Hydroliquefaction kinetics of coal-derived preasphaltenes catalyzed by FeS and S
2021-04-28KANGShigangGAOBinSHUIHengfuWANGZhicaiLEIZhipingRENShibiaoYANJingchongLIZhankuPANChunxiuYANHongleiWANGXiaoling
KANG Shi-gang ,GAO Bin ,SHUI Heng-fu ,WANG Zhi-cai ,LEI Zhi-ping ,REN Shi-biao ,YAN Jing-chong ,LI Zhan-ku ,PAN Chun-xiu ,YAN Hong-lei ,WANG Xiao-ling
(School of Chemistry and Chemical Engineering, Anhui Province Key Laboratory of Coal Clean Conversion and High Valued Ultilization, Anhui University of Technology, Ma'anshan 243002, China)
Abstract: Hydroliquefaction behavior of preasphaltenes, derived from direct coal liquefaction, was carried out in a 30 mL autoclave with FeS + S catalyst and tetralin at initial hydrogen of 5.0 MPa, residence time of 0-60 min and reaction temperature of 380-440 °C in order to optimize the conditions of direct coal liquefaction and improve oil yield.The products distribution and kinetic parameters of preasphaltenes catalytic hydroliquefaction were investigated.A new kinetic model was established to simulate the preasphaltenes hydroliquefaction catalyzed by FeS + S catalyst using lump kinetic model.It was found that preasphaltenes were hydroliquefaction into asphaltenes and char directly, and then asphaltenes were hydrocracked into oil + gas products.Regressive reactions of preasphaltenes to char and asphaltenes to preasphaltenes occurred at higher temperatures.Higher temperature and longer time were favorable for increasing the conversion of preasphaltenes and the oil +gas yield.The hydroliquefaction of preasphaltenes under 440 °C and 60 min reached 79.45% with 34.7% of oil + gas yield.The hydroliquefaction conversions calculated from the model agreed well with the experimental data, and the activation energies ranged within 50-245 kJ/mol.
Key words: preasphaltenes;hydroliquefaction;kinetic;activation energies
Direct coal liquefaction is an approach for the efficient and clean utilization of coal, which produces liquid fuels, especially for China with abundant coal resources and insufficient petroleum resources.Heavy intermediates preasphaltenes (PA) or asphaltenes (AS)of direct coal liquefaction play an important role in the liquefaction process.The hydroliquefaction behavior of heavy intermediates is crucial to the coal liquefaction mechanism and optimize the liquefaction conditions.
Heavy intermediates AS and PA, separated from coal liquefaction products, need to be hydrogenated further into oil or other chemicals.Hydrogenation of AS and its kinetics have been extensively investigated in the past decades[1-4].Compared with the AS hydrogenation and its kinetics, the hydro-conversion of PA has been studied scarcely, especially the kinetics of PA hydroliquefaction.Ouchi et al[5]believed that the hydro-conversion of PA to AS plus oil with red mud and sulphur as catalyst was mainly due to the splitting of ether linkages and the partial saturation of aromatic rings with hydrogen.Kemp et al[6]studied four preasphaltenes had been hydropyrolysed with or without 9,10-dihydroanthracene.Significant differences were observed in the yields of products, and the fate of nitrogen and sulphur during these processes had been investigated.Meanwhile, Steedman et al[7]pointed out that the heteroatom contents of PA decreased progressively with the extent of hydrogenation, while the non-phenolic oxygen content remained constant,and the hydrogen and carbon type distributions in products were different.PA have higher aromaticity than AS.Zhang et al[8]thought the conversion of PA followed a different pathway compared to the coal since the PA was converted more selective to oil during hydroliquefaction.Wang et al[9]studied the hydroconversion of PA and its kinetics catalyzed bysolid acid.The results indicated that the content of condensed aromatic rings increased, and the contents of hydrogen, oxygen and aliphatic side chains of PA decreased with the rise of liquefaction temperature.The conversion of PA hydro-conversion under 425 °C, for 40 min reached 81.3% with 51.2% of oil + gas yield.The activation energy of PA conversion into AS was 72 kJ/mol.
In our previous work, FeS + S catalyst in the Xiaolongtan (XLT) lignite hydroliquefaction showed higher catalytic activity than FeS[10].Thesolid acid showed outstanding catalytic activity liquefaction of Shenhua subbituminous coal compared to FeS and FeS + S[11].In order to probe the kinetics of PA, the hydroliquefaction experiments of PA with FeS +S were carried out in this paper.
