Diagnostic value of 5 serum biomarkers for hepatocellular carcinoma with different epidemiological backgrounds: A large-scale, retrospective study
2021-04-22DongmingLiuYiLuoLuChenLiweiChenDuoZuoYueguoLiXiaofangZhangJingWuQingXiGuangtaoLiLishaQiXiaofenYueXiehuaZhangZhuoyuSunNingZhang0TianqiangSongWeiLuHuaGuo
Dongming Liu*, Yi Luo*, Lu Chen*, Liwei Chen, Duo Zuo, Yueguo Li, Xiaofang Zhang, Jing Wu, Qing Xi, Guangtao Li, Lisha Qi, Xiaofen Yue, Xiehua Zhang, Zhuoyu Sun, Ning Zhang0, Tianqiang Song, Wei Lu, Hua Guo
1Department of Hepatobiliary, Liver Cancer Research Center for Prevention and Therapy; 2Department of Tumor Cell Biology; 3Clinical Laboratory, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin’s Clinical Research Center for Cancer, Tianjin 300060, China; 4Medical Laboratory, Tianjin Medical University General Hospital, Tianjin 300052, China; 5Clinical Laboratory, Tianjin Third Central Hospital, Tianjin 300170, China; 6Department of Pathology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin’s Clinical Research Center for Cancer, Tianjin 300060, China; 7Department of Tianjin Research Institute of Liver Diseases, Tianjin Second People’s Hospital, Tianjin 300192, China; 8Department of Infectious Diseases, The First Affiliated Hospital of Baotou Medical College, Inner Mongolia University of Science and Technology, Baotou 014010, China; 9Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin 300070, China; 10The Center for Translational Cancer Research, Peking University First Hospital, Beijing 100034, China
ABSTRACT Objective: Hepatocellular carcinoma (HCC) is a lethal global disease that requires an accurate diagnosis. We assessed the potential of 5 serum biomarkers (AFP, AFU, GGT-II, GPC3, and HGF) in the diagnosis of HCC.
KEYWORDS Hepatocellular carcinoma; serum; biomarker; AFP; AFU
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide, accounting for more than 600,000 deaths each year1-3. The prognoses of HCC patients are generally poor, and the median survival of patients is only 6-20 months4,5. The main reason for the poor prognosis of HCC is the lack of a timely and accurate diagnosis6,7. Thus, according to the epidemiological characteristics of HCC, we divided our research protocol into the diagnoses of NBNC-HCC patients (patients who are negative for hepatitis B surface antigen and hepatitis C antibody)8and the diagnosis of hepatitis (such as HBV or HCV)-related HCC patients9,10. Although hepatitis-related HCC accounts for the greatest percentage of HCC patients in China, the percentage of NBNC-HCC patients is rapidly increasing8,11. Thus, we recruited this type of HCC patient. We also enrolled patients with a history of alcohol use, aflatoxin exposure, or nonalcoholic steatohepatitis as the “healthy controls,” when compared with the NBNCHCC patients. This study was conducted because it is critical to develop novel assays to identify NBNC-HCC patients, to increase the likelihood of effective treatments.
When diagnosed at an early stage, HCC can be treated with surgery, transplantation, or radiofrequency ablation, which results in a 5-year survival of 40%-70%12, whereas the lack of effective treatments for patients diagnosed with mid- or latestage disease is associated with a dramatic decrease in survival. Despite the low sensitivity, α-fetoprotein (AFP) is a unique serum biomarker for HCC. Unfortunately, the level of AFP may be elevated in patients with nonmalignant chronic liver diseases, including approximately 40% of patients with hepatitis and 30% of patients with cirrhosis13. Thus, only approximately 10%-40% of HCC patients are diagnosed at an early stage using the current AFP-based procedures14. This limitation restricts the early diagnoses of HBV-HCC and HCV-HCC based on serum AFP levels.
Over the past several years, serum microRNA panels have become a promising approach for diagnosing early-stage HCC. These panels differentiate HCC patients from healthy and at-risk controls, and provide prognostic values for HCC15. However, these panels often require the accurate detection of several serum miRNA levels, which may be complicated and costly for HCC screening in large populations. Thus, use of serum protein biomarkers is still a reliable and economic approach for screening HCC in a large population. In the past decade, many studies of serum biomarkers for detecting HCC have been documented12,16-18. More studies have been focused on HBV-HCC, with few studies associated with various etiologies, such as hepatitis C virus infection, alcohol-related liver disease, or nonalcoholic steatohepatitis.
Numerous protein serum biomarkers have been suggested for diagnosing HCC, including α-L-fucosidase (AFU), γ-glutamyl transferase isoenzyme II (GGT-II), glypican-3 (GPC3), and hepatocyte growth factor (HGF)19-22. AFU is a lysosomal enzyme detected in most mammalian cells, and is related to the degradation of fucose-containing fucoglycoconjugates23. The expression of AFU was higher in HCC samples than in healthy controls and in patients with chronic hepatic disease24. GPC3 is a component of heparin sulfate proteoglycans25. It is highly expressed in HCC cells and tissues26. Recent studies reported that GPC3 was examined in HCC cells, but not in benign liver tissues27. GGT-II acted as the second candidate serum marker and was shown to have a higher sensitivity and specificity for hepatoma patients. Surprisingly, it was almost undetectable in other chronic liver diseases28. Cui et al.19observed a lower sensitivity and specificity of GGT-II of 74% and 82.2%, respectively. However, their findings still showed GGT-II might be a promising supplemental biomarker for HCC diagnosis. HGF is many times dysregulated, playing an essential role in malignant tumors, including HCC29. Kim et al.30reported that the combination of serum bFGF and HGF levels might be candidate biomarkers for HCC patients who could benefit from sorafenib therapy. However, limitations such as small sample sizes and single-center designs have prevented their widespread application.
