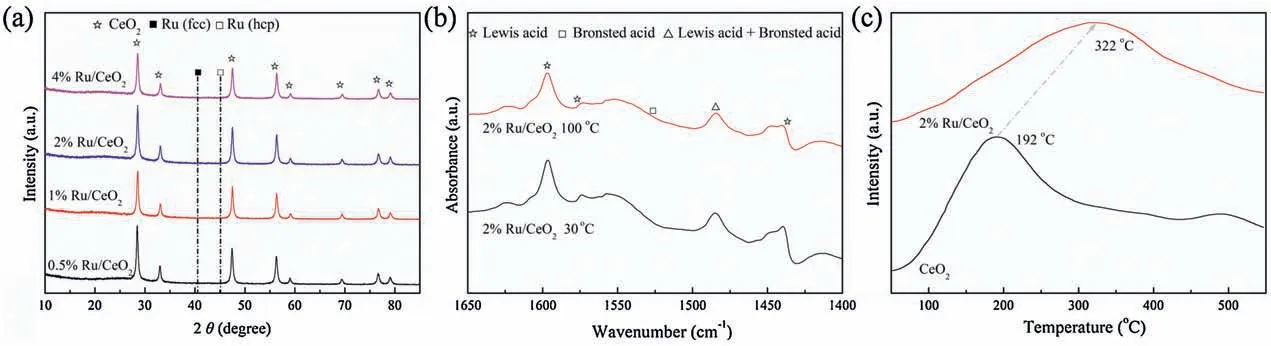
Fig.1.(a) XRD patterns for different Ru-based catalysts.(b) Pyridine adsorption IR of Ru/CeO2catalysts.(c) CO2-TPD curves of 2% Ru/CeO2catalyst and CeO2support.
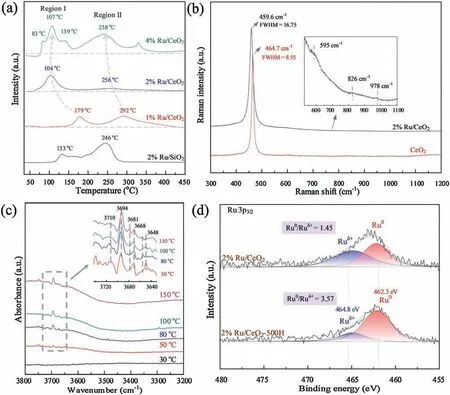
Fig.3.Supported Ru catalysts (a) H2-TPR profiles, (b) in-situ H2-DRIFTS spectra, (c) Raman spectra and (d) XPS spectra of Ru 3p3/2.
The Raman spectroscopy(Fig.3b)showed that there was a peak at 978which can be attributed to Ru-O-Ce [10,14], but the intensity was quite weak due to the relative low defects density of stable (111) planes of ceria [15].The broad TPR peak region II at high temperature can be attributed to the reduction of Ru species which strongly interact with ceria.The reduction peak shifted to lower temperature with increase of Ru content.This peak decreased from 292to 238when the Ru amount increased from 1%to 4%.These Ru species were oxidized to a greater extent by the higher affinity of oxygen which was provided by supports(i.e., higher absorption enthalpy of oxygen)[16].And thus,these Ru species, which strongly interact with support, are relatively not easy to be reduced.could benefit greatly from the H2readily dissociation and transferring by spilling-over.


Hydrogenation of aniline was carried out with prepared catalysts (Table 1).As shown, the 2% Ru/CeO2catalysts had a good catalytic performance,providing as high as conversion of 95%and selectivity of 99%toward cyclohexylamine(entry 3).The sideproducts by hydrodealkylation or condensation reaction (e.g., dicyclohexylamine)were not observed in the reaction.Comparingwith oxide support or commercial activated carbon, the ceria showed better both activity and selectivity.The catalyst was also effective for MDA hydrogenation process.As shown in Table S1 (Supporting information ), comparing with the existing catalysts(or catalyst systems), the 2% Ru/CeO2catalyst in this work exhibited quite high catalytic efficiency and selectivity under milder conditions.The catalyst also showed good stability and applicability of other aromatic compound with different functional groups (Figs.S7 and S8 in Supporting information).
