Highly efficient photocatalytic Suzuki coupling reaction by Pd3P/CdS catalyst under visible-light irradiation
2021-05-14HuiQingYngQinQinChenFuliLiuRuiShiYongChen
Hui-Qing Yng,Qin-Qin Chen,Fuli Liu,Rui Shi,Yong Chen,*
a Key Laboratory of Photochemical Conversion and Optoelecctronic Materials & CAS-HKU Joint Laboratory on New Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China
b University of Chinese Academy of Sciences, Beijing 100049, China
ABSTRACT Monodispersed palladium phosphide (Pd3P) (5.2 ) was firstly applied to photocatalytic Suzuki coupling reaction under visible light irradiation with CdS nanoflake as a photosensitizer.This heterogeneous system exhibited high yields to corresponding products and excellent stability in alcohol solvent at room temperature.
Keywords:Pd3P CdS Photocatalysis Suzuki coupling reaction
The formation of carbon-carbon (CC) bonds via coupling reactions has been applied to synthesize a wide range of organics,such as natural products, pharmaceuticals, perfumes and dyes[1–6].Recently, photocatalytic CC coupling reactions have attracted considerable attention due to its sustainability.Especially,Pd nanoparticles(NPs)displayed high conversion and selectivity in these photocatalytic CC coupling reactions [7–9].However,the relatively high cost of individual Pd NPs is disadvantageous to its widespread application [10,11].To improve the atomic utilization, Kim and coworkers studied the size effect of Pd NPs on visible-light-driven Suzuki coupling reaction and found that 5.5 nm Pd NPs supported on exfoliated 2H-WS2exhibited an extraordinary photocatalytic activity, where polyvinylpyrrolidone(PVP)was used to stabilize the highly active ultrafine Pd NPs[12].Guo and coworkers reported that PdCu alloy with a Pd/Cu molar ratio of 3:1 could not only enhance the photocatalytic activity toward Sonogashira CC coupling reaction but also reduce the use of metal Pd [13].
Metal phosphides (MPs) are composed of zero-valent metal and phosphorus; metal-rich phosphides have similar characteristics to zero-valent metals[14].We and others recently reported that Ni2P, CoP, Cu3P, etc.could act as excellent cocatalysts in a myriad of photocatalytic reactions for hydrogen evolution[15–17],reduction of nitroarenes[18]and CO2reduction[19].In view of the fact that Pd3P NPs have similar characteristics to Pd NPs, the following question naturally arises: Can Pd3P NPs be used as cocatalysts for photo-driven CC coupling reactions?Our present study shows that photocatalytic Suzuki coupling reactions are indeed highly efficient with Pd3P as a cocatalyst, CdS as a photosensitizer, 4-iodotoluene and phenylboronic acid as the coupling partners and ethanol as an electron donor, in which a yield of up to 98% can be achieved under optimized conditions at room temperature.To the best of our knowledge,it is the first time that Pd3P is used in catalyzing Suzuki coupling reactions.The high catalytic activity and excellent stability of this present system highlights its promising application in other cross-coupling reactions.

Fig.1.(a) TEM image and (b) HRTEM image of CdS.(c) TEM image and (d) HRTEM image of Pd3P/CdS (Inset (c): size distributions of Pd3P NPs).(e, f) TEM image and corresponding TEM-EDS elemental mapping images of hybrid Pd3P/CdS.
