Pancreatic cancer: genetics, disease progression,therapeutic resistance and treatment strategies
2021-05-11KarnikaSinghGauriShishodiaHariKoul
Karnika Singh, Gauri Shishodia, Hari K. Koul
1Department of Radiation Oncology, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210, USA.
2Department of Otolaryngology/Head & Neck Cancer Surgery, LSU Health Sciences Center, Shreveport, LA 71103, USA.
3Department of Biochemistry & Molecular Biology, Urology and Stanley S Scott Cancer Center School of Medicine LSU Health Sciences Center, New Orleans, LA 70112, USA.
#Authors contributed equally.
Abstract Pancreatic cancer is a deadly disease and the third-highest cause of cancer-related deaths in the United States. It has a very low five-year survival rate (< 5%) in the United States as well as in the world (about 9%). The current gemcitabine-based therapy soon becomes ineffective because treatment resistance and surgical resection also provides only selective benefit. Signature mutations in pancreatic cancer confer chemoresistance by deregulating the cell cycle and promoting anti-apoptotic mechanisms. The stroma-rich tumor microenvironment impairs drug delivery and promotes tumor-specific immune escape. All these factors render the current treatment incompetent and prompt an urgent need for new, improved therapy. In this review, we have discussed the genetics of pancreatic cancer and its role in tumor evolution and treatment resistance. We have also evaluated new treatment strategies for pancreatic cancer, like targeted therapy and immunotherapy.
Keywords: Pancreatic cancer, signature mutations, gemcitabine, desmoplasia, therapeutic resistance,immunotherapy
INTRODUCTION
Pancreatic cancer is a fatal disease that currently ranks third in the list of cancer-related deaths in the United States after lung cancer and colon cancer[1]. It has a low five-year survival rate of < 5% and a poor patient prognosis[2]. Different factors contribute towards the poor prognosis of pancreatic cancer such as lack of early stage-specific symptoms, dearth of definite screening tests, shortage of biomarkers, lack of effective therapy and acquired resistance[3,4]. The estimates show that by 2030 pancreatic cancer will be the second most common cause of cancer-related deaths in the United States, just after lung cancer[4].
Pancreatic cancer can originate in either the exocrine or endocrine portion of the organ. The exocrine pancreatic cancer includes pancreatic ductal adenocarcinoma (PDAC), which is also the most commonly detected histological type in the clinic (~90% patients)[5]and displays histology of the ductal cells of the pancreas, hence the name[6]. Other less common forms include acinar cell carcinoma, solid pseudopapillary tumors, serous cystadenoma, and pancreatoblastoma,etc.[7,8]. Pancreatic endocrine tumors originate in the endocrine glands of the pancreas. These tumors are rare and makeup < 5% of all pancreatic cancer cases[9].Older age (> 50 years) is the major risk factor associated with pancreatic cancer[4]. Other risk factors include smoking (15%-30%), obesity (16%), diabetes mellitus, family history (5%-10%)[4,10], and heavy alcohol consumption[11]. Some hereditary diseases like, Peutz-Jeghers syndrome, Lynch syndrome, and pancreatitis also raise the risk of pancreatic cancer[12]. The current treatment modalities provide a median survival of only 6 months[3]. Surgery combined with radiation and/or chemotherapy (preoperative or post-operative) is the only treatment option for patients diagnosed at advanced stages, which prolongs survival by 20%-25% in eligible patients[13,14]. Therefore for patients with metastatic disease, chemotherapy remains the only alternative[15]. This comprehensive review focuses on different gene mutations, therapeutic resistance, and the current treatment modalities for pancreatic cancer.
ROLE OF SIGNATURE MUTATIONS IN PANCREATIC CANCER
Pancreatic cancer involves around 63 genetic mutations, bulk of which are point mutations. These mutations contribute to the dysregulation of at least 12 signaling pathways that are frequently altered in pancreatic tumors[16]. This causes heterogeneity in pancreatic tumors leading to aggressiveness and lack of targeted therapy. Pathological and molecular analysis of pancreatic tumors has identified the following signature mutations; mutations inKRAS,TP53,CDKN2A, andSMAD4genes[4,7]. These genetic lesions fuel uncontrolled growth and survival of pancreatic cancer cells by deregulating the cell cycle during different phases of pancreatic tumor development. These mutations also contribute to therapeutic resistance[17]. These signature mutations occur temporally over the course of pancreatic tumor development and steer the progress from PanIN to adenocarcinoma[2]. Table 1 shows the signature mutations involved in different stages of pancreatic cancer.