Herein, we evaluated the sensitivity and specificity of these biomarkers in a large-scale, retrospective study to identify a more accurate diagnostic method for NBNC-HCC and hepatitis-related HCC screening in normal populations and at-risk populations. Our results showed that the combination of AFU and AFP protein biomarkers detected NBNC-HCC in the normal population and in hepatitis-related HCC in the at-risk population with stable and reliable cut-off values. Moreover, the combination maintained diagnostic specificity and improved the sensitivity for the detection of NBNC-HCC and hepatitis-related HCC populations, when compared with AFP alone.
Materials and methods
Ethical approval
Our experiments on human subjects were in accordance with the ethical standards of the Helsinki Declaration (amended in 2000) of the World Medical Association. In addition, this study was approved by the Ethics Committees of Tianjin Medical University Cancer Institute and Hospital (Approval No. bc2020083). All patients were informed about the study, and gave their consent for participation.
Study design and patients
A total of 996 subjects who visited the Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University General Hospital or Tianjin Third Central Hospital between July 2012 and April 2014 were recruited in this study for different cohorts (Supplementary Figure S1). Patients with HCC were diagnosed based on ultrasound, computed tomography, or magnetic resonance imaging, and the diagnoses were confirmed histopathologically according to the AASLD guidelines. According to different etiologies, we divided the HCC patients into the hepatitis-related HCC and NBNC-HCC groups. Tumor stage was defined according to the Barcelona Clinic Liver Cancer (BCLC) staging system. For the purpose of this study, we classified tumors with BCLC stage 0 + A as early-stage hepatitis-related HCC and patients who were suffering from chronic hepatitis or liver cirrhosis as at-risk patients. None of the patients underwent any treatment, including surgery, chemotherapy, or radiotherapy, before blood sampling. The diagnosis of cirrhosis was confirmed by liver biopsy and/or clinical, laboratory, and imaging evidence. Chronic hepatitis was defined as chronic necroinflammatory disease of the liver caused by persistent HBV or HCV infection. Healthy controls were used for comparison with NBNC-HCC patients. They were recruited from the Physical Examination Center at Tianjin Medical University Cancer Institute and Hospital, and were eligible if they had no viral hepatitis and no malignant disease. Participants with a history of alcohol use, aflatoxin exposure, or nonalcoholic steatohepatitis also met the criteria for healthy controls. Patients were excluded for the following reasons: (1) 27 patients had primary liver cancer other than HCC, (2) 2 patients had metastatic liver cancer, (3) 11 patients had liver sarcoma or adenocarcinoma, and (4) 35 patients did not have available clinical data. Thus, 468 patients, including 150 HCC patients (123 hepatitis-related HCC patients and 27 NBNC-HCC patients), 82 chronic hepatitis (CH) patients, 61 liver cirrhosis (LC) patients, and 175 healthy controls (HC), were recruited from the three hospitals as the test group between July 2012 and June 2013. When we finished the analysis of the test group, 453 patients who were matched for age and sex with the test group were recruited from the same hospitals as the validation group. The validation cohort was comprised of 121 hepatitis-related HCC patients, 72 CH patients, and 61 LC patients as one subgroup and 27 NBNC-HCC patients and 172 HC patients as another subgroup. The data involving 5 factors and demographic characteristics such as sex and age of patients are listed in Supplementary Table S1-S5.
Statistical analysis
Statistical analyses were performed using SPSS statistical software for Windows, version 25.0 (SPSS, Chicago, IL, USA) and MedCalc, version 18.2.1 (https://www.medcalc.org/). Differences between two independent groups were tested using the Mann-Whitney U test (continuous variables and nonparametric analyses). P values < 0.05 were considered to be significant, and all P values were two-sided. To assess whether the combination of AFU and AFP was better than either of them alone, a new variable predicted probability (P) for HCC was created on the basis of an equation obtained by binary logistic regression:
(a). all-stage HBV-HCC and HCV-HCC vs. CH and LC in the test cohort: (P): 0.007668*AFP + 0.033718*AFU-1.347426
(b). all-stage HBV-HCC and HCV-HCC vs. CH and LC in the validation cohort(P): 0.001227*AFP + 0.017566*AFU-0.957458
(c). early-stage HBV-HCC and HCV-HCC vs. CH and LC in the test cohort(P): 0.005890*AFP + 0.018753*AFU-1.557863
(d). early-stage HBV-HCC and HCV-HCC vs. CH and LC in the validation cohort(P): 0.000921*AFP + 0.011742*AFU-1.166897
(e). NBNC-HCC vs. HC in the test cohort(P): 0.059672*AFP + 0.403175*AFU-8.669705
(f). NBNC-HCC vs. HC in the validation cohort(P): 0.047177*AFP + 0.211019*AFU-5.707287
Nomogram for the hepatitis-related HCC and NBNC-HCC populations
A nomogram was formulated based on the results of logistic regression analyses and by using the rms package of R, version 3.0 (http://www.r-project.org/). The nomogram was based on proportionally converting each regression coefficient in multivariate logistic regression to a total points scale. For the diagnosis of HCC based on the model, the total score for each participant was calculated with the nomogram. We could preliminarily predict the likelihood of a participant suffering from HCC based on the probability.
Blood samples
Blood samples were obtained by peripheral venous puncture before any surgical or chemotherapeutic treatment. After clotting and within 1 h of collection, the blood samples were centrifuged at 3,000 × g for 5 min, and serum aliquots were stored at -80 °C until analysis.
Serum tumor marker detection
The AFP, AFU, GGT-II, GPC3, and HGF serum levels were analyzed according to the manufacturer’s instructions using ELISA kits (Cusabio, Wuhan, China and eBioscience, San Diego, CA, USA). All assays were performed in duplicate.