Pd3P was prepared by a one-step annealing reaction of Pd(Ac)2and NaH2PO2with a mass ratio of 1:5 under Ar atmosphere.As revealed by X-ray diffraction (XRD) pattern in Fig.S2 (Supporting information), the sample synthesized at 400has well-defined peaks of orthorhombic Pd3P (JCPDS No.089-3046) [20–22]without other impurities.Further, X-ray photoelectron spectroscopy (XPS) was performed to analyze the valence state and electronic properties of Pd3P NPs.As shown in Fig.S3c(Supporting information), two discernible peaks of P 2p region located at 129.8 eV and 130.6 eV can be assigned to P 2p3/2and 2p1/2of Pd3P,respectively.The peak at 134.6 eV can be attributed tobond,which originates from surface oxidation of Pd3P [23].Compared with the P0peaks at 130.1 and 131.4 eV [20,21,24], P element in Pd3P owns a lower binding energy,indicating its slightly negative charge in Pd3P NPs.In addition,the peaks of Pd 3d region located at 335.9 eV and 341.2 eV belong to Pd 3d5/2and Pd 3d3/2, respectively.Compared with binding energies(335.2 eV and 340.5 eV)of metal Pd [12,25], the higher binding energies of Pd species in Pd3P NPs indicate that Pd element is in the form of slightly positive valence state.Finally,the effect of calcination temperature on the structure and composition of as-prepared samples was explored and corresponding XRD patterns of samples synthesized at 300400and 550are shown in Fig.S2.We failed to obtain pure Pd3P above or below 400.For example,incomplete phosphidation exists in the sample synthesized at 300,in which two peaks at 39.4and 45.8ascribed to Pd NPs are observed[26].However,PdO and other impurities such as PdP2and PdO are detected in the sample synthesized at 550
CdS was fabricated via a hydrothermal route according to literature methods.As shown in Fig.S3a(Supporting information),XRD pattern reveals that CdS is present in hexagonal phase(JCPDS No.77-2306)[27].Furthermore,hybrid Pd3P/CdS was obtained by mechanical mixing of a certain amount of Pd3P and CdS.In the XRD pattern of hybrid Pd3P/CdS in Fig.S3a,the weak diffraction peaks of Pd3P at 39.9,40.3and 42.6prove that Pd3P NPs are successfully incorporated onto CdS.Finally, to clarify the optical property of CdS, Pd3P and Pd3P/CdS, ultraviolet-visible diffuse reflectance spectra were performed.As shown in Fig.S3b (Supporting information),Pd3P/CdS shows an enhanced absorption than naked CdS in the visible region.
The morphological information of CdS and hybrid Pd3P/CdS was characterized by transmission electron microscopy (TEM).TEM image of Cds in Fig.1a exhibits the flake-like shape.A highresolution TEM (HRTEM) image (Fig.1b) shows that CdS has the distinct lattice fringes, suggesting its excellent crystallinity.Obviously, as revealed by the TEM image of hybrid Pd3P/CdS in Fig.1c,ultrasmall Pd3P NPs are uniformly dispersed on the surface of CdS nanosheets and the average size of Pd3P NPs is about 5.20.5 nm based on statistical analysis of sizes of 120 Pd3P NPs.HRTEM image of hybrid Pd3P/CdS is also shown in Fig.1d.The high-resolved lattice fringes with distances of 0.316 nm and 0.356 nm can be well indexed to the(101)and(002)planes of CdS,respectively, and 0.226 nm corresponds to (112) plane of Pd3P, which well correspond to XRD results.Meanwhile, the HRTEM result confirms the intimate contact of CdS nanosheets and Pd3P NPs.To further prove the distribution of Pd3P NPs on CdS nanosheet, the TEM-EDS elemental mapping images of Pd3P/CdS are illustrated in Fig.1f and indicate the existence and homogenous distribution of Cd, S, Pd and P elements in Pd3P/CdS.