1 Experimental
1.1 Sample and reagents
PA used in this work was obtained from consecutive solvent extraction as discussed in our previous work[10].PA was pulverized to pass through a 200-mesh sieve,stored under nitrogen atmosphere and dried in vacuum overnight at 80 ℃ before use.Table 1 shows the elemental analysis of PA.All the chemical reagents were commercially purchased.
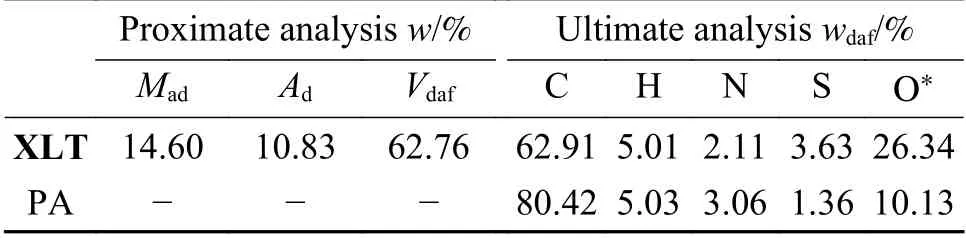
Table 1 Proximate and ultimate analyses of samples
1.2 Experimental procedure
Each experiment for PA hydroliquefaction was carried out in a 30 mL stainless-steel tube reactor.In each run, 1.0 g PA, 5 mL tetralin, 0.05 g FeS + S(molar ratio S∶FeS = 1∶1) were charged.After replacing air in the autoclave and being pressurized with H2to a pressure (5.0 MPa), the autoclave was heated to a prescribed temperature (380, 400, 420 and 440 ℃) and kept at the temperature for 0, 5, 10, 20, 30, 60 min.Then the autoclave was cooled to room temperature in an ice-water bath.
1.3 Fractionation and analyses
Each reaction mixture was transferred from the autoclave with tetrahydrofuran (THF) into a Soxhlet extractor and extracted exhaustively to produce char(THF-inextractable) and THF-extractable portion.The THF-extractable portion was sequentially extracted withn-hexane and toluene to afford oil (n-hexaneextractable), AS (n-hexane-inextractable but tolueneextractable) and PA (toluene-inextractable but THFextractable)[12].The above char was dried in vacuum at 80 ℃ for 24 h.The resulted AS and residual PA were also dried under the same conditions.Considering that the resulted oil may contain some species of volatile as solventsn-hexane and THF, the oil was not subjected to vacuum drying.Instead, the sum of oil + gas was calculated by difference of PA and the above residue,AS and residual PA.All runs were duplicated.The errors in the yields of oil + gas, AS and PA were within ± 5%.
2 Results and discussion
2.1 Hydroliquefaction of preasphaltenes at different temperatures
Isothermal liquefactions of preasphaltenes at four different temperatures, i.e.380, 400, 420 and 440 ℃were carried out.Due to the complexity of PA and diversity of liquefaction products, it is difficult to descript the PA liquefaction process using a simple chemical kinetic reaction.Lumped kinetic model is an effective method to study kinetics of coal liquefaction[13].In this work, four lumped liquefaction products were used that included un-converted PA, AS, oil+gas (Og) and char(C).Table 2 shows the hydroliquefaction conversions and product distributions of PA at different temperatures.