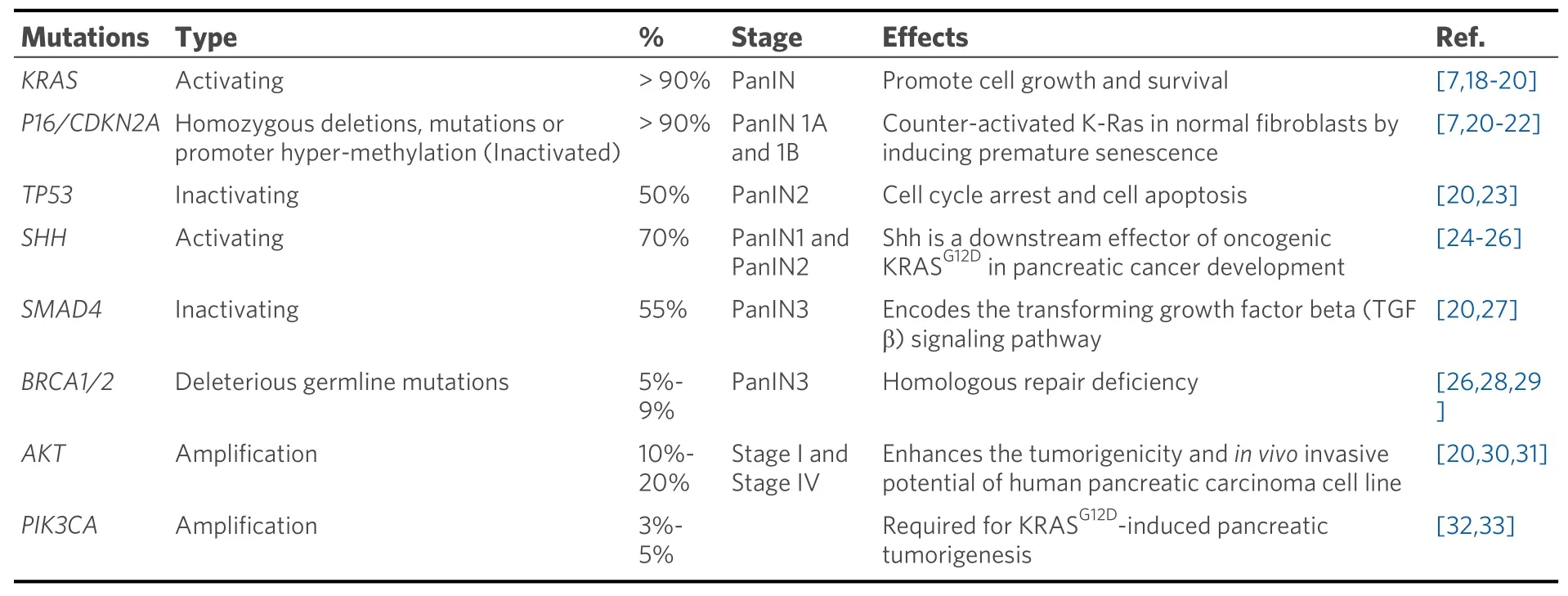
Table 1. Most commonly occurring mutations in different stages of pancreatic cancer
The driver mutation happens inKRASof the normal pancreatic cells. As the disease progresses, mutations inKRASaccumulate and is thus observed in > 90% of pancreatic cancer cases[7].KRASis also frequently mutated in other human cancers (~85%) like colorectal (52%) and lung (31%) adenocarcinomas[34].
Given the central role of KRAS in the initiation, growth, and progression of pancreatic cancer, it is imperative to target this oncogene. The use of KRAS as a therapeutic target for pancreatic cancer has been studied extensively in recent years. Several strategies have been developed to target mutant KRAS protein either genetically or using small molecules afterin silicoandin vitroscreenings and assays[35]. In pancreatic cancer KRAS is known to upregulate Raf-MEK-ERK and PI3K/Akt signaling pathways[18]which promote cell growth and survival of pancreatic cancer cells. The constitutive Ras signaling in pancreatic cancer cells causes aberrant activation of ERK, which facilitates the process of cell proliferation and tumor initiation[36].
In conclusion, constitutive activation of Raf-MEK-ERK signaling by mutant KRAS leads to increased levels of G1 cyclins that confer a survival advantage to these cells. This drives pancreatic carcinogenesis by inducing PanIN formation.
As pancreatic cancer approaches the low grade PanIN stage (PanIN 1A and 1B), mutations are acquired inCDKN2Agene in the form of homozygous deletions, mutations, or promoter hyper-methylation[7,37].CDKN2Acodes for p16 (INK4A) tumor suppressor that limits G1 to S transition by inhibiting the formation of Cyclin D1-CDK4/6 complex. Loss of p16 function is observed in 80%-90% of pancreatic cancer cases[23]and also associates with poor patient prognosis[38]. P16 has been shown to counteract activated KRAS in normal fibroblasts by inducing premature senescence[21,22]. Therefore, it is speculated that pancreatic cancer cells lose p16 activity in order to gain the survival advantage offered by mutantKRAS.Additionally, loss of p16 has been implicated in chemoresistance[39].
During the stage of medium grade PanIN (PanIN2), inactivating mutations inTP53are acquired. These are usually missense mutations that occur in the DNA binding domain of p53 and are encountered in about 50% of pancreatic cancer patients[23]. In pancreatic cancer, inactivation of p53 results in excessive genomic instability, which is often observed in this disease. Therefore, in the event of p53 inactivation, the pancreatic cancer cells accumulate any genetic abnormalities inflicted upon them. Loss of function of p53 also leads to chemoresistance to gemcitabine by preventing DNA damage-induced apoptosis (discussed in later sections). The majority of mutations found in PDAC forTP53gene are missense mutations leading to stable and highly expressed mutant p53 proteins[40]. A recent study showed that mutant p53 interacts with CREB1 upon KRAS activation, which hyperactivates several pro-metastatic transcriptional networks that drive PDAC metastasis[41]. Another study showed that upregulation of platelet-derived growth factor (PDGF)receptor beta mediates mutant p53 to drive the invasive phenotype of PDAC[42].