The performance of Pd3P/CdS photocatalyst for photocatalytic Suzuki coupling reaction was assessed under the irradiation of white LED light (425 W) at room temperature.Typically, the system was constructed with 4-iodotoluene and phenylboronic acid as the coupling partners,K2CO3as base,EtOH and H2O with a volume ratio of 3:2 as solvent.As shown in Table 1,4-iodotoluene can be converted into desirable 4-phenyltoluene with 98% yield(entry 1).Notably, the control experiments showed that the reaction could hardly occur when either components of Pd3P,CdS,light or Pd3P/CdS was absent(entries 2–5),which confirms that the Suzuki coupling reaction is photocatalyzed by hybrid Pd3P/CdS under visible light.In the absence of K2CO3, no desirable productwas detected(entry 6),because the Suzuki coupling reaction needs base to achieve deprotonation of aryl boric acid and by-product hydrogen halide [28].Therefore, the addition of an appropriate amount of K2CO3is necessary to drive this reaction.In addition,to eliminate the effect of photo-induced heat to Suzuki coupling reaction, the control experiment was carefully operated under heating condition without visible light irradiation (entry 7).Onlytrace desirable product was detected,which further suggests that the reaction is actually photo-driven.Moreover, the cycling experiment under the “standard condition” was performed carefully.Hybrid Pd3P/CdS after 4 cycles can maintain the activity almost equivalent to the first cycle (Fig.S4 in Supporting information), indicating its excellent stability.
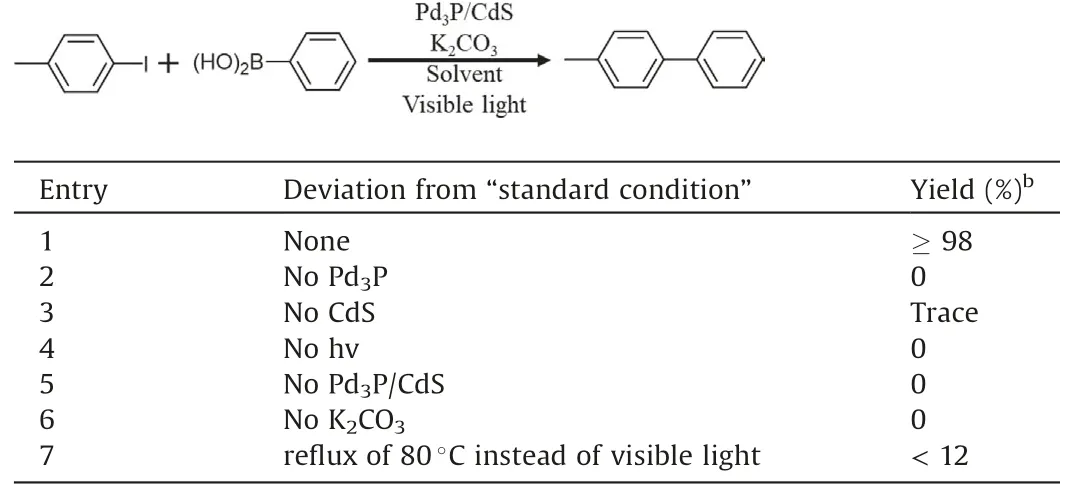
Table 1 Photocatalytic Suzuki coupling reaction over Pd3P/CdS.a
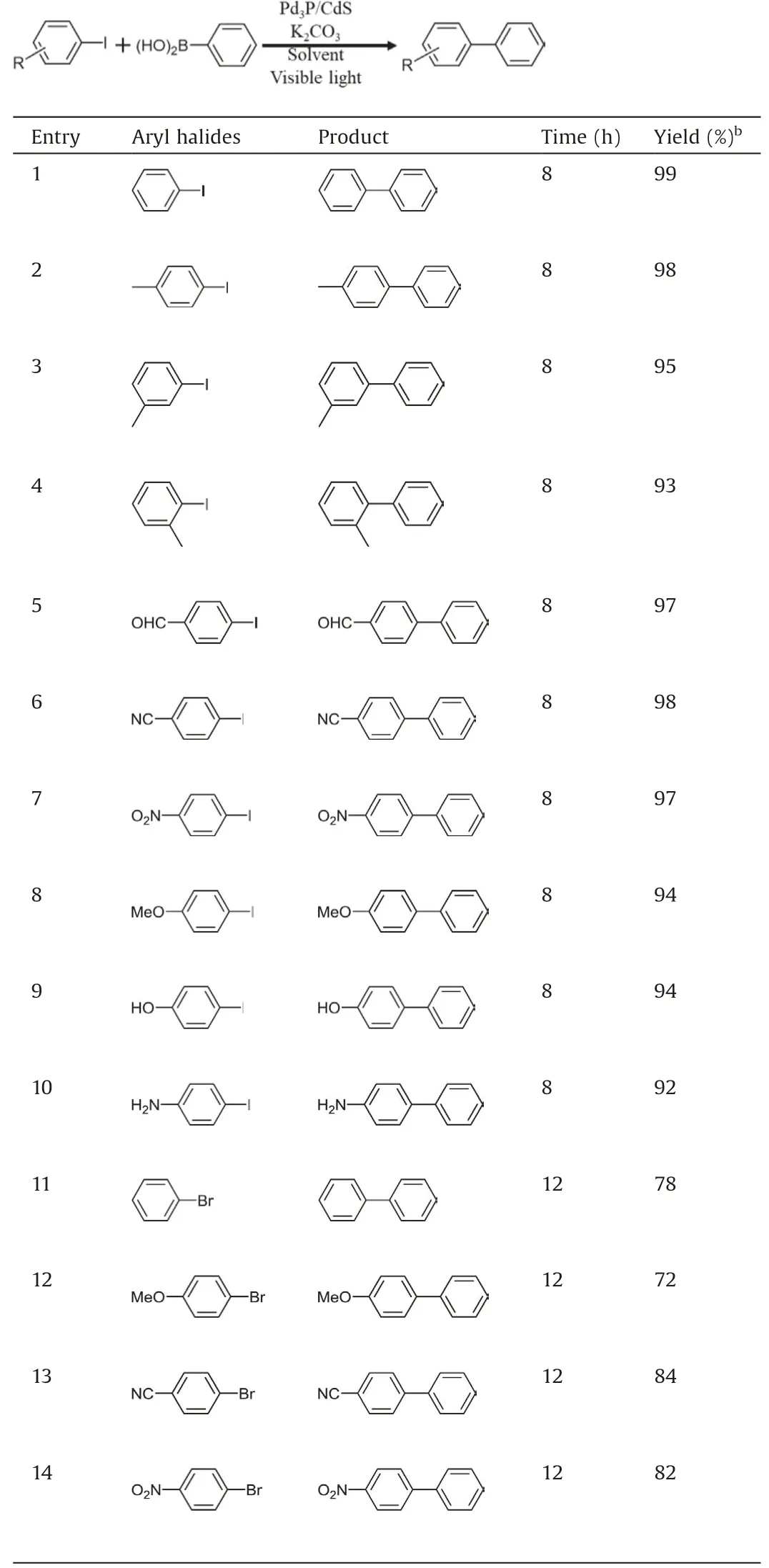
Table 2 Photocatalyzed Suzuki coupling reactions with various aryl halides by Pd3P/CdS.a
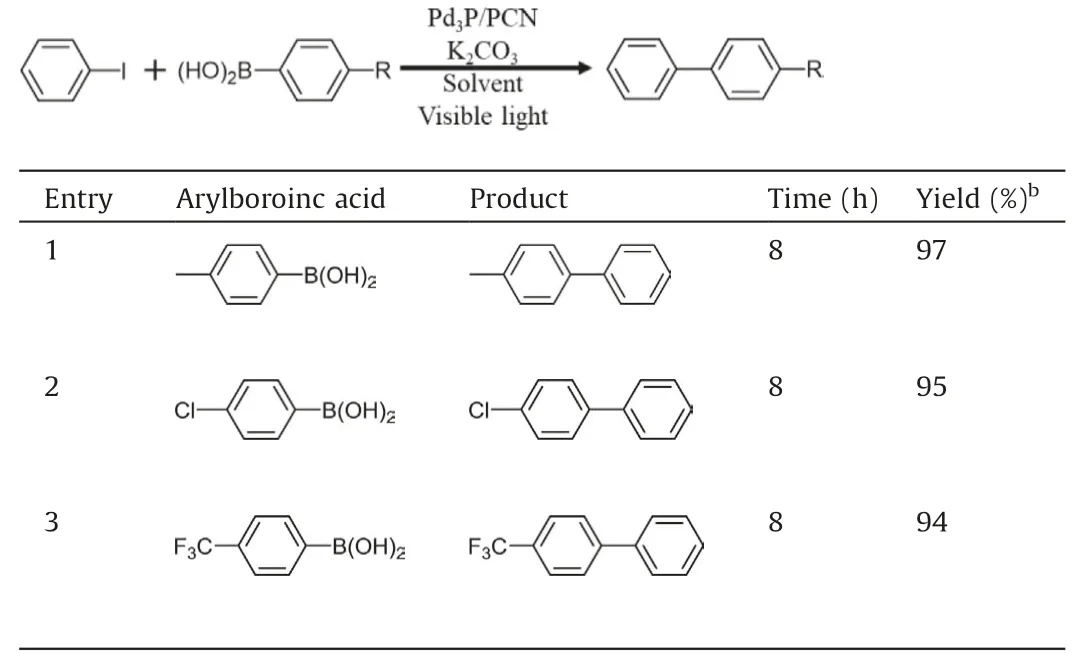
Table 3 Photocatalyzed Suzuki coupling reactions with several aryl-boronicacids by Pd3P/CdS.a
With the optimal conditions in hand,we sought to evaluate the generality of photocatalytic Suzuki coupling reaction by Pd3P/CdS.Various aryl halides and boronic acids are cross-coupled under the standard condition (Tables 2 and 3).1H NMR spectroscopy was used for product