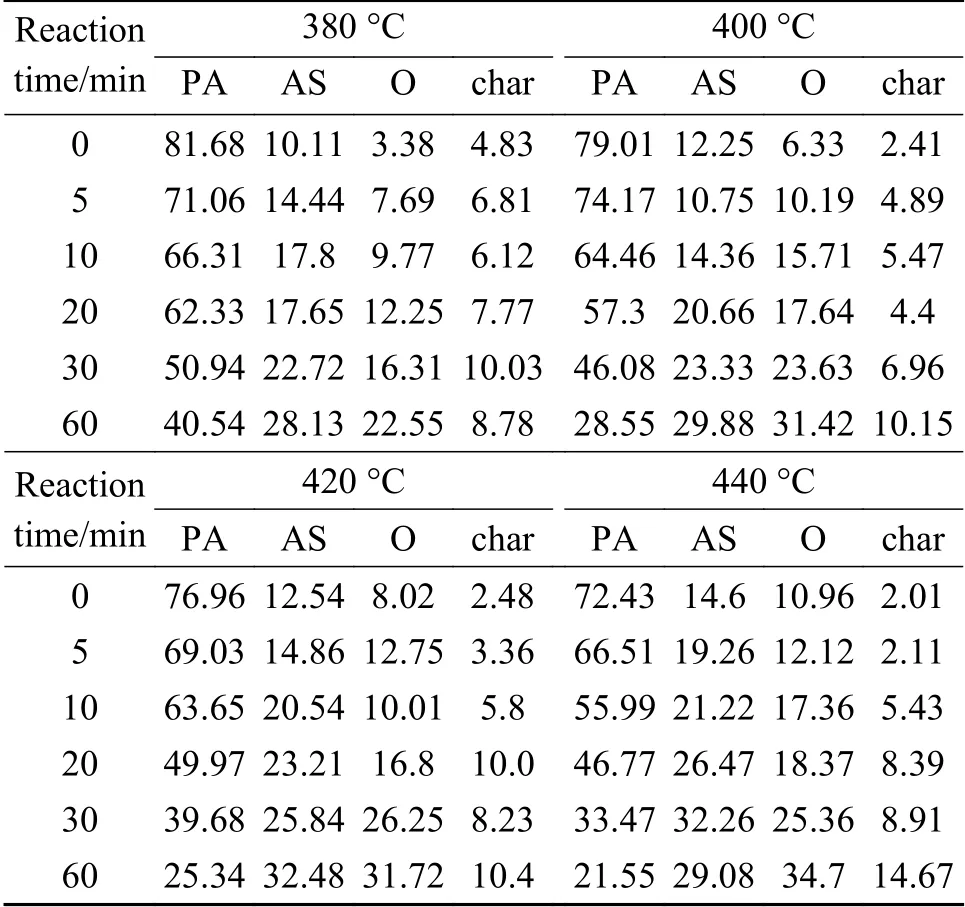
Table 2 Conversions (%) and liquefaction product distributions (%) of PA liquefaction catalyzed by FeS + S at 380, 400, 420 and 440 °C
2.2 Preasphaltenes hydroliquefaction lump kinetic model establishment and solution
The PA hydroliquefaction is a complex multiphase catalytic process containing of consecutive, parallel and retrogressive reactions.Based on the kinetic models[1-4,8,14-22]of coal hydroliquefaction and its derivatives, the PA hydroliquefaction model were proposed in this study, as Figure 1 shown.
In order to solve the model, three main assumptions were made:
(1) All the reactions were the pseudo first-order.
(2) The rate constants fitted the Arrhenius law.
(3) The influences of mass transfer and other reaction conditions except temperature and time were ignored at the specified conditions.
There were four apparent reaction rate constants,kpc,kpa,kapandkaoshown in Figure 1.According to the model, the kinetic model differential equations of PA hydroliquefaction experiments with FeS + S as catalysts were obtained as shown in Equations(1)-(4):
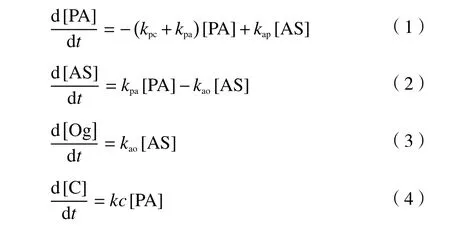
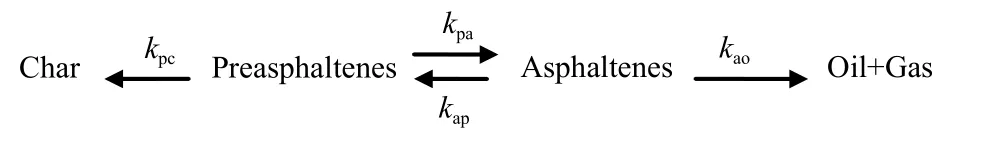
Figure 1 Hydroliquefaction model of preasphaltenes
where PA, AS, Og and Char were un-converted PA (%), AS yield (%), Oil + gas (%) and char yield(%), respectively.These differential equations were solved for the rate constants using the experimental data of Table 2.The method involved integration of the differential equations and the least-squares fitting to directly determine the rate constants.The integrals were estimated by fourth-order Runge-Kutta method.The procedure details were available in the literature[9,22].
2.3 Kinetic model analysis
Figures 2-5 show the comparing results between theoretical values fitted by the model and the experimental data of PA hydroliquefaction catalyzed by FeS + S at four temperatures.Clearly, the model satisfyingly fitted the experimental data.The rate constants at different temperatures and Arrhenius activation energies (Ea) were calculated as shown in Table 3.
