A practical synthesis of α-bromo/iodo/chloroketones from olefins under visible-light irradiation conditions
2021-04-02ZhihuiWangLeiWangZhimingWangPinhuaLiYichengZhang
Zhihui Wang,Lei Wang,c,*,Zhiming Wang,Pinhua Li,Yicheng Zhang,**
a Advanced Research Institute and Department of Chemistry, Taizhou University, Taizhou 318000, China
b Department of Chemistry, Huaibei Normal University, Huaibei 235000, China
c State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Shanghai 200032, China
ABSTRACT A practical synthesis of α-bromo/iodo/chloroketones from olefins under visible-light irradiation conditions has been developed.In the presence of PhI(OAc)2 as promoter and under ambient conditions,the reactions of styrenes and triiodomethane undergo the transformation smoothly to deliver the corresponding α-iodoketones without additional photocatalyst in good yields under sunlight irradiation.Meanwhile,the reactions of styrenes with tribromomethane and trichloromethane generate the desired α-bromoketones and α-chloroketones in high yields by using Ru(bpy)3Cl2 as a photocatalyst under blue LED (450-455 nm) irradiation.
Keywords: α-Bromoketones α-Iodoketones α-Chloroketones Olefins Visible-light irradiation
α-Haloketones are not only useful building blocks and important intermediates in organic synthesis but also in medicinalchemistry [1-3].A range of pharmaceutically important heterocycles including thiazoles, imidizoles and indoles were prepared from α-haloketones as starting materials [2-3].In addition, α-haloketones can be transformed into a number of useful organic compounds,such as aldols stereospecifically in the presence of Cr(II)-salt [4], α-allyl carbonyl compounds with allyl-Ga and allyl-In reagents [5], arylacetic acids by Ag-promoted rearrangement of α-bromo-alkylarylketones [6] and α-diketones through organic Zn-reagents [7].In general, α-haloketones are prepared from the corresponding ketones.For the synthesis of αbromoketones from ketones, a number of procedures have been recommended, including the reactions of ketones with Br2/acid[8],silica/dioxane/Br2[9],[hydroxy(tosyloxy)iodo]benzene/MgBr2[10], N-methylpyrrolidin-2-one hydrotribromide [11], CuBr2[12],and NBS [13].On the other hand, a variety of synthetic methods have been developed for the direct conversion of ketones to their corresponding α-iodoketones from reactions of ketones with I2[14a], I2/CAN [14b], I2/HgCl2[15], I2-SeO2[16], I2/H2O2[17],I2-trimethyloxymethane [18] and NIS [19].However, the direct synthesis of α-haloketones from olefins, which are less expensive than ketones is more preferable comparing their synthesis from ketones.For the preparation of α-bromoketones, the developed transformations are finished through the reactions of olefins with KBr/K2S2O8[20], IBX/TBAB [21], NBS/IBX [22], bromide/bromate[23],HBr-H2O2-KBr/V[24],HBr-H2O/oxidant[25],NaBrO2[26]and TsNBr2[27].Meanwhile, α-iodoketones are synthesized by the reactions of olefins with I2/Ag2CrO4[28], I2/PDC [29], I(collidine)2BF4/DMSO [30], and I2/O2/UV [31].
In general,the direct conversion of ketones into α-chloroketones can be accompanied by using chlorination agents including CuCl2[32], p-toluenesulfonylchloride [33], sulfurylchloride [34], tetraethylammoniumtrichloride [35] and NCS [36], as well as the combinations of TMSCl/KNO3[37], TMSCl/DMSO [38], TMSCl/SeO2[39],NCS/DMSO[40],NCS/Amberlyst-15[41],NCS/Lewis acid[42], NCS/thiourea [43], [Hmim][NO3]/HCl [44], AlCl3/urea/H2O2[45] and NaClO2/Mn(acac)3/Al2O3[46].However, most of these systems have drawbacks, such as long reaction time, use of extra oxidizing agent, tedious procedures, relatively harsh reaction conditions,and the consumption of large amounts of organometallic reagents.It should be noted that a general procedure for the preparation of α-bromoketones, α-iodoketones and α-chloroketones is still scarce.Therefore,the development of a facile synthesis of α-bromo/iodo/chloroketones from olefins under mild reaction conditions is highly desirable.