detection.Overall, all aryl iodides and aryl bromides showed high yields (Table 2, entries 1–14).It can be found that the position of substituents on the benzene ring has a slight effect on the product (entries 1–4), in which the yield of para-substituted substrate is slightly higher than that of ortho- and meta-substrates(entries 2–4).Surprisingly,whether the substituent is electron-withdrawing or electron-donating, the reaction yields are all over 90% (entries 5–10).Aryl bromides are also evaluated under the same condition,but have moderate yield even with long reaction time (entries 11–14).Aryl chlorides result in very poor yields because the strong C-Cl bond is more difficult to break [12].Additionally, three different aryl-boronic acid compounds and 4-iodotoluene can be successfully cross-coupled,and all of them have high yields (Table 3).
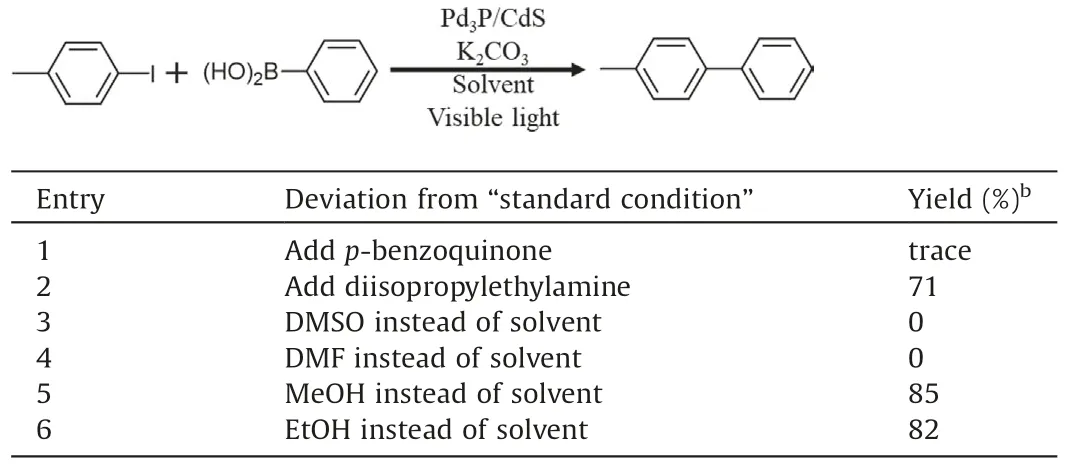
Table 4 Photocatalytic Suzuki coupling reaction over Pd3P/CdS.a
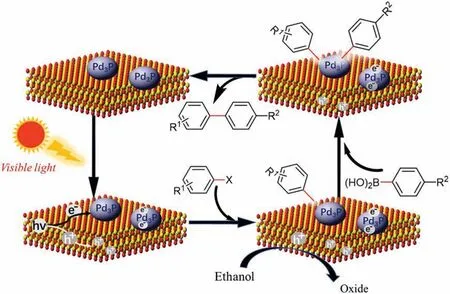
Fig.2.Proposed mechanism responsible for photocatalytic Suzuki coupling reactions over Pd3P/CdS.