At the stage of high-grade PanIN (PanIN3),SMAD4also gets altered. It is seen mutated or deleted in around 55% of pancreatic cancers and is also correlated with poor patient prognosis[27]. SMAD4 is a transcriptional regulator that is a key component in the transforming growth factor β (TGFβ) pathway.Since TGFβ signaling blocks cell growth and promotes differentiation, it is often mutated in cancers[43,44].One of the functions of TGFβ is to cause G1 phase cell cycle arrest by inducing the expression of p27 (CKI)and prevent its degradation by downregulating Skp2 protein levels[45]. Therefore, it can be understood that the inactivation of Smad4 in pancreatic cancer cells removes p27 protein from the equation contributing to the disabling of G1/S checkpoint.
In conclusion, all the above-described mutations deregulate the cell cycle in pancreatic cancer cells, mainly at G1 to S transition [Figure 1].KRASmutation upregulates cyclin D1 (G1 cyclin), whereas the mutations inCDKN2A,TP53, andSMAD4inactivate the tumor suppressors, p16, p21, and p27 respectively. All these events render the G1/S checkpoint dysfunctional and set the stage for malignant transformation.
PANCREATIC CANCER AND THERAPEUTIC RESISTANCE
Adjuvant chemotherapy after surgical resection remains the primary treatment for early pancreatic cancer patients. For the past two decades, gemcitabine (gemzar®) has been the mainstay of pancreatic cancer treatment. Gemcitabine was approved by FDA in 1996 on the basis that it increased survival in five-fold more patients over 5-FU (5-Fluorouracil), the previously used drug for pancreatic cancer chemotherapy[46].Here we will discuss the metabolic actions of gemcitabine and the mechanisms involved in the therapeutic resistance of gemcitabine.
Metabolism of gemcitabine
Gemcitabine is a deoxycytidine analog that functions by interfering with the DNA synthesis pathway and eventually inducing apoptosis. Gemcitabine is a prodrug that is taken up into the cells mainly by two human nucleoside transporters, equilibrative nucleoside transporters (ENT), and concentrative nucleoside transporters (CNT)[47]. Inside the cells, it gets converted into dFdCDP and dFdCTP by a series of reactions initiated by deoxycytidine kinase (dCK) enzyme, which obstructs DNA replication by inhibiting DNA polymerase[46,48], culminating in DNA damage-induced apoptosis[46]. Metabolism of gemcitabine and the components affected by resistance mechanisms are shown in Figure 2 and summarized in Table 2.

Table 2. Mechanisms of gemcitabine resistance
Mechanisms of gemcitabine resistance
It has been observed that pancreatic cancer patients acquire resistance to gemcitabine therapy soon after the starting of treatment resulting in poor patient response[49](highlighted in Figure 2). The mechanisms of gemcitabine resistance can be classified into two categories: (1) mechanisms that impede gemcitabine metabolism and (2) mechanisms that intercept gemcitabine-induced apoptosis[49]. The first category involves mechanisms like downregulation of CNT1 and ENT1 transporters in pancreatic cancer cells to decrease the uptake of gemcitabine[50,51]. Overexpression of cytidine deaminase (CDA) is also observed in pancreatic cancer cells along with MRP-1 (multidrug resistance-associated protein) transporter responsible for causing an efflux of clinically relevant drugs[52]. Another mechanism is the downregulation of dCK enzyme, which prevents the breakdown of gemcitabine into its active metabolites. Studies have shown that levels of dCK correlate with the overall survival of pancreatic cancer patients[53]. Increased RNR expression is associated with sustained dCTP pools and inhibition of gemcitabine-incorporation[54,55]. The second category of gemcitabine resistance mechanisms involves upregulation of survival pathways like PI3K/Akt and unfolded protein response (UPR) interfering with gemcitabine induced apoptosis[56,57]. PI3K upregulation is associated with poor patient prognosis[58,59]and is known to prevent gemcitabine induced apoptosis[60]. The inhibition of PI3K/Akt pathway has shown promise in sensitizing pancreatic cancer cells towards apoptosis induced by gemcitabine as well as other chemotherapeutics bothin vitroandin vivo[61].Other mechanism includes inactivation of p53 tumor suppressor protein by mutations resulting in inhibition of DNA damage-induced apoptosis (discussed previously).