Table 3 shows that the consecutive reactions of PA→AS→Og are the main reactions of PA hydroliquefaction.Oil + gas is mainly produced from asphaltenes→oil + gas(kao).At lower temperature(380 ℃), the four rate constants are small.Meanwhile,kpa(PA→AS) is smaller thankap(AS→PA) indicating the retrogressive reactions are not negligible.With the temperature rises to higher than 400 ℃, the retrogressive reactions from PA to Char and AS to PA occure markedly.At higher temperature, a great number of parallel and competitive reactions occur, such as pyrolysis, cleavage of bridge bonds between structural units, dehydration and ring opening[12].Compared with the PA hydroliquefaction catalyzed bysolid acid[9], there are not only the retrogressive reactions from AS to PA but also retrogressive reactions from PA to Char in the work.All thekpa,kapandkaoare smaller than those of the corresponding constants,perhaps duo to the different feeds and catalysts used.It was reportedsolid acid catalyst was more active to catalyze the reaction of PA into AS[23].
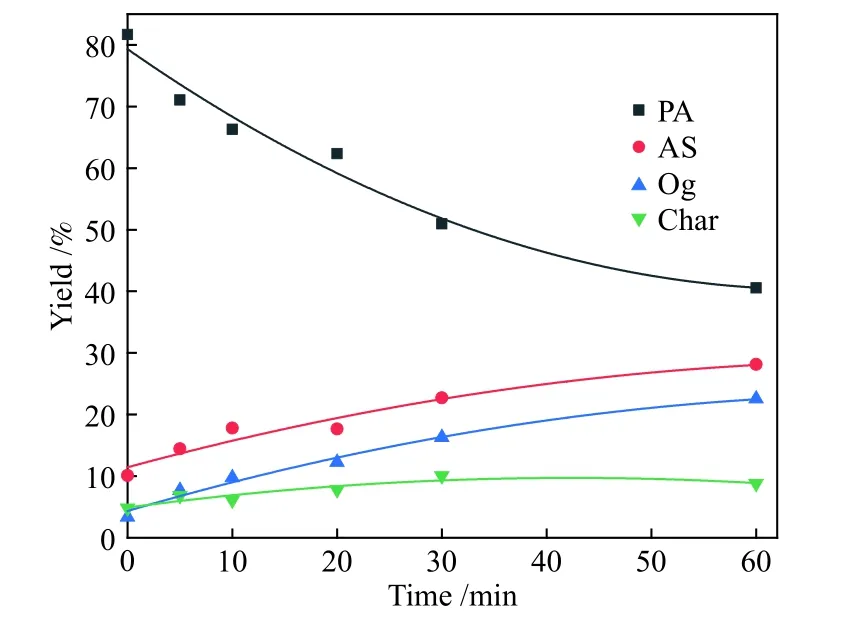
Figure 2 Comparison of model-fited curves with experimental data of PA hydroliquefaction at 380 °C
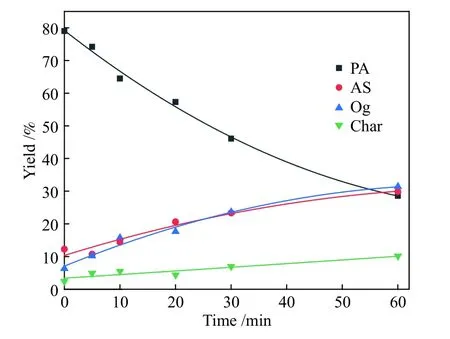
Figure 3 Comparison of model-fited curves with experimental data of PA hydroliquefaction at 400 °C
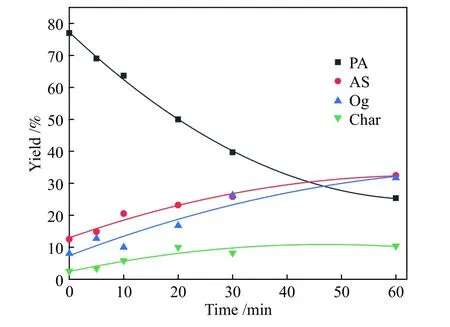
Figure 4 Comparison of model-fited curves with experimental data of PA hydroliquefaction at 420 °C
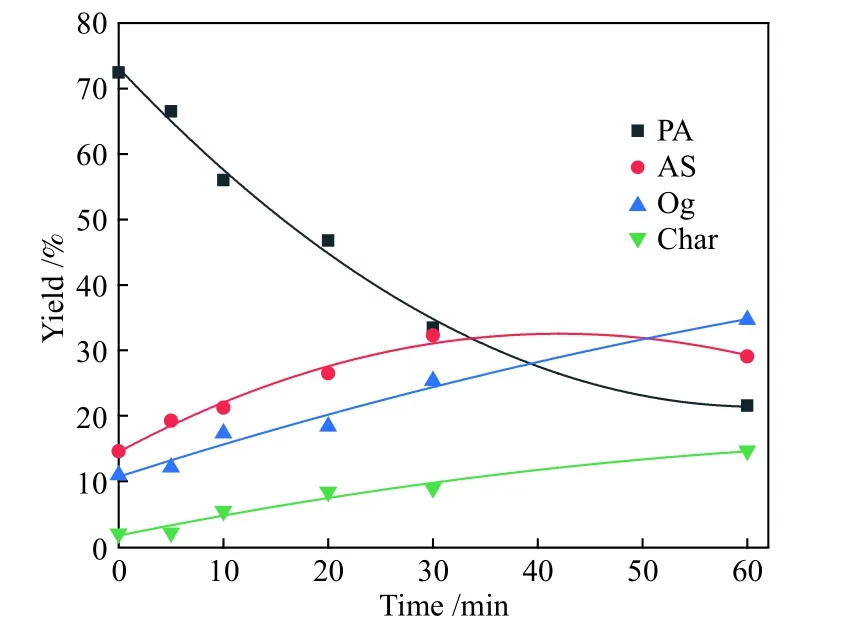