Herein, we wish to report a practical and efficient strategy toward synthesis of α-bromo/iodo/chloroketones from olefins under visible-light irradiation conditions.In the presence of PhI(OAc)2as promoter and under ambient conditions, the reactions of styrenes and triiodomethane undergo smoothly to deliver the corresponding α-iodoketones without additional photocatalyst in good yields under sunlight irradiation.Meanwhile, the reactions of styrenes with tribromomethane and trichloromethane generate the desired α-bromoketones and αchloroketones in high yields by using Ru(bpy)3Cl2as photocatalyst under blue LED irradiation (Scheme 1).
To optimize the reaction conditions for the preparation of αiodoketones, styrene (1a) and triiodomethane (2a) were selected as the model substrates.When the model reaction was performed in the presence of Ru(bpy)3Cl2(1.0 mol%) as a photocatalyst and PhI(OAc)2(50.0 mol%)as a promoter under ambient conditions in acetone with blue LED (450-455 nm) irradiation for 6 h, the desired product 2-iodo-1-phenylethanone(3a)was isolated in 57%yield(Table 1,entry 1).The solvent plays an important role in the reaction.After examination of the selected solvents, it was found that dioxane gave the highest reactivity (entry 2).Other solvents,such as DMC, CH3CN, DME and PhMe exhibited less reactivity,generating the product 3a in 55%-23% yields (entries 3-6).However,DCE,CH3OH,DMF,DMSO and THF showed no reactivity(entries 7-11).To our delight,when the model reaction was carried out in the absence of additional photocatalyst under blue LED (410-415 nm) or sunlight irradiation, the excellent yields of product 3a were obtained (entries 12 and 13).Addition of TBHP as promoter showed poor reactivity in the reaction (entry 14).As the model reaction was performed without light irradiation(in the darkness), no desired product 3a was observed (entry 15).In the absence of PhI(OAc)2, the model reaction afforded 17% yield of 3a(entry 16).The model reaction exhibited no reactivity under green LED (530-535 nm) irradiation (entry 17).Further investigation indicated that optimal amount of PhI(OAc)2is in the range of 70-100 mol% (Table 1, entries 18-20).
Based on the optimized conditions in our hand,the generality of α-iodoketones preparation from olefins was investigated,as shown in Scheme 2.In general,a number of styrenes(1)bearing different substituents on the benzene rings underwent the reaction with triiodomethane smoothly to yield the corresponding products in good yields.It should be noted that styrenes with an electrondonating group(Me,t-Bu)at the para-position of the phenyl rings,are excellent substrates,which could react with 2a to complete the reactions, affording the desired products (3b -3c) in 88%-89%yields.It was found that styrenes with an electron-withdrawing group(F,Cl,Br,CO2Me,CF3,NO2or CN)at the para-position of the benzene rings, generating the corresponding products (3d -3j) in 87%-93% yields.When styrenes containing a Me, F or Br at the ortho-position of the aromatic rings were employed, the desired products (3k -3m) were achieved in 73%-83% yields, indicating steric effect.Meanwhile, styrenes with a F, Cl or Br at the metaposition of the phenyl rings reacted with triiodomethane to afford the products (3n -3p) in 85%-88% yields.The employment of 2-vinylnaphthalene and 2-vinylthiophene afforded the desired products (3q and r) in 95% and 96% yield, respectively.When aliphatic alkenes,such as cyclohexene and 1-octene were used,no desired products (3 s and 3t) were obtained.
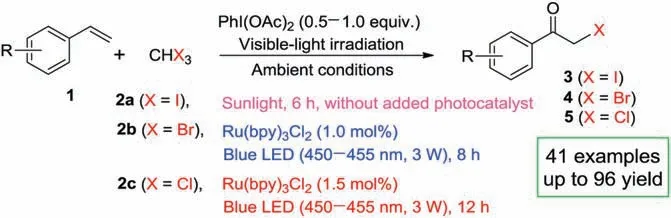
Scheme 1.Synthesis of α-bromo/iodo/chloroketones from olefins under visiblelight irradiation conditions.