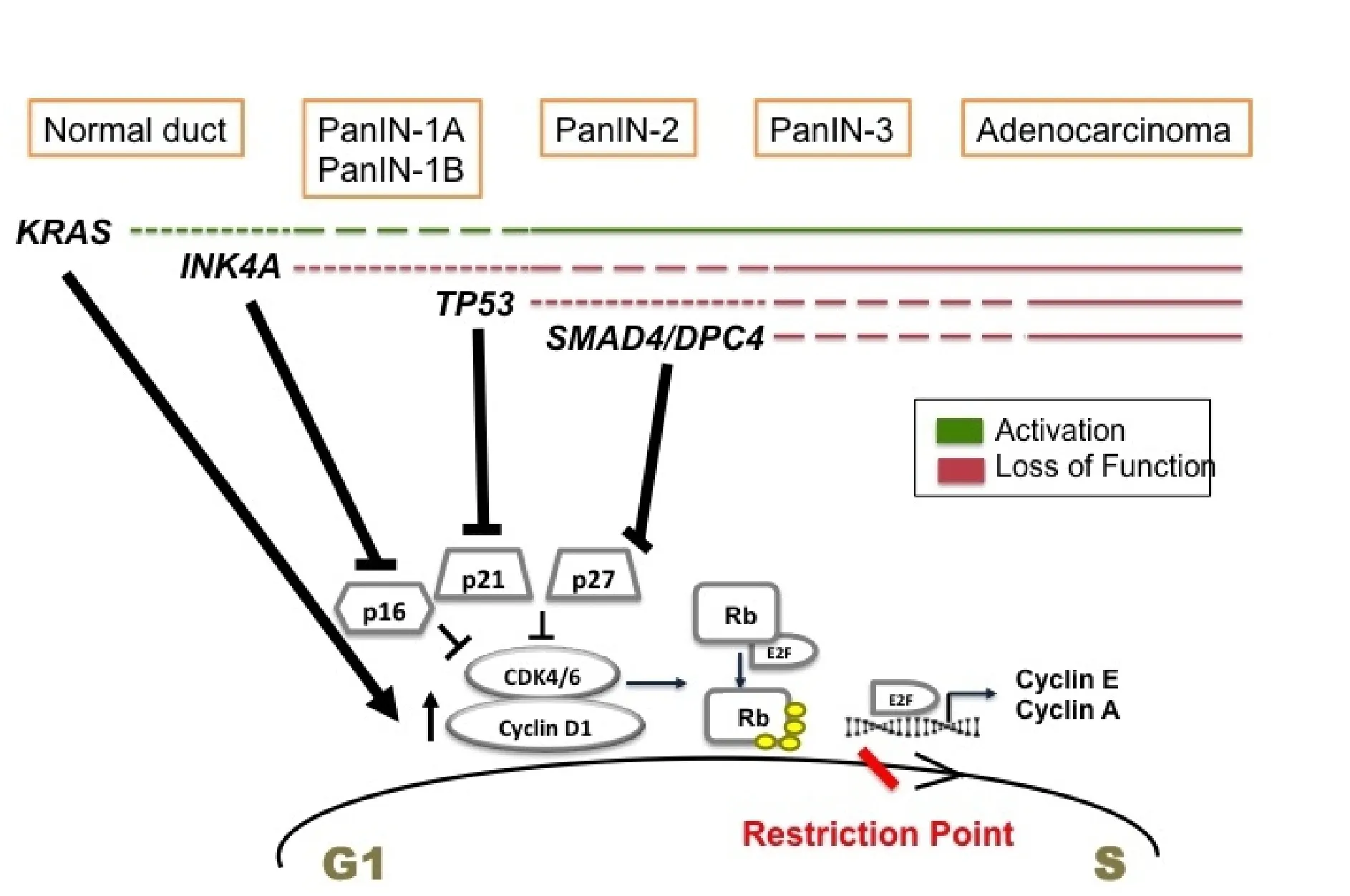
Figure 1. Temporal occurrence of signature mutations in pancreatic cancer and its effect on G1 to S transition. Driver mutations occur in KRAS of normal pancreatic cells initiating tumor formation. These mutations promote cell proliferation by upregulating cyclin D1. During the stages of low grade PanIN, INK4A mutations are acquired. It inactivates p16 tumor suppressor. As the tumor progresses to high grade PanIN, TP53 and SMAD4 are mutated mediating the inactivation of p21 and p27 CKIs. All these events deregulate G1 to S transition promoting uncontrolled proliferation and pancreatic cancer proceeds to full blown adenocarcinoma.

Figure 2. The gemcitabine metabolism and its mechanism of resistance. Gemcitabine is taken into cells by nucleoside transporters and converted by a series of reactions into dFCTP. It is incorporated into replicating DNA resulting in chain termination. The incorporated dFdCTP leads to dislodgement of DNA polymerase one nucleotide downstream of the dFdCTP. This extra nucleotide masks the break site and makes it imperceptible to the DNA repair enzymes leading to DNA damage[49]; whereas dFdCDP inhibits ribonucleotide reductase (RNR) enzyme leading to reduced pools of dCTP thus creating a positive feedback loop ensuring gemcitabine incorporation.The steps affected by the resistance mechanisms are starred (⋆) in blue.