Immunohistochemistry (IHC) staining
IHC staining was used to examine the expression levels of AFU in paraffin-embedded samples of HCC tissues according to previously described methods31. An anti-AFU (FUCA2) antibody was purchased from Bioss (bs-16192R, 1:200; Bioss, Woburn, MA, USA). The IHC score was used to evaluate the correlation between AFU expression and overall survival (OS) and disease-free survival (DFS) of HCC patients.
Bioinformatic analysis
Correlation between AFU or AFP/AFU combination expression and overall/DFS in HCC patients was based on the Kaplan-Meier method (http://kmplot.com/analysis/). The threshold of significance was set at P < 0.05.
Results
The serum levels of AFP, AFU, GPC3, GGT-II, and HGF in the test group
In the test cohort, all 463 patients were tested for serum levels of AFP, AFU, GPC3, GGT-II, and HGF. The median plasma levels of all 5 tumor markers were found to be significantly higher in the NBNC-HCC subgroup than in the healthy controls (Figure 1A-1E). In the HBV-HCC and HCV-HCC subgroups, the levels of AFU, GPC3, and GGT-II were significantly higher in the LC group than in the CH group (Figure 1B-1D), suggesting that elevated levels of these three biomarkers may be associated with the progression of hepatitis to liver cirrhosis. The HBV-HCC and HCVHCC patient median plasma levels of AFP, AFU, and HGF were found to be significantly higher than those of the CH and LC patients (Figure 1A, 1B, and 1E), indicating that a high expression of these biomarkers was associated with the progression of liver disease. Generally, a high level of these 3 candidate markers was associated with the onset of HBVHCC and HCV-HCC.
The combination of AFP and AFU had high accuracy in the detection of NBNC-HCC
The area under the curve (AUC) values of AFP, AFU, GPC3, GGT-II, and HGF were 0.792, 0.967, 0.825, 0.824, and 0.759, respectively (Figure 2A-2E). Each serum biomarker could be a candidate serum biomarker combined with AFP in diagnosing NBNC-HCC. Thus, we determined the different values of the receiver operating characteristic (ROC) curve with various combinations of serum biomarkers (AFP, AFU, GPC3, GGT-II, and HGF). The 5 biomarker combinations performed well (AUC: 0.989, sensitivity: 92.6%, specificity: 98.9%) (Figure 2F). The best 4/3/2 biomarker combinations had a similar AUC, sensitivity, and specificity compared with the 5 biomarker combination (AUC: 0.989, sensitivity: 92.6%, specificity: 99.4%; AUC: 0.989, sensitivity: 92.6%, specificity: 99.4%; AUC: 0.986, sensitivity: 92.6%, specificity: 98.9%, respectively) (Figure 2G-2I). The combination results of the remaining serum indicators are shown in Supplementary Figure S2-S4. After combining various factors (such as AUC, sensitivity, and specificity), we chose the combination of AFP and AFU as the diagnostic combination for NBNC-HCC. The predictive values and likelihood ratios for AFU and AFP in the diagnosis of NBNC-HCC are shown in Table 1. The combination improved the sensitivity of AFP in diagnosing NBNCHCC, while the specificity was relatively unchanged.
According to the stable cutoff value of AFP and AFU in detecting NBNC-HCC, we verified the results of the test cohort in the validation cohort. First, the trends of AFP and AFU concentrations in healthy controls and NBNC-HCC patients were consistent with those in the test cohort (Figure 1F and 1G). Furthermore, the AUC, sensitivity, specificity, PPV (positive predictive value), NPV (negative predictive value), positive LR (likelihood ratio), and negative LR of AFP, AFU, and their combinations were similar to those in the test cohort at the optimum cut-off value (Supplementary Figure S5 and Table 1). The AUC of the combination was better than any other single biomarker (only AFP or AFU) of NBNC-HCC in the test and validation groups (Figure 3A and 3B). We used a nomogram model for the clinical application of these 2 serum markers (Figure 3C). For example, if the AFP and AFU values of a “healthy person” (including individuals with a history of alcohol, aflatoxin exposure, or nonalcoholic steatohepatitis) were 50 ng/mL and 20 mU/mL, respectively, then based on the nomogram model, the probability of this participant developing NBNC-HCC was nearly 90%.
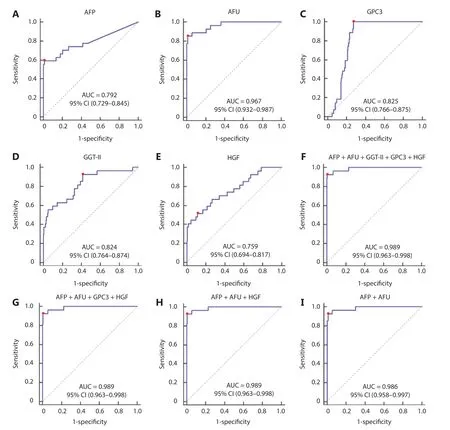
Figure 2 The receiver operating characteristic curve of AFP (A), AFU (B), GPC3 (C), GGT-II (D), HGF (E), AFP + AFU + GGT-II + GPC3 + HGF (F), AFP + AFU + GPC3 + HGF (G), AFP + AFU + HGF (H), and AFP + AFU (I) in the detection of the NBNC-HCC test group. The sensitivity and specificity represented by the red dots are shown in detail (lower). (A). AFP sensitivity: 59.3% and specificity: 98.9%; (B). AFU sensitivity: 85.2% and specificity: 98.9%; (C). GPC3 sensitivity: 100.0% and specificity: 72.6%; (D). GGT-II sensitivity: 92.6% and specificity: 58.3%; (E). HGF sensitivity: 51.9% and specificity: 88.6%; (F). AFP + AFU + GPC3 + GGT-II + HGF sensitivity: 92.6% and specificity: 98.9%; (G). AFP + AFU + GPC3 + HGF sensitivity: 92.6% and specificity: 99.4%; (H). AFP + AFU + HGF sensitivity: 92.6% and specificity: 99.4%; (I). AFP + AFU sensitivity: 92.6% and specificity: 98.9%.