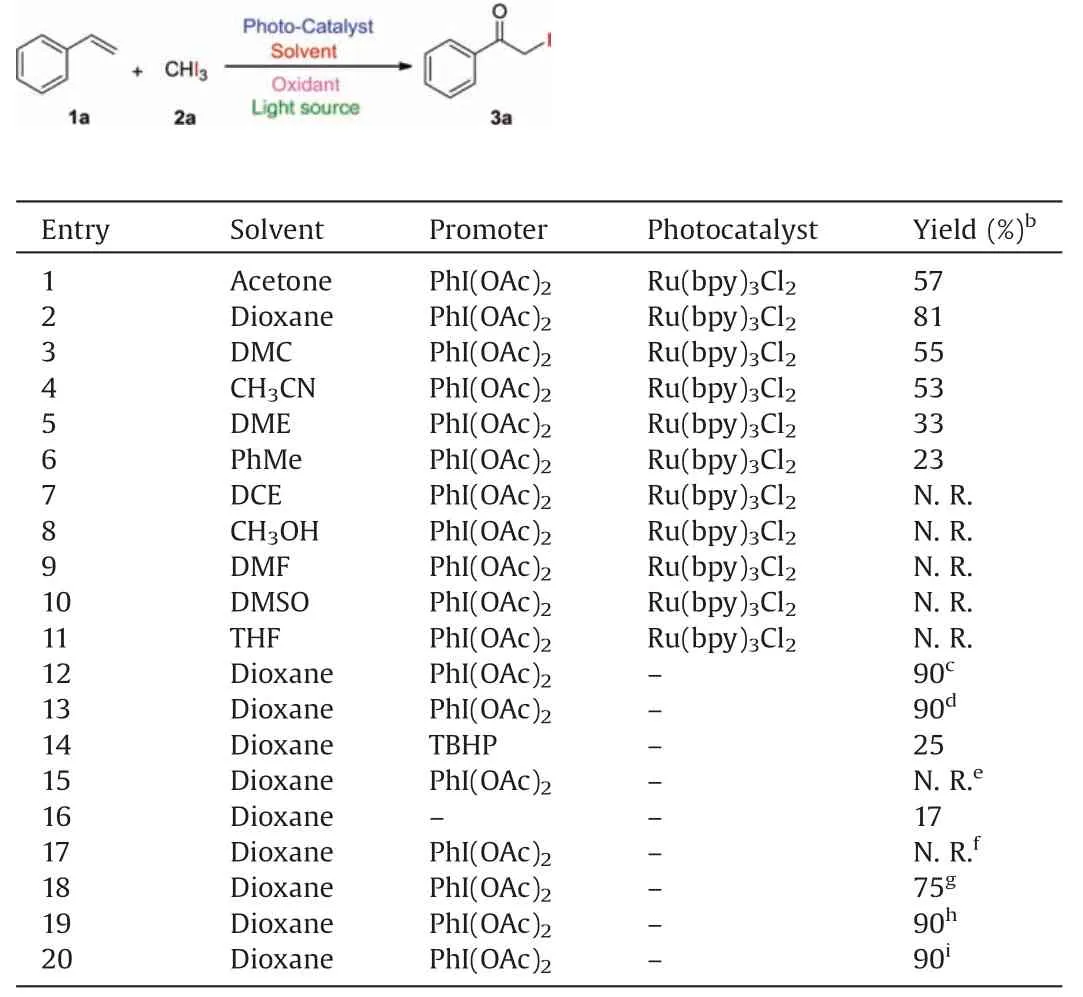
Table 1 Optimization of the reaction conditions.a
Then,the scope of α-bromoketones synthesis from olefins was evaluated using this visible-light induced protocol.As can be seen from the Scheme 3, a variety of styrenes (1) including styrene, 4-methylstyrene,4-fluorostyrene,4-chlorostyrene,4-bromostyrene,3-chlorostyrene and 3-bromostyrene underwent the reaction with tribromomethane to generate the corresponding products(4a -4h)in good yields.It important to note that the reactions performed in the presence of Ru(bpy)3Cl2under blue LED (450-455 nm)irradiation are more efficient than that carried out in the absence of Ru(bpy)3Cl2under sunlight irradiation.
In order to extend the scope of α-chloroketones preparation from olefins, the model reaction of styrene (1a) with trichloromethane(2c)under the reaction conditions in the absence of any additional photocatalyst, no desired product was detected.Interestingly, when Ru(bpy)3Cl2(1.0 mol%) as a photocatalyst was added to the reaction, and the desired product (5a) was isolated in 80%yield,as shown in Scheme 4.When the synthesis of α-chloroketones was performed in the presence of Ru(bpy)3Cl2(1.5 mol%) as a photocatalyst and PhI(OAc)2(1.0 equiv.) under ambient conditions with blue LED (450-455 nm, 3 W) irradiation for 12 h,the corresponding products 2-chloro-1-arylethanones(5b -5o) were obtained in 54%-86% yields (Scheme 4).It should be noted that styrenes bearing electron-rich groups such as Me and t-Bu or electron-poor groups including Cl, Br, CO2Me, CF3, NO2, CN and F on the para-, meta-or ortho-position of the benzene rings reacted with trichloromethane to afford the according products(5b -5n)in satisfactory yields.The reaction of 2-vinylnaphthalene also generated the corresponding product (5o) in 86% yield.