Role of desmoplasia in pancreatic cancer chemoresistance
Desmoplasia or inflammatory fibrotic reaction is considered as the histological hallmark of pancreatic cancer, which makes up to 90 percent of total tumor volume[62]. The pancreatic stroma is composed of both cellular and acellular components; the cellular components are fibroblasts, myofibroblasts, pancreaticstellate cells (PSCs), and immune cells, and acellular components are blood vessels, extracellular matrix(ECM), cytokines, and growth factors[63]. The desmoplastic stroma is primarily composed of cancerassociated fibroblasts (CAFs), immune cells, small blood vessels, and ECM[64]. In normal pancreatic tissue,the PSCs are found in a quiescent state[64]. Upon tissue injury, the PSCs are activated by pancreatic tumor cells and acquire a myofibroblast-like appearance[63]. Factors like aberrant TGFβ signaling due toSMAD4deletion combined withKRASmutation, PDGF, tumor necrosis factor α, and several interleukins (ΙL-1, 6 and 10) can initiate the desmoplastic reaction. The pancreatic tumor cells secrete these factors, which bind to their respective receptors present on the PSC and activate them by their specific signaling resulting in increased ECM deposition. The activated PSCs create an autocrine loop and promote tumor growth and migration[62,64]. CAFs also overexpress SMO and have a hyperactive Hh pathway that further contributes to their maintenance[65].
The activated PSCs or CAFs also secrete ECM components like collagens, laminins, fibronectin, hyaluronic acid (HA),etc. This results in the development of dense stroma around the tumor that acts as a structural barrier to drug delivery[66,67]. In addition, stromal fibroblasts lead to increased interstitial fluid pressure (IFP)by acquiring contractile properties and increasing contraction of the interstitial matrix, thus posing a physical barrier to drug delivery in pancreatic tumors[67,68]. It can be understood that desmoplasia is another contributory factor to drug resistance in pancreatic cancer. Several stromal components like CAFs, HA,collagen (type 1),etc., also exclusively contribute to gemcitabine resistance by various mechanisms promoting apoptosis resistance[69]. The pancreatic stroma also induces tumor microenvironment-associated stresses which upregulate UPR and promote survival in pancreatic cancer cells. Therefore, the pancreatic stroma is another attractive target for therapy.
Till date, various studies have targeted different components of the pancreatic stroma. The anti-fibrotic drug pirfenidone, approved for the treatment of pulmonary fibrosis, inhibits fibroblasts and production of TGFβ, PDGF, and collagen type 1 in PDAC mouse model[70]. Another study inKPCmouse model demonstrated that targeting HA lowers IFP in the tumors and inhibits tumor growth due to improved drug delivery[71,72]. A phase 1b clinical trial was done to test the safety and efficacy of Pegylated recombinant human hyaluronidase (PEGPH20) and gemcitabine combination in stage IV PDAC patients. PEGPH20 depleted interstitial HA, which caused a decrease in IFP and improvement in drug delivery[73]. In 2005, the FDA approved the use of nanoparticle- albumin-bound paclitaxel (nab®-paclitaxel) (trade name:ABRAXANE) for the treatment of the pancreatic cancer. It was later shown that nab-paclitaxel causes disruption of pancreatic stroma by softening the tumor by decreasing its CAF content[74]. The MPAC trial in 2013 by Von Hoffet al.[75]combined nab-paclitaxel with gemcitabine for the treatment of metastatic pancreatic cancer patients showing improved overall survival over gemcitabine alone. In the same year,nab®-paclitaxel plus gemcitabine combination received FDA approval for the treatment of metastatic pancreatic cancer. Various studies targeting different components of pancreatic stroma are summarized in Table 3.