The combined AFP/AFU panel showed an improvement in the diagnostic sensitivity for the detection of all-stage and early-stage hepatitis-related HCC
The AUC values of AFP, AFU, GPC3, GGT-II, and HGF in the all-stage HBV-HCC and HCV-HCC groups were 0.780, 0.752, 0.520, 0.547, and 0.735, respectively (Figure 4A-4E). Because there was no significance between GGT-II and GPC3 in detecting all-stage HBV-HCC and HCV-HCC, we chose AFP, AFU, and HGF in a combination model (Figure 4F-4I). The diagnostic performance of the serum biomarkers in different subgroups was further evaluated. Among these combinations, the AFP/AFU panel outperformed the others and exhibited agreater diagnostic sensitivity and specificity for the differentiation of all-stage HBV-HCC and HCV-HCC patients from CH and LC patients [AUC: 0.835 (0.784-0.877), sensitivity: 69.1%, specificity: 87.4%] (Figure 4G). The diagnostic values of serum AFP and AFU were 42.34 ng/mL and 13.94 mU/mL, respectively. Regarding early stage HBV-HCC and HCV-HCC, the AUC values of AFP, AFU, GPC3, GGT-II, and HGF were 0.741, 0.666, 0.517, 0.510, and 0.665, respectively (Figure 5A-5E). We observed similar results in this test cohort with the all-stage HBV-HCC and HCV-HCC groups. AFP, AFU, and HGF were selected for the combination model (Figure 5F-5I). The AFP/AFU combination was also notable for early-stage HBV-HCC and HCV-HCC [AUC: 0.776 (0.712-0.831), sensitivity: 52.5%, specificity: 91.6%] in the test cohort (Figure 5G). In summary, the AFP/AFU panel improved the diagnostic sensitivity without a loss of specificity in the detection of all-stage HBV-HCC and HCV-HCC (Table 2: AFP vs. AFP + AFU: sensitivity 52.8% vs. 69.1%, specificity 93.7% vs. 87.4%) and early-stage HBV-HCC and HCV-HCC (Table 2: AFP vs. AFP + AFU, sensitivity: 44.3% vs. 52.5%, specificity: 93.7% vs. 91.6%) among at-risk patients.
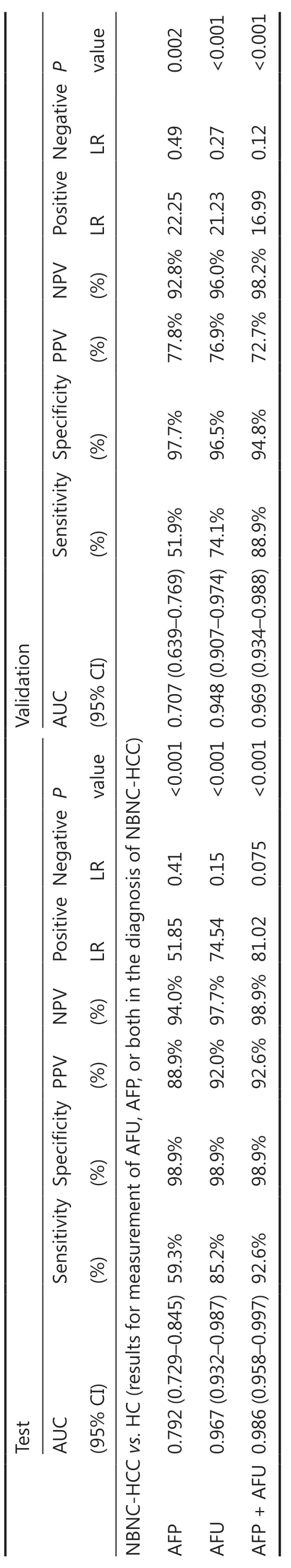
Table 1 Results for the measurement of serum AFU, AFP, or both, in the diagnosis of NBNC-HCC
In the validation cohort, the concentrations of AFP and AFU in CH-, LC-, and hepatitis-related HCC patients are shown in Figure 1F and 1G. The results were similar to those in the test cohort. The ROC curves of single serum markers and combined serum markers in the validation group are shown in Supplementary Figure S6 (the results for all-stage HBV-HCC and HCV-HCC are shown in A, B, and E; the results for early-stage HBV-HCC and HCV-HCC are shown in C, D, and F). Compared with the optimum diagnostic cutoff values of AFP and AFU for HBV-HCC and HCV-HCC in the test group, the parameter values in the validation group for all-stage and early-stage hepatitis-related HCC are summarized in Table 2 [AUC: 0.841 (0.790-0.884), sensitivity: 71.9%, specificity: 86.5% in the validation cohort for all-stage HBV-HCC and HCV-HCC; AUC: 0.791 (0.728-0.845), sensitivity: 75.4%, specificity: 73.7% in the validation cohort for early-stage HBV-HCC and HCV-HCC]. The AUC of the AFP/AFU combination was better than any other single biomarker (only AFP or AFU) of all-stage (Figure 6A and 6B) and early-stage (Figure 6C and 6D) hepatitis-related HCC in the test and validation groups. We also constructed a nomogram model for the clinical application of these 2 serum markers in HBV-HCC and HCV-HCC (Figure 6E). For example, if the AFP and AFU values of an “at-risk person” (such as an individual with HBV or HCV) were 60 ng/mL and 25 mU/mL, respectively, then based on the nomogram model, the probability of this individual developing HBV-HCC and HCVHCC was nearly 75%.