Figure 5 Comparison of model-fited curves with experimental data of PA hydroliquefaction at 440 °C
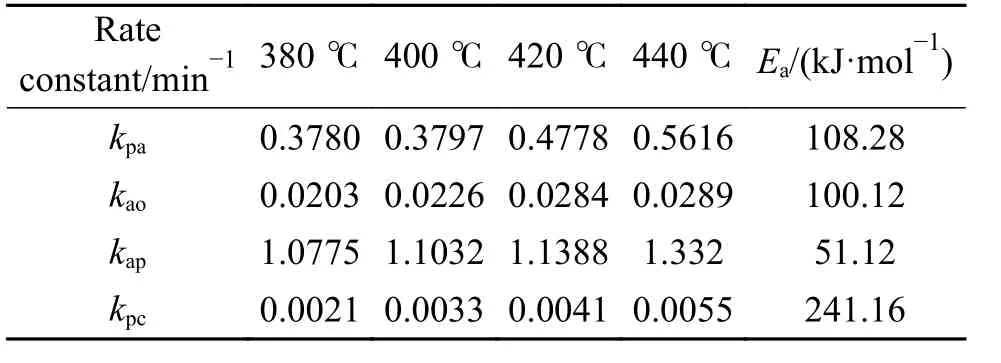
Table 3 Kinetic parameters of PA hydroliquefaction calculated by the model
The activation energies in Table 3 are within 50-110 kJ/mol except for the regressive reaction of PA to char.Table 3 shows that activation energy of PA to char is highest,kpcis the lowest indicating that consecutive reactions of PA→AS→Og are the main reactions in PA hydroliquefaction.The activation energies of PA→AS and AS→Og are 108.28 kJ/mol and 100.12 kJ/mol, respectively, which are higher than the corresponding values of 72.3 kJ/mol and 73.2 kJ/mol in this work, because thesolid acid catalyst was more effective than FeS + S in cracking reactions[9,24].
3 Conclusions
Preasphaltenes were hydrocracked to asphaltene and char directly, and then asphaltenes were further hydrocracked into oil + gas products.Obvious regressive reactions of PA to char and AS to PA occur at higher temperatures.Higher temperature and longer time were favorable for increasing the conversion of PA and the oil + gas yield.The hydroliquefaction of preasphaltenes under 440 °C and 60 min reached 79.45% with 34.7%of oil + gas yield.The established model agreed well with the experimental data under the experimental conditions, and the activation energies varied within 50-245 kJ/mol.
Acknowledgements
This work was supported by the Anhui Provincial Innovative Group for Processing and Clean Utilization of Coal Resource, Anhui Province Key Laboratory of Coal Clean Conversion and High Valued Ulitilization.