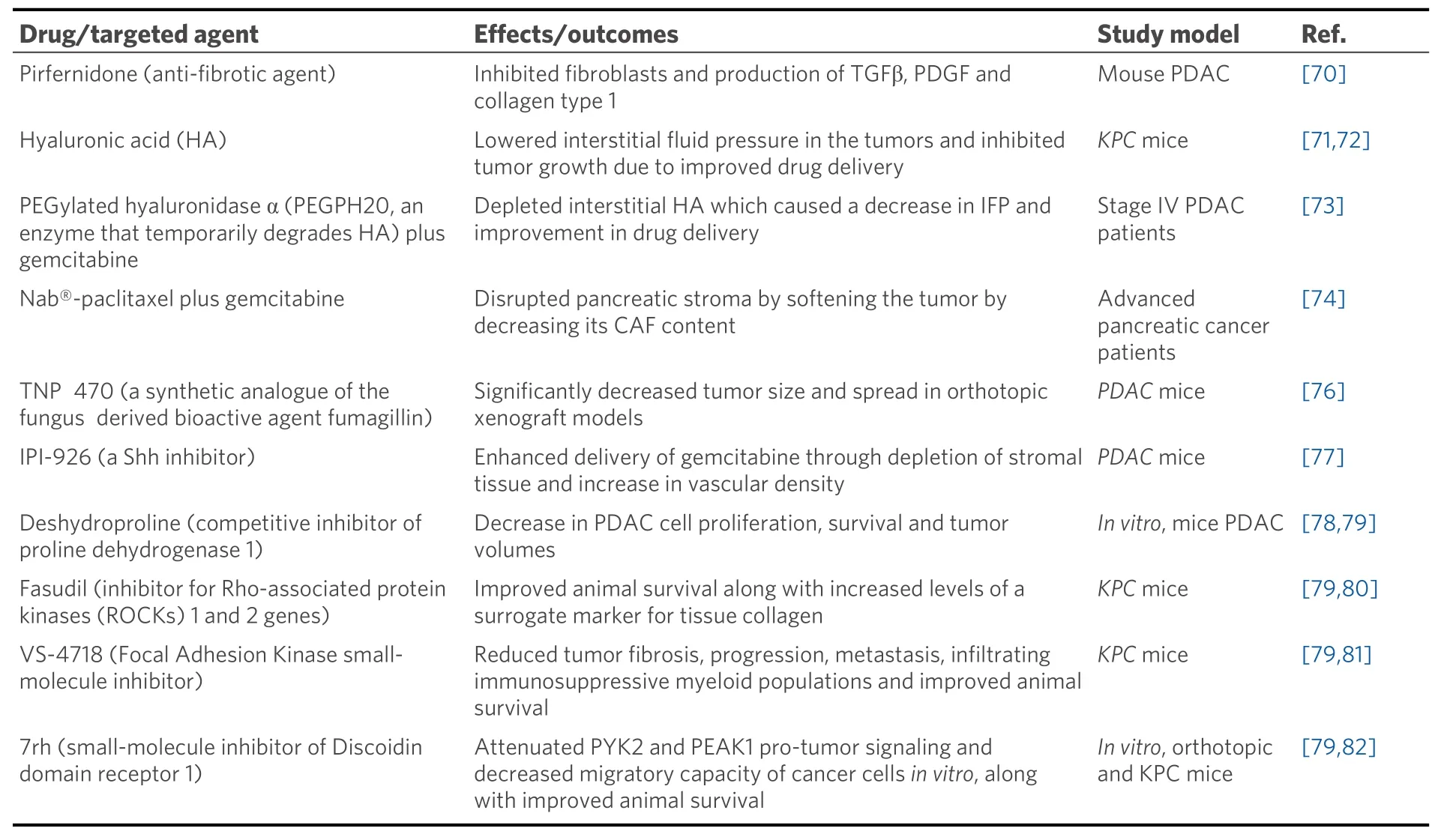
Table 3. Studies showing targeting different components of pancreatic stroma
BARRIERS TO CURRENT CHEMOTHERAPY IN PANCREATIC CANCER
Pancreatic cancer is a deadly disease with disappointing statistics. The current treatment options are limited and largely ineffective. The reasons for therapy failure in pancreatic cancer are multifold and need to be considered while designing new therapies. This section highlights some of the inherent features of pancreatic tumors that present a barrier to chemotherapy in general. The signature mutations encountered in pancreatic cancer are not only responsible for its progression but also chemoresistance. As highlighted in previous sections, driver mutations inKRASresult in aberrant activation of downstream signaling pathways like Raf-MEK-ERK MAPK and PI3K/Akt signaling[18]. These survival pathways promote resistance to chemotherapy by causing apoptotic resistance through the upregulation of anti-apoptotic proteins in the cell in response to chemotherapeutic agents. For example, the ERK mediates induction of anti-apoptotic proteins like Bcl-2, Mcl-1, and Bcl-X(L), which prevent chemotherapy-induced apoptosis in pancreatic cancer cells[83,84]. Inactivating mutations inTP53tumor suppressor lowers the ability of the cell to sense the DNA damage induced by gemcitabine incorporation, therefore, particularly inhibiting gemcitabine-induced apoptosis in pancreatic cancer cells[23]. Additionally, p21 (CKI) is not induced by a non-functional p53, and cell cycle is not arrested for the DNA repair[85]. Similarly, mutations inINK4AandSMAD4cause the inactivation of p16 and p27 (CKIs) tumor suppressors respectively, and lead to an uninterrupted cell cycle[39,44]. Due to the highly proliferative and secretory nature of pancreatic cancer cells, UPR pathway is also expected to play a protective role in these cells by maintaining protein folding quality control[86]. The desmoplastic microenvironment of pancreatic tumors inflicts stresses like hypoxia and nutrient starvation which upregulate UPR in these cells to prevent stress-induced apoptosis[87]. Furthermore, the presence of dense stroma around the tumor poses a physical barrier to drug delivery[66]. The individual stromal components also induce gemcitabine resistance by inhibiting the apoptosis of pancreatic cancer cells[69].Several intrinsic mechanisms also exist in pancreatic cancer cells, which interfere with the metabolism of gemcitabine and prevent its incorporation into the DNA. These mechanisms include decreased expression of NTs to prevent gemcitabine uptake into the cells. For example, inactivation of dCK enzyme to prevent gemcitabine breakdown, upregulation CDA to promote metabolic deactivation of gemcitabine and its consequent efflux through overexpressed ABC pumps, and upregulation of RNR to counteract the effect of gemcitabine by maintaining the dCTP pools in the cell[46,50-55]. In all, it is evident that pancreatic tumors have evolved diverse mechanisms to protect themselves from gemcitabine treatment and chemotherapy in general. Therefore, to efficiently treat pancreatic cancer, new improved therapies need to be designed that can cross the presented hurdles.