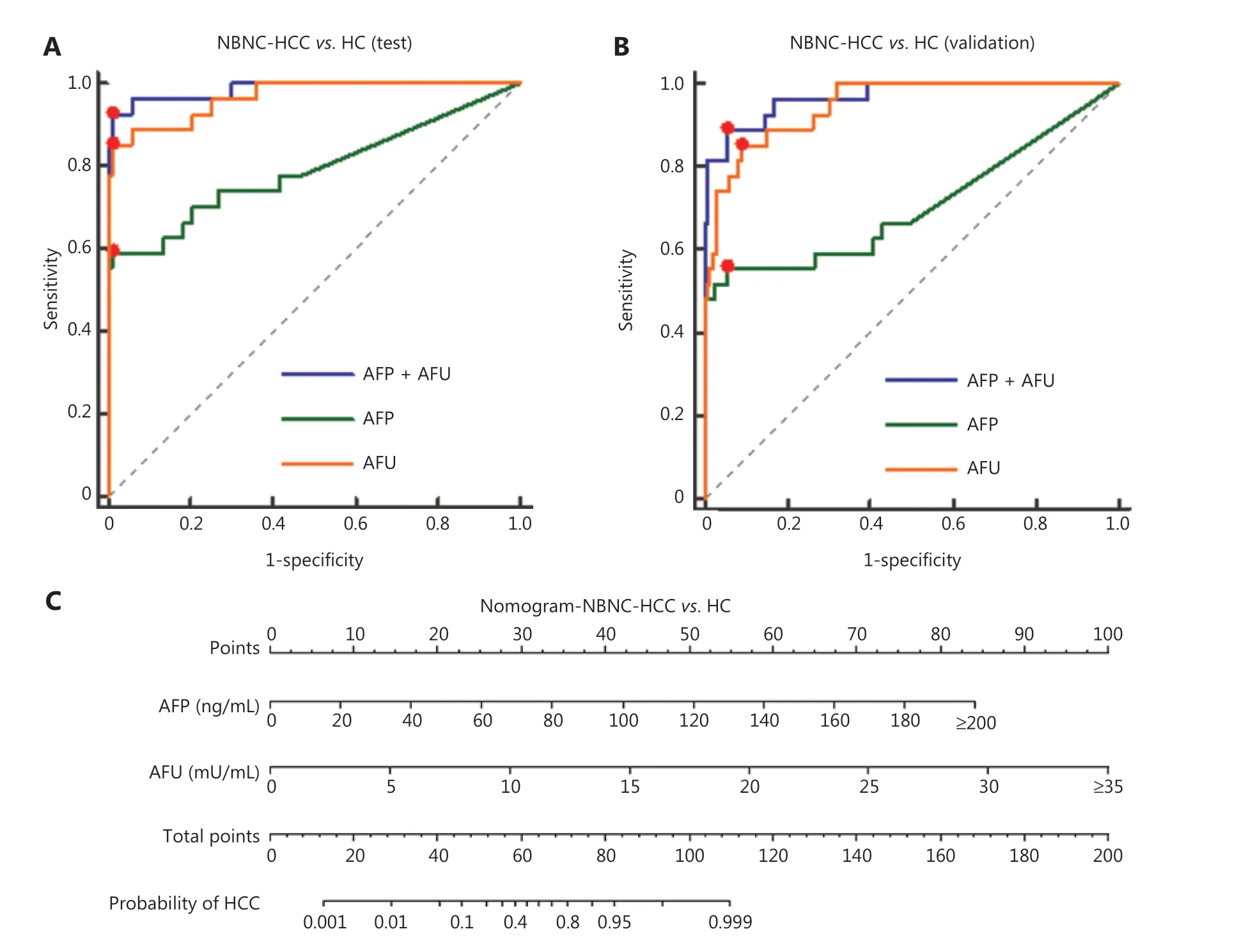
Figure 3 Diagnostic outcomes and nomograms for the combination of serum AFP and AFU in the diagnosis of NBNC-HCC. (A). Receiver operating characteristic curves (ROCs) for AFU, AFP, or both for all patients with NBNC-HCC vs. HC in the test cohort. (B). ROC curves for AFU, AFP, or both for all patients with NBNC-HCC vs. HC in the validation cohort. (C). Nomogram of the combined AFP/AFU in diagnosing NBNC-HCC.
Overall, the AFP/AFU panel improved the accuracy for diagnosing all-stage and early-stage hepatitis-related HCC compared to any other single marker. Moreover, the inclusion of demographic characteristics assisted in the detection of disease.
The AFP/AFU combination was effective in predicting the survival of HCC patients
We evaluated the AFU levels in predicting HCC patient prognoses based on a KM plotter database. The results showed that patients with low expression of AFU might have better prognoses (Supplementary Figure S7A and B). Thus, we assessed the value of the AFP/AFU combination in forecasting survival of HCC patients. The 5-year overall/DFS of HCC patients with low expression of the AFP/AFU combination was almost 60% and 40%, respectively, while the survival of patients with high expression was approximately 40% and 25%, respectively (Supplementary Figure S7C and D). Overall, the results showed that the AFP/AFU combination was effective in predicting HCC prognosis.
We also verified the results of the KM plotter based on our IHC data. First, we found that patients with high AFU levels had worse prognoses (Supplementary Figure S7E and F). In addition, the IHC results of the AFP/AFU combination in predicting HCC prognoses were consistent with those in the KM plotter database (Supplementary Figure S7G and H). Thus, the AFP and AFU panel was effective in predicting the survival of HCC patients.
