Photo-induced anti-Markovnikov hydroalkylation of unactivated alkenes employing a dual-component initiator
2021-05-14YchoZhngLingLingMoSifnHuYiLunHunCong
Ycho Zhng,Ling-Ling Mo,Sifn Hu,Yi Lun,**,Hun Cong,c,*
a School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, China
b Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences,Beijing 100190, China
c School of Future Technology, University of Chinese Academy of Sciences, Beijing 100190, China
ABSTRACT Metal-free anti-Markovnikov hydroalkylation of unactivated alkenes with cyanoacetate has been developed, featuring the use of a dual-component initiator containing an organic photocatalyst and a radical precursor.When combined, the two components can undergo visible light-induced singleelectron transfer, and serve as a versatile and effective alkyl radical generator.
Keywords:Photocatalysis Anti-Markovnikov Alkene hydroalkylation Single-electron transfer Radical
Hydrofunctionalization of unactivated alkenes represents a productive strategy to rapidly access diverse molecular structures from abundant and low-cost synthetic feedstocks [1].Dictated by the empirical Markovnikov’s rule, classic olefin electrophilic addition involving proton as the electrophile can afford regioselective hydrofunctionalization products(Scheme 1a) [2].Complementary approaches have been investigated showing anti-Markovnikov regioselectivity: (1)transition-metal hydride-mediated olefin insertion [3] and (2)radical addition to alkenes followed by hydrogen atom transfer(HAT) [4].
The facile generation of radical species under mild conditions is essential to the success of visible light photoredox catalysis through photo-induced single-electron transfer (SET) processes[5].In order to achieve efficient SET, the excited states of the photocatalysts (PC) are usually required to match the redox properties of the starting materials[6],which may limit the scope of applicable reaction substrates.We envisioned that a dualcomponent initiator system wherein visible light-induced SET between a photocatalyst and a radical precursor could provide a versatile radical source [7].The resulting radicals would further interact with starting materials to initiate the desired reactions.Therefore, the formation of radical intermediates would be less dependent on the redox properties of the starting materials,which may offer opportunity for new reaction development(Scheme 1b).
Here we report the photo-induced anti-Markovnikov hydroalkylation of unactivated alkenes with cyanoacetate, featuring metal-free conditions(Scheme 1c).Specifically,an organic dye(i.e.,eosin Y-Na2)[8]and N-hydroxyphthalimide(NHP)ester[9]prove to be effective as the photocatalyst and the radical precursor,respectively.As a proof of concept,such dual-component initiator can indeed serve as an economical and environmentally friendly alkyl radical generator under visible light irradiation.
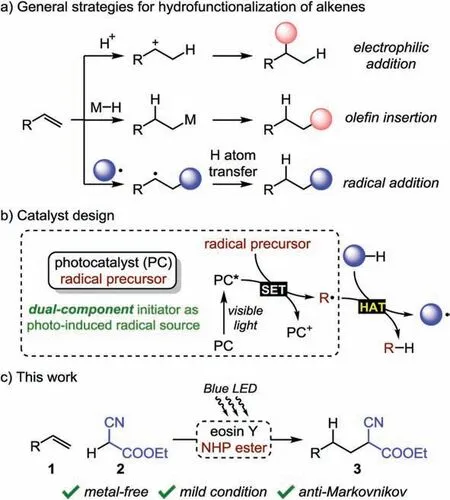
Scheme 1.Background and design of the photo-induced anti-Markovnikov hydroalkylation.
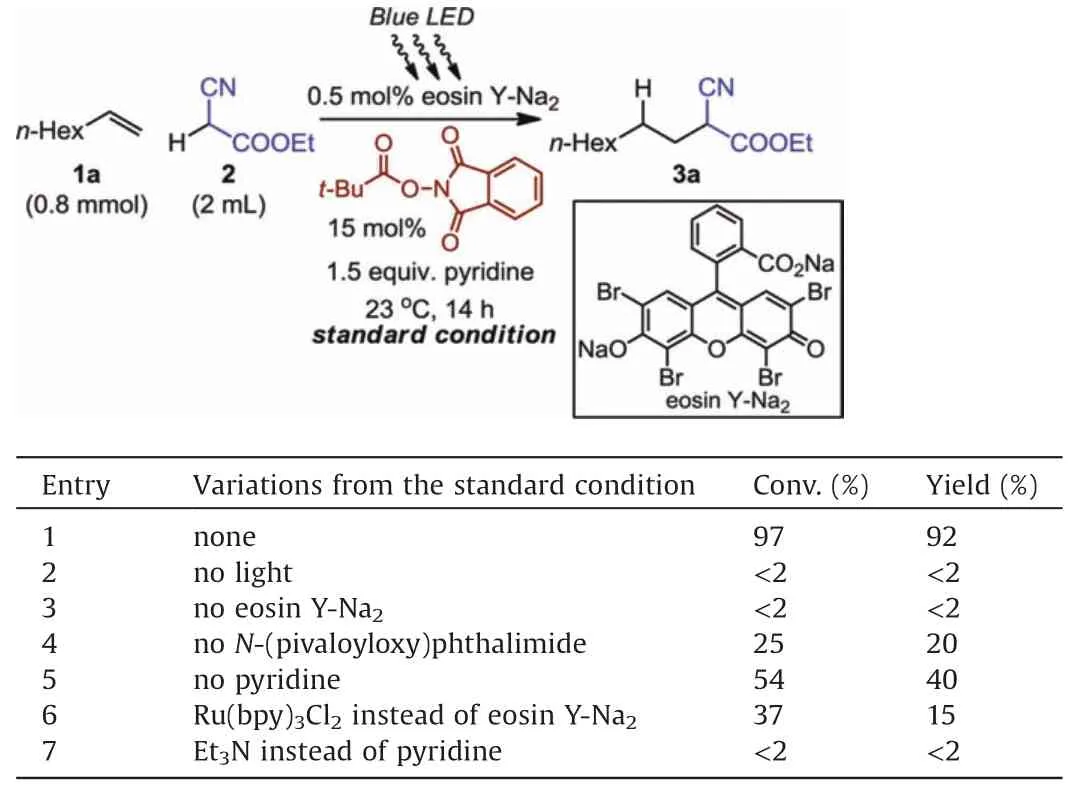
Table 1 Reaction optimization.