DIFFERENT STRATEGIES TO COMBAT PANCREATIC CANCER
Immunotherapy
Immunotherapy is the latest addition to the treatment design for solid tumors. It involves targeting immune checkpoint molecules CTLA-4 (Cytotoxic T-lymphocyte associated antigen 4), PD-1 (Programmed cell death-1), PD-L1 (Programmed cell death ligand-1) using monoclonal antibodies. CTLA-4 inhibitors have been tested in melanoma, renal cell cancer, NSCLC, SCLC, ovarian cancer,etc.[88]. PD-L1 expression has been detected by IHC in a variety of solid tumors, including pancreatic cancer[89]. The desmoplastic microenvironment of pancreatic tumors protects them from host innate immunity in many ways.Pancreatic tumor stroma has been shown to have an activated CD40 pathway which is involved in establishing tumor-specific T cell immunity[90]. Reports have also shown that the prevalence of CD4+ Th2 cells in the pancreatic tumor stroma is associated with poor patient prognosis[91]. However, the presence of CD4+ and CD8+ TIL together is an indicator of a good prognosis in surgically resected PDAC patients[92].
Several immune checkpoint inhibitors have been tested in pancreatic cancer, alone and combined with radiation and chemotherapy. Ipilimumab, a CTLA-4 inhibitor, alone and in combination with gemcitabine or Nivolumab (PD-1 inhibitor) and has been tested in unresectable/locally advanced/ metastatic stage III or IV pancreatic cancer[93]. Ipilimumab alone did not show any improvement in patient survival[94]. However,Ipilimumab and gemcitabine combination had an OS of 8.5 months[95]. Other CTLA-4 inhibitors like Tremelimumab and PD-1 inhibitors like Pembrolizumab and Atezolizumab have also been tested alone and in combination in advanced pancreatic cancer. These studies have shown varying OS in different phases of clinical trials[93].
Anti-cancer vaccines are the other therapeutic modalities that have been tried in pancreatic cancer. Several kinds of vaccines exist like whole-cell vaccines, peptide-based vaccines, dendritic cell vaccines, DNA vaccines (plasmid vaccines, virus-based vaccines, bacterial vectors, and yeast-based recombination vaccines), and mRNA vaccines. Various vaccines are being tested in clinical trials at Pre-clinical/Phase I/Phase II stages for metastatic pancreatic cancer. Some of these vaccines include OCV-C01, GVAX, synthetic Ras peptides, Mucin-1 peptides,etc.[96]. OCV-C01, in combination with gemcitabine, has shown better DFS of 15.8 months over gemcitabine alone (12 months)[97]. GVAX is a whole tumor vaccine that is engineered to express GM-CSF (granulocyte macrophage- colony-stimulating factor). This causes induction of APC antigen uptake and T cell priming. In at least 5 clinical trials, GVAX (in combination) showed the antitumor response in tumors and increased OS in patients with low or minimum toxicity[98]. A combination of CTLA-4 inhibitor, Ipilimumab, and cancer vaccine, GVAX has also been tested in previously treated advanced pancreatic cancer. The patients in which OS > 4.3 months showed an increase in peak mesothelinspecific T-cells and T-cell repertoire[99].
The above-discussed studies suggest that although immunotherapy is still in its preliminary stages, it holds a strong potential to be developed as a therapeutic for pancreatic cancer treatment.
Poly (ADP-ribose) polymerase inhibitors
Pancreatic cancer is the third most common cancer related to early-onset mutation in the breast cancer(BRCA) gene. Approximately 4%-7% of patients with PDAC have germline BRCA1/2 mutations(gBRCA1/2)[100,101]. These mutations have potential therapeutic implications as they confer increased sensitivity to platinum-based chemotherapy and poly (ADP-ribose) polymerase inhibitors (PARPi)[102].Cancer cells with mutations that prevent homologous recombination repair, such as BRCA1/2 loss-offunction mutations, are often synthetically lethal with PARPi due to significantly lower DNA damage response[103]. PARPi causes unrepaired accumulation of single-strand DNA breaks, which eventually culminate into double-strand breaks, causing the death of the BRCA1/2-mutant cancer cells[104]. PARPi have become the most commonly used drug to target BRCA mutations. The use of PARPi in PDAC is an activearea of investigation which is developing from being used as monotherapies to combination therapy with other classes of therapeutic agents. Olaparib, a small molecule PARPi, has proven efficacy against germline BRCA-mutated metastatic pancreatic cancer patients[105]; and is the only accepted PARPi for clinical application in pancreatic cancer[106]. Several trials are in the clinic using olaparib as monotherapy in advanced disease of PDAC[104]. In addition to PARPi alone, clinical trials are currently underway to evaluate PARPi combinations with other classes of therapies causing DNA damage in pancreatic cancer patients[107].