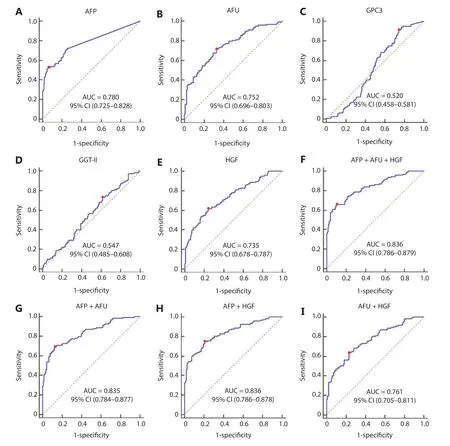
Figure 4 The receiver operating characteristic curves of AFP (A), AFU (B), GPC3 (C), GGT-II (D), and HGF (E), AFP + AFU + HGF (F), AFP + AFU (G), AFP + HGF (H), AFU + HGF (I) in the detection of all-stage HBV-HCC and HCV-HCC of the test group. The sensitivity and specificity represented by the red dots are shown in detail (lower). (A). AFP sensitivity: 52.8% and specificity: 93.7%; (B). AFU sensitivity: 71.5% and specificity: 67.1%; (C). GPC3 sensitivity: 91.1% and specificity: 25.9%; (D). GGT-II sensitivity: 73.2% and specificity: 38.5%; (E). HGF sensitivity: 61.8% and specificity: 75.5%; (F). AFP + AFU + HGF sensitivity: 65.9% and specificity: 89.5%; (G). AFP + AFU sensitivity: 69.1% and specificity: 87.4%; (H). AFP + HGF sensitivity: 74.8% and specificity: 79.0%; (I). AFU + HGF sensitivity: 63.4% and specificity: 76.9%.
Discussion
There is a consensus that early diagnosis is the key to improving the survival of HCC patients4. Several preliminary studies have suggested that serum biomarkers, including AFP, AFU, GGT-II, HGF, and GPC3, may be used for the diagnosis of HCC19-22. However, these markers are not currently included in routine clinical assessments because of the lack of largescale, multicenter clinical investigations.
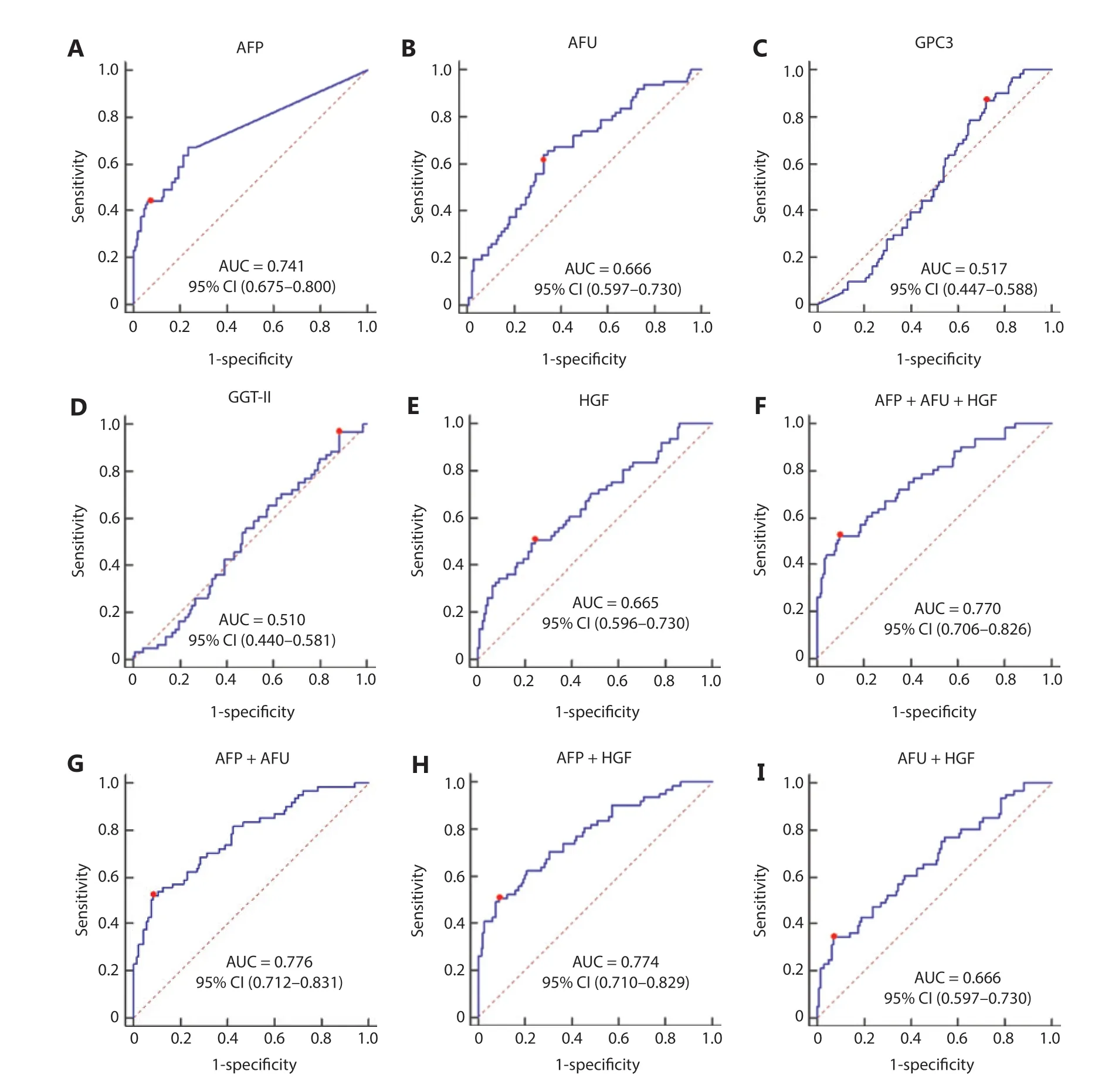
Figure 5 The receiver operating characteristic curves of AFP (A), AFU (B), GPC3 (C), GGT-II (D), and HGF (E), AFP + AFU + HGF (F), AFP + AFU (G), AFP + HGF (H), and AFU + HGF (I) in the detection of early-stage HBV-HCC and HCV-HCC of the test group. The sensitivity and specificity represented by the red dots are shown in detail (lower). (A). AFP sensitivity: 44.3% and specificity: 93.7%; (B). AFU sensitivity: 63.9% and specificity: 67.1%; (C). GPC3 sensitivity: 86.9% and specificity: 28.0%; (D). GGT-II sensitivity: 96.7% and specificity: 11.9%; (E). HGF sensitivity: 50.8% and specificity: 75.5%; (F). AFP + AFU + HGF sensitivity: 52.5% and specificity: 90.2%; (G). AFP + AFU sensitivity: 52.5% and specificity: 91.6%; (H). AFP + HGF sensitivity: 50.8% and specificity: 90.9%; (I). AFU + HGF sensitivity: 34.4% and specificity: 93.0%.