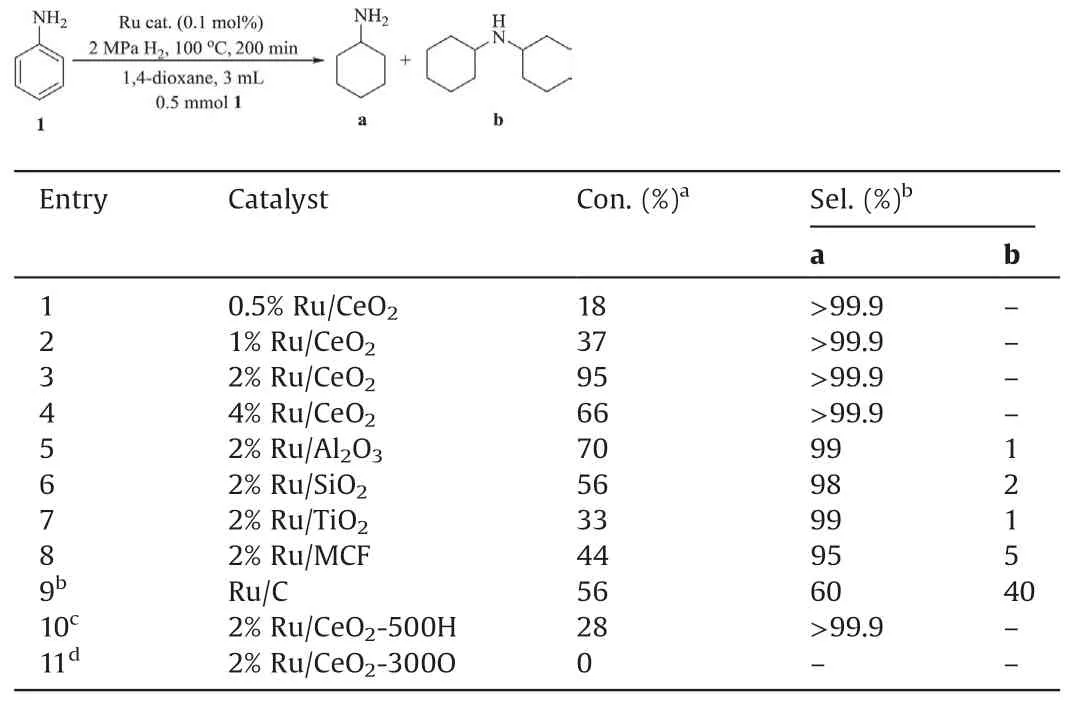
Table 1 Hydrogenation performance using various catalysts.


The high selectivity and low condensation side-products can be explained by to the coexistence of solid frustrated Lewis acid-base pair sites over ceria [7].For a general acidic support, aniline and cyclohexylamine molecules have strong adsorption on acidic sites[25,26].And condensation reaction may take place between the neighboring molecules.However,regarding with ceria,which has a fluorite-like cubic structure.Each cerium atom possessing the Lewis acid nature is surrounded by the surface and subsurface alkaline oxygen sites (and even enhanced by oxygen vacancies).This"acidic site isolation"of Lewis acid sites by alkaline sites could effectively suppress condensation of amines.Similar results also observed in selective hydrogenation of alkynes [17], a low degree of oligomerization of intermediates was found in the conversion of alkynes to olefins over CeO2.
In summary, the well-tuned Ru/CeO2was demonstrated as efficient catalyst for aromatic amines hydrogenation with excellent activity and selectivity.Morphology, electronic and chemical properties of Ru/CeO2was well were analyzed by using different characterization techniques.Due to the special chemical nature of Ru/CeO2, the catalyst exhibited good conversion of 95% and selectivity of 99.9% toward cyclohexylamine.The catalyst also showed superior stability and applicability of other aromatic amines and heteroarenes containing different functional groups.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was financially supported by the Youth Innovation Promotion Association CAS (No.2018453) and the National Natural Science Foundation of China (No.91645118 for L.He,21773270 for G.Zhu).Supports from the NSF of Jiangsu Province(No.BK20180249) are also gratefully acknowledged.
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.05.045.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- One-step straightf oward solid synthesis of high yield white fluorescent carbon dots for white light emitting diodes
- Free-standing nitrogen doped graphene/Co(OH)2composite films with superior catalytic activity for aprotic lithium-oxygen batteries
- Amorphous silicon from low-temperature reduction of silica in the molten salts and its lithium-storage performance
- Two 2D uranyl coordination complexes showing effective photocatalytic degradation of Rhodamine B and mechanism study
- Recent advances in electrochemical sensors for antibiotics and their applications