Gemcitabine is widely used as a radiosensitizer for PDAC treatment and other cancers[108,109]. It is known to induce tumor cells S-phase arrest and thus sensitize cells to DNA damage[108]. PARPi could sensitize cells to exogenous DNA damage inducer treatment, such as irradiation in pancreatic cancer cell’s[110]or gemcitabine in non-small-cell lung cancer[111]. Combination treatment of PARPi- olaparib with gemcitabine and proton therapy significantly enhanced tumor response and progression-free survival in pancreatic cancer mice model[112]. Taken together, these studies provide crucial evidence that a combination of PARPi with gemcitabine for radiosensitization could be used as an improved therapeutic regimen for overcoming the therapeutic resistance in pancreatic cancer.
Cancer-associated fibroblasts
CAFs have emerged as key players in mediating drug resistance due to their presence within the PDAC tumor, along with their secreted factors. CAFs and their generated ECM can function as a physical barrier and thus prevent efficient drug delivery[113]. Targeting CAFs is becoming a promising therapeutic strategy owing to their involvement in the progression of tumorigenesis and drug resistance[113], their genetic stability and relative abundance among stromal cells[114]. Currently, numerous clinical trials based on CAF-directed anticancer therapies with a goal of either normalizing CAFs or reduce their secretion are going on[115].
CAFs have been shown to exert immunosuppressive effects through different mechanisms[116-118].Francesconeet al.[119], investigated the role of CAFs in PDAC tumorigenesis. They showed that Netrin G1 expression in CAFs creates an immunosuppressive microenvironment that inactivates natural killer (NK)cells and protects PDAC cells from NK cell-mediated death[119]. Their data suggest an important role of CAFs in the microenvironment (i.e., ECM) in PDAC cell survival. Fibroblast Activation Protein (FAP) is frequently (90%) expressed, predominantly in CAFs, with pancreatic cancer patients[120]. High expression of FAP is associated with shorter overall survival and disease-free survival in pancreatic cancer patients.Several clinical trials targeting FAP in metastatic pancreatic cancer and other cancers are underway[121]. A recent study highlighted the importance of stromal macropinocytosis to support CAF cell fitness and providing amino acids in sustaining PDAC cell survival[122]. Macropinocytosis is a form of endocytosis that mediates non-selective fluid-phase uptake and represents a survival strategy in PDAC patients. Targeting macropinocytosis is another potential area to explore in pancreatic cancer since the pancreatic tumors exhibit high levels of macropinocytosis[123], and selective disruption of macropinocytosis in CAFs helps suppress PDAC tumor growth[122]. All these studies reinforce the importance of considering the stroma as a promising therapeutic option in PDAC.
CONCLUSION
Pancreatic cancer is a complex disease that has developed many shields to combat therapy. Overcoming gemcitabine resistance has been the focus of many conventional therapies, including adjuvant therapy,neoadjuvant therapy, targeted therapy as well as immunotherapy[124]. Within the last decade, several clinical trials have shown benefit in pancreatic cancer patients after using gemcitabine with other agents. For example, the patients treated with gemcitabine/nab-paclitaxel had an overall survival of 5.5 months compared to 3.7 months for the gemcitabine alone group[75], and patients treated with 5-fluorouracil/leucovorin with irinotecan and oxaliplatin (FOLFIRINOX) survived for 6.4 months compared to 3.3 months survival of gemcitabine alone group[125]. These studies have shown improved survival outcomes in patients, but the improvement is still not huge. Despite the improved prognosis of advanced pancreatic cancer using the above treatments, the development of chemoresistance severely limits the effectiveness of the chemotherapy[56]. Other factors like unavailability of efficient screening method, lack of specific symptoms or biomarkers, and aggressive nature of this disease also contribute to the difficulty treating pancreatic cancer. Most of the cases are diagnosed only after metastasis, which not just limits surgical resection, but also lowers the chances of survival. Therefore, in order to efficiently treat this disease,a bi-directional strategy needs to be followed. One direction should aim at early detection of the tumor, and the other direction should focus on designing efficient therapy with all the resistance mechanisms in mind.Thus, it is important to investigate new methods and targets that can act as a catalyst in pancreatic cancer treatment.
DECLARATIONS
Authors’ contributions
Wrote the manuscript: Singh K, Shishodia G
Helped draft the manuscript: Singh K, Shishodia G, Koul HK
Made substantial contributions to the data analysis and interpretation: Singh K, Shishodia G, Koul HK
All authors have revised and approved manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported in part by funds from Carroll W. Feist Endowed Chair in Cancer (Koul HK) and LSUHSC-graduate stipend to Singh K. Koul HK is supported in part by the NIH/NCI RO1 R01CA242839.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
©The Author(s) 2021.
杂志排行
Journal of Cancer Metastasis and Treatment的其它文章
- The unclear role of VEGF in POEMS syndrome:therapeutic implications of neoangiogenesis in a rare plasma cell disorder
- Regulation and function of angiogenic factors in chronic lymphocytic leukemia
- The role of immune checkpoint inhibitors in triplenegative breast cancer: recent developments and future perspectives
- Cancer field surgery for locoregional tumor control of cervical carcinoma
- Uterine cervical carcinoma treated with chemoradiotherapy: impact of three-month MRI follow-up on clinical management and outcome
- AUTHOR INSTRUCTIONS