Over the past 2 decades, infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) has been associated with approximately 85% of worldwide HCC32. Due to the promotion of antiviral therapy, the number of patients with other causes of HCC (hepatitis B virus surface antigen-negative and hepatitis C virus antibody-negative or NBNC-HCC) is increasing8,11. In our study of 401 subjects (202 in the test cohort and 199 in the validation cohort), the levels of all 5 markers were significantly higher in NBNC-HCC patients than in healthy controls. We therefore further studied the diagnostic capabilities of these 5 markers for NBNC-HCC. It is worth mentioning that the healthy controls in this study only referred to individuals who had not been infected with HBV or HCV. The healthy controls may have suffered from alcohol-related liver disease, nonalcoholic steatohepatitis, or aflatoxin exposure33. The combination of AFP and AFU was uniquely associated with the progression of NBNC-HCC (HC to NBNC-HCC). This combination showed promising characteristics as a diagnostic marker for NBNC-HCC. Their diagnostic capability outperformed that ofany other serum marker in this study (AUC: 0.986, 95% CI: 0.958-0.997, sensitivity: 92.6%, specificity: 98.9% in the test cohort; AUC: 0.969, 95% CI: 0.934-0.988, sensitivity: 88.9%, specificity: 94.8% in the validation cohort). Considering various factors, such as the incidence of NBNC-HCC, our study is the first large-scale, retrospective analysis of serum biomarkers in NBNC-HCC patients.
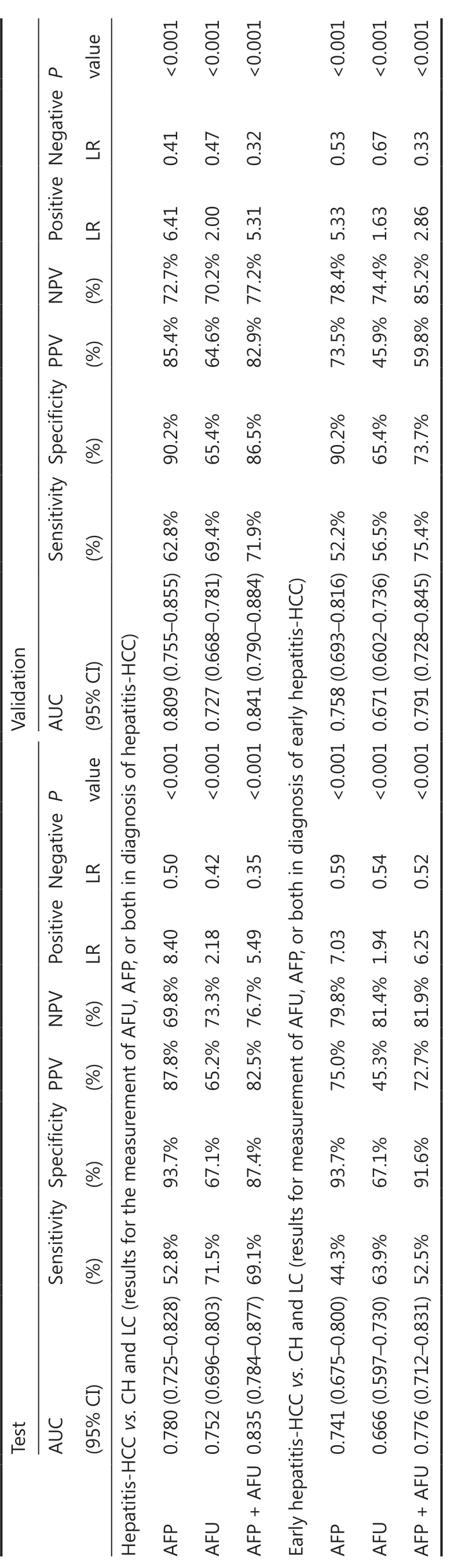
Table 2 Results for measurement of serum AFU, AFP, or both, in the diagnosis of HBV-HCC and HCV-HCC
In China, the incidence and mortality of hepatitis-related HCC is still high34. The HBV-HCC and HCV-HCC patient median plasma levels of AFP, AFU, and HGF were found to be significantly higher than those of CH and LC patients. We showed that the rise of these 3 serum biomarkers may be related to the occurrence of HBV-HCC and HCV-HCC. Thus, we paid particular attention to these 3 serum markers for differentiating HBV-HCC and HCV-HCC patients from at-risk (CH and LC) patients. This differentiation has also been the focus of current research worldwide35. In our study, AFU showed promising accuracy in identifying HBV-HCC and HCV-HCC patients from the at-risk population. We found that the combination of AFP and AFU uniquely reflected the progression of HBV-HCC and HCV-HCC (CH to LC to HBVHCC and HCV-HCC). For all-stage hepatitis-related HCC vs. CH and LC, the ROC curves showed that the AFP/AFU combination had an AUC of 0.835 (95% CI: 0.784-0.877), a sensitivity of 69.1%, and a specificity of 87.4%. Our results are comparable with other promising markers, especially in terms of diagnostic sensitivity (e.g., DKK1: 74.8% vs. 69.1% in all-stage detection)12. Similar results were also shown in early-stage HBV-HCC and HCV-HCC (AUC: 0.776, 95% CI: 0.712-0.831, sensitivity: 52.5%, specificity: 91.6%). Most importantly, the AFP/AFU panel improved the diagnostic sensitivity in the absence of a loss of specificity in the detection of HBV-HCC and HCV-HCC. Notably, this strategy showed an advantage for using an AFP/AFU panel. Our findings are consistent with the results of basic and clinical research studies23,36,37. AFU was also considered to be a prognostic and disease recurrence marker and has been shown to be associated with metastasis and reduced overall survival38.