On the basis of the aforementioned initiator design, our investigation commenced with the model reaction between 1-octene(1a) and ethyl cyanoacetate(2).As an abundant industrial feedstock($2.7 USD per mole,based on the list price from Energy Chemical Co.,one of the leading lab chemical suppliers in China,as of April 2020),ethyl cyanoacetate exhibits versatile utility for fine chemical synthesis because of its multiple orthogonal reactive sites, but alkene hydroalkylation involving cyanoacetate has thus far been reported with limited success [10].After screening the reaction parameters, we have determined that the desired hydroalkylation reaction can proceed under the irradiation of blue LED light at ambient temperature, showing exclusive anti-Markovnikov regioselectivity and almost quantitative yield(Table 1,entry 1).Notably,best reaction performance was obtained in the presence of excess ethyl cyanoacetate without external solvent.Under the optimized condition, the dual-component initiator is composed of eosin Y-Na2and N-(pivaloyloxy)phthalimide, with control experiments confirming the necessity of light and each component (entries 2).Following the control experiment of Table 1, entry 4, prolonging the reaction time to 24 h in the absence of N-(pivaloyloxy)phthalimide afforded 73%of alkene conversion and 40%yield of the product based on calibrated GC analysis.The substantial product formation catalyzed by eosin Y-Na2alone indicates that parallel HAT processes may also be possible[11].In addition,the use of pyridine as base is beneficial to achieve enhanced reaction efficiency (entries 1 vs.5).Upon investigating an array of photocatalysts, we discovered that eosin Y-Na2, with a relatively low loading (0.5 mol%), provided superior results compared to other organic dyes (Table S1 in Supporting information)and the more expensive Ru-bipyridyl complex(entry 6).Evaluation of a series of NHP esters (Table S2 in Supporting information) and bases (entry 7 and Table S3 in Supporting information) further established the metal-free standard condition.
We next examined the scope of the standard condition for photo-induced anti-Markovnikov hydroalkylation with ethyl cyanoacetate.A range of functionalized unactivated alkenes were found to be suitable substrates, including a demonstration of scalability with a gram-scale reaction (Scheme 2), but styrenes were found to be unproductive substrates.Certain reactive or labile functional groups such as hydroxy (3c), ketone (3d), imide (3g),and acetal (3j) are compatible with the standard condition,affording good yields.In addition to mono-substituted olefins, a cyclic internal alkene (3l) and 1,1-disubstituted alkenes (3m and 3n)can also undergo hydroalkylation smoothly.For all substrates,the anti-Markovnikov hydroalkylation products were exclusively observed.
The α-cyanoester products are highly useful compounds which can be converted into important synthetic building blocks.For example,the N-Boc amino ester 4 can be rapidly prepared through reduction [12] of cyanoester 3c followed by Boc-protection.The unprotected hydroxyl group remained intact under the lowtemperature reductive condition of sodium borohydride in the presence of nickel chloride.Subsequent tosylation of 4 and SN2 with potassium phthalimide furnished the β2-amino acid derivative 6, a key intermediate of a biologically active somatostatin analog (Scheme 3) [13].
To gain insight into whether radical-based mechanism is operating,we conducted a radical clock experiment using the 1,6-diene 1o as the substrate [14].As shown in Scheme 4, our observation that the 5-exo cyclized product 3o', rather than the linear compound 3o,was isolated suggests the existence of radical intermediates.