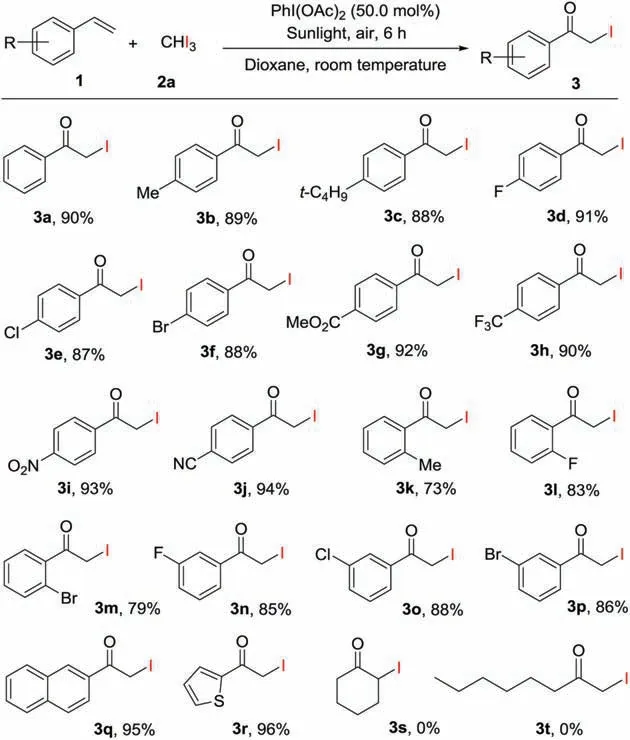
Scheme 2.Preparation of α-iodoketones from olefins.Reaction conditions:styrene(1, 0.20 mmol), triiodomethane (2a, 0.20 mmol), dioxane (2.0 mL), PhI(OAc)2(50.0 mol%)at room temperature under sunlight irradiation in air for 6 h; isolated yields of the products.
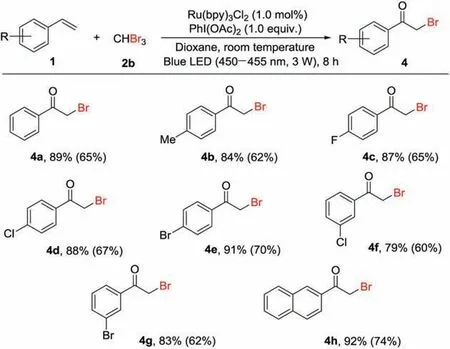
Scheme 3.Preparation of α-bromoketones from olefins.Reaction conditions:styrene(1, 0.20 mmol),tribromomethane(2b, 0.20 mmol),Ru(bpy)3Cl2(1.0 mol%),PhI(OAc)2(1.0 equiv.),dioxane(2.0 mL)at room temperature under blue LED (450-455 nm,1.5 W)irradiation in air for 8 h;isolated yields of the products.Yields in the brackets were from the reaction in the absence of Ru(bpy)3Cl2 under sunlight irradiation in air for 12 h.
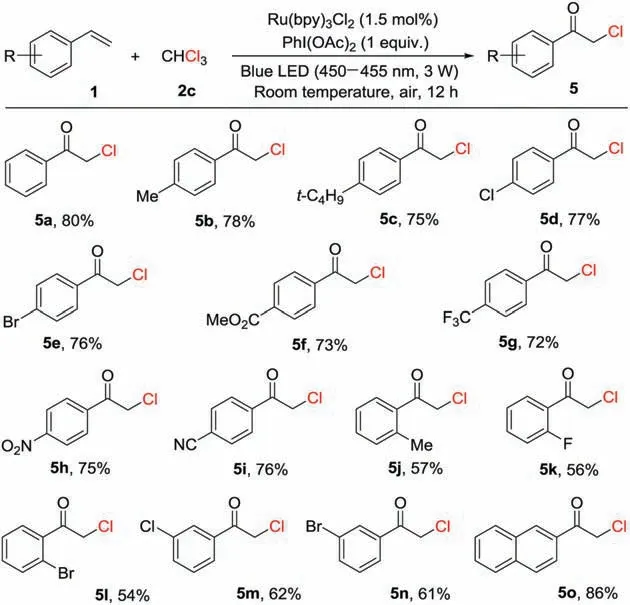
Scheme 4.Preparation of α-chloroketones from olefins.Reaction conditions:styrene (1, 0.20 mmol), trichloromethane (2c, 2.0 mL), Ru(bpy)3Cl2 (1.5 mol%),PhI(OAc)2 (1.0 equiv.) at room temperature under blue LED (450-455 nm, 1.5 W)irradiation in air for 12 h; isolated yields of the products.