To get an in-depth understanding of photocatalytic Suzuki coupling reaction mechanism over Pd3P/CdS, reference experiments were performed with p-benzoquinone and diisopropylamine under standard condition,respectively[8,12,29].The former is an electronic quenching agent, which competes with reactant for photoexcitation electrons from photosensitizers CdS, thus the photoexcitation electrons cannot successfully transfer to 4-iodotoluene to break C–I bonds.The latter is a good hole quenching agent, which can greatly quench the holes from photosensitizer CdS in the system and prevent the hole to transfer to phenylboronic acid.As shown in Table 4, the reaction with addition of p-benzoquinone can hardly proceed, whereas the yield of the corresponding product is still 71% upon addition of diisopropylamine.These results imply that photogenerated electrons play a crucial role in these photocatalyzed Suzuki coupling reactions.To further figure out the effect of solvents,the reactions in different solvents were carried out and the results were observed carefully.Withethanolandmethanolassolvents,ithasahighyieldof more than 80%.However, there is almost no products when using DMSO and DMF, which are excellent solvents for the thermalcatalytic Suzuki coupling reaction(Table 4,entries 5 and 6)[30,31].The experimental results indicate that the alcohol solvents(methanol,ethanol,etc.)play a key role in photocatalytic Suzuki coupling reaction.We speculate that alcohols may act as sacrificial agents oxidized by photogenerated holes to promote photocatalytic reaction,but holes did not transfer to phenylboronic acid[32].
Based on the above experimental results, we propose a plausible mechanism of photocatalytic Suzuki cross-coupling reaction by Pd3P/CdS (Fig.2).Under visible light irradiation,photogenerated electron-hole pairs can be produced and separated within CdS.Then photogenerated electrons transfer to Pd3P to break the C–X bonds,while photogenerated holes are consumed by alcohol solvent.At the same time,oxidation addition of aryhalide can occur on the surface of Pd3P, and then arylboronic acid undergoes transmetalation.Finally, the corresponding products are obtained by reductive elimination of intermediate species[28,33].
In summary, a highly efficient catalytic system with hybrid Pd3P/CdS as photocatalyst was established for Suzuki coupling reaction.The reaction product was obtained in a facile way.The introduction of Pd3P cocatalyst displayed significantly enhanced activity with bare CdS.Moreover,compared with naked metal Pd,Pd3P achieves a reduction in the usage of noble- metal Pd.Given the sustainability,high activity and economics,Pd3P is expected to be extended to other similarcoupling reactions as a highly effective catalyst.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the financial support from the Strategic Priority Research Program of the Chinese Academy of Sciences(No.XDB17000000) and the National Natural Science Foundation of China (Nos.21773275 and 21971250).Y.Chen acknowledges the financial support from K.C.Wong Education Foundation and the CAS-Croucher Funding Scheme for Joint Laboratories.
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.06.022.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- One-step straightf oward solid synthesis of high yield white fluorescent carbon dots for white light emitting diodes
- Free-standing nitrogen doped graphene/Co(OH)2composite films with superior catalytic activity for aprotic lithium-oxygen batteries
- Amorphous silicon from low-temperature reduction of silica in the molten salts and its lithium-storage performance
- Two 2D uranyl coordination complexes showing effective photocatalytic degradation of Rhodamine B and mechanism study
- Recent advances in electrochemical sensors for antibiotics and their applications