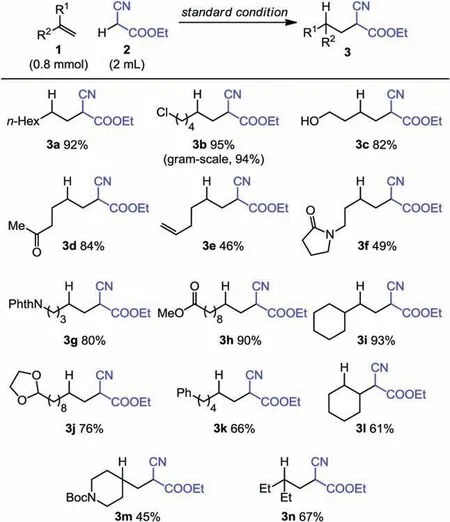
Scheme 2.Substrate scope.
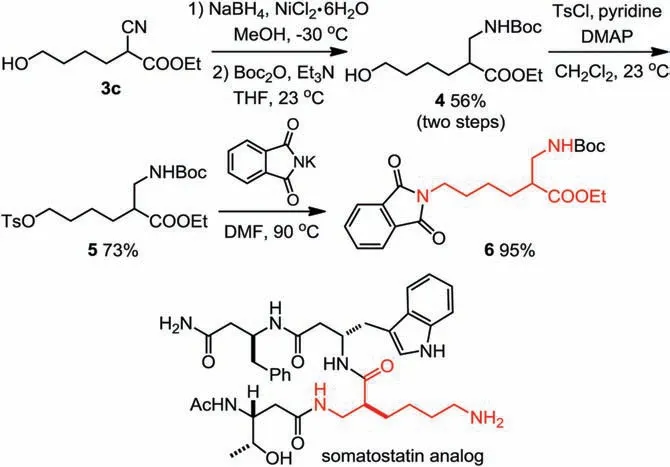
Scheme 3.Synthetic application.
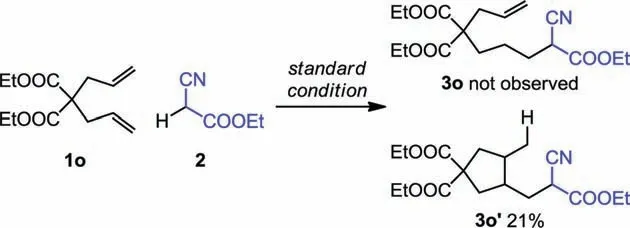
Scheme 4.Radical clock experiment.
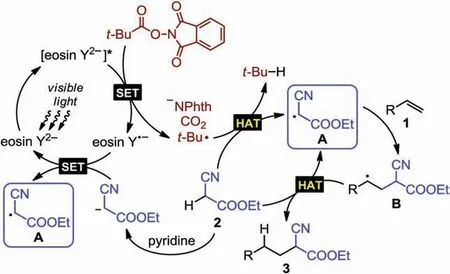
Scheme 5.Plausible mechanism.
We proposed a plausible reaction mechanism (Scheme 5)wherein the visible light-induced SET between the photo-excited eosin Y dianion and NHP ester leads to eosin Y radical anion formation and homolyticbond cleavage[15].The NHP ester decomposes with release of phthalimide anion and CO2(confirmed by GC-MS analysis,Figs.S3 and S4 in Supporting information),and concurrently generates a tert-butyl radical, which undergoes HAT with cyanoacetate 2 to generate isobutane (confirmed by GC-MS analysis, Fig.S4) and alkyl radical A.Radical A then initiates the radical chain propagation process which entails radical addition to olefin 1.Subsequent HAT between the resulting radical B and another cyanoacetate 2 regenerates radical A and provides the hydroalkylation product 3 [16].In parallel, cyanoacetate 2, upon deprotonation by pyridine, is subjected to SET with the eosin Y radical anion, affording radical A and regenerating the photocatalyst.
In summary, the environmentally benign hydroalkylation of unactivated alkenes with cyanoacetate has been developed under visible light irradiation.This method features the dual-component initiator containing an organic photocatalyst and a radical precursor as an effective alkyl radical generator, thereby avoiding the use of conventional toxic radical initiators and metal-based reagents.Further studies on the applications of this versatile photoredox initiator design would inspire the development of novel radical-based synthetic methods.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences(No.XDB17000000),the National Natural Science Foundation of China(Nos.21672227,21922113), the National Key Research and Development Program of China (No.2017YFA0206903), Beijing Natural Science Foundation (No.L182020), Fundamental Research Funds for the Central Universities(No.FRF-TP-19-013B1),K.C.Wong Education Foundation, and the TIPC Director’s Fund.We thank Profs.Li-Zhu Wu(TIPC-CAS), Wenxiong Zhang (PKU), and Congyang Wang (ICCAS)for the help with instrumental analysis.
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.06.026.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- One-step straightf oward solid synthesis of high yield white fluorescent carbon dots for white light emitting diodes
- Free-standing nitrogen doped graphene/Co(OH)2composite films with superior catalytic activity for aprotic lithium-oxygen batteries
- Amorphous silicon from low-temperature reduction of silica in the molten salts and its lithium-storage performance
- Two 2D uranyl coordination complexes showing effective photocatalytic degradation of Rhodamine B and mechanism study
- Recent advances in electrochemical sensors for antibiotics and their applications