Figure 6 Diagnostic outcomes and nomogram for the combination of serum AFP and AFU of all-stage and early stage hepatitis-related hepatocellular carcinoma (HCC). (A). Receiver operating characteristic curves (ROCs) for AFU, AFP, or both for all patients with all-stage HBVHCC and HCV-HCC vs. CH and LC in the test cohort. (B). ROC curves for AFU, AFP, or both for all patients with all-stage HBV-HCC and HCV-HCC vs. CH and LC in the validation cohort. (C). ROC curves for AFU, AFP, or both for all patients with early-stage HBV-HCC and HCV-HCC vs. CH and LC in the test cohort. (D). ROC curves for AFU, AFP, or both for all patients with early-stage HBV-HCC and HCV-HCC vs. CH and LC in the validation cohort. (E). Nomogram of the combined AFP/AFU in diagnosing HBV-HCC and HCV-HCC.
Zhang et al.39assessed the diagnostic value for HCC in combination with AFU, AFP, and TK1. They enrolled participants including 116 patients with HCC, 109 patients with benign hepatic diseases (such as hepatitis and liver cirrhosis), and 104 normal subjects. The results showed that the AUC was 0.718 for AFU, 0.832 for AFP, 0.773 for TK1, and 0.900 for the combination of these markers. The results were similar to our data in the detection of HBV-HCC and HCV-HCC (0.780 for AFP, 0.752 for AFU, and 0.835 for the combination). In addition, we showed that the AFP/AFU combination was effective in detecting NBNC-HCC patients. Zhu et al.40found that the AUCs were 0.80, 0.80, and 0.87 for serum AFU, 5′-NT, and AFP, respectively. The correlation of AFU and AFP was significant. However, they did not identify the combination of these markers. In addition, the number of participants was too low (36 for HCC and 36 for healthy controls). Xing et al.24reported that a combination of AFU and AFP (AUC: 0.582) did not improve the diagnostic efficacy compared with AFP (AUC: 0.764) alone for HCC patients. They showed that the majority of HCC patients (85.5%) had chronic HBV and only 13 patients (6.9%) had chronic HCV, so there were some NBNC-HCC patients (7.6%) who were enrolled in the HCC cohort. Based on the etiology of HCC, patients with hepatitis do not progress to NBNC-HCC. This part of NBNC-HCC patients might therefore cause bias in the results. However, patients with benign disease would not evolve to HBV or HCV-related HCC. This phenomenon might lead to a low AUC of AFU and its combination. We enrolled patients with hepatitis or liver cirrhosis as controls of hepatitis-related HCC patients. In addition, healthy controls were used for comparison with NBNC-HCC patients. Thus, the results regarding the AUC of the AFP/AFU combination in our study were more convincing.
Conclusions
To the best of our knowledge, this is the first report showing the potential of AFU in diagnosing NBNC-HCC and hepatitis-related HCC, based on a study with a large sample size and independent validation. Wang et al.21reported that preoperative serum AFU is a prognostic predictor of HCC based on survival prognosis data. We showed that AFU was a promising diagnostic marker for NBNC-HCC and hepatitis-related HCC, with a high degree of accuracy and clinically applicable cut-off concentrations; AFU could also serve as a reliable second-line marker for the detection of HCC. The AFP/AFU panel had a high degree of accuracy for differentiating NBNC-HCC from healthy controls and hepatitis-related HCC in patients at risk for developing HCC. The assays, which are easy to perform and cost effective, can be translated into a standard protocol for the clinical diagnosis of HCC, which may identify asymptomatic patients early for curative treatments. We are currently conducting a prospective study to confirm the present findings and to determine the potential utility of measuring AFP/AFU levels to monitor therapeutic responses, and for the prognostic diagnosis of HCC.
Grant support
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81972656 and 31671421), the Key Project of Tianjin Natural Science Foundation (Grant No. 18JCZDJC35200), the State Key Project on Infectious Diseases of China (Grant No. 2018ZX10723204), and the National 135 Major Project of China (2018ZX10302205).
Conflict of interest statement
No potential conflicts of interest are disclosed.
杂志排行
Cancer Biology & Medicine的其它文章
- Competing endogenous RNAs in lung cancer
- The role and its mechanism of intermittent fasting in tumors: friend or foe?
- Association of systemic inflammation and body mass index with survival in patients with resectable gastric or gastroesophageal junction adenocarcinomas
- Exosome-mediated cellular crosstalk within the tumor microenvironment upon irradiation
- Predictive value of MGMT promoter methylation on the survival of TMZ treated IDH-mutant glioblastoma
- Patient-derived non-small cell lung cancer xenograft mirrors complex tumor heterogeneity