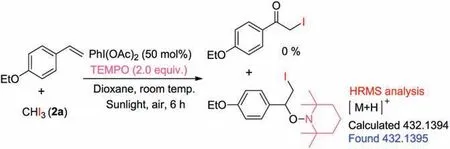
Scheme 5.Control experiment.
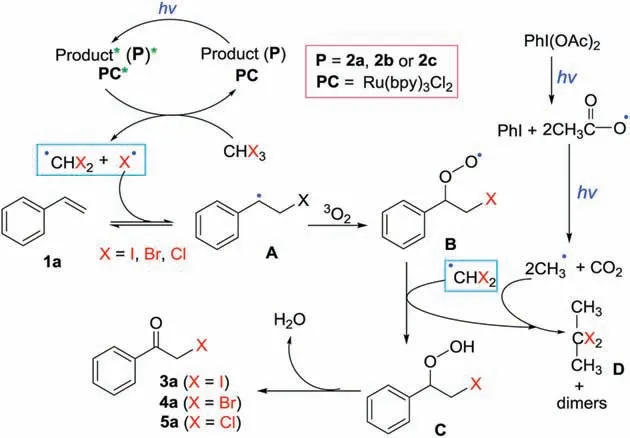
Scheme 6.The proposed mechanism.
To gain insight into the reaction mechanism, a control experiment was conducted, as shown in Scheme 5.The reaction of 1-ethoxy-4-vinylbenzene with 2a was completely inhibited when a radical scavenger, 2,2,6,6-tetramethyl-1-piperidinyloxy(TEMPO)was added to the reaction,along with the formation of a TEMPO adduct(Scheme 5),which was detected by HRMS analysis(Fig.S2 in Supporting Information).The result indicated a radical process was involved in the reaction.
On the basis of above investigation on the control experiment and related papers [47], a possible reaction mechanism is proposed, as in Scheme 6.Photocatalyst (PC) or product αiodoketone(P),which has more efficient absorbance in the range of visible light wavelength than that of α-bromoiodoketone and α-chloroiodoketone(Fig.S1 in Supporting Information),is excited by visible-light irradiation to generate the excited-stateof(PC)*or P*.The formed (PC)* or P* then undergoes an energy transfer process with reagents 2a/2b/2c, generating the corresponding(CHX2)·and X·radicals via a hemolytic cleavage of C -X bond(reaction order:C -I >C-Br >C-Cl).The obtained X·undergoes an addition to styrene 1a to afford a radical intermediate A,which subsequently reacts with3O2to result in the formation of an intermediates B.The formed B abstracts an hydrogen radical form(CHX2)·to generate an intermediate C,which loses H2O to provide the desired product 3a/3a/5a.On the other hand, PhI(OAc)2as a promoter undergoes a homolysis to generate PhI and acetoxy radical (CH3CO2·), which proceeds an decarboxylation to methyl radical and carbon dioxide under light irriadiation [48].The obtained methyl radical reacts with(CHX2)·, and undergoes further reaction with B and itself to give the more stable compound D along with the formation of dimers.
In summary, we have developed a practical synthesis of αbromo/iodo/chloroketones from the corresponding olefins under visible-light irradiation conditions.In the presence of PhI(OAc)2,the reactions of styrenes and triiodomethane undergo to deliver the desired α-iodoketones without additional photocatalyst in high yields under sunlight irradiation and ambient conditions.In addition, the reactions of styrenes with tribromomethane and trichloromethane generate the corresponding α-bromoketones and α-chloroketones in good yields using Ru(bpy)3Cl2as a photocatalyst under blue LED irradiation.Further studies on mechanism are currently underway.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We gratefully acknowledge the National Natural Science Foundation of China (No.21772062) for financial support.
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.02.022.
杂志排行
Chinese Chemical Letters的其它文章
- Diverse synthesis of the C ring fragment of bryostatins via Zn/Cu-promoted conjugate addition of α-hydroxy iodide with enone
- Directly conversion the biomass-waste to Si/C composite anode materials for advanced lithium ion batteries
- Mechanism and selectivity of copper-catalyzed borocyanation of 1-aryl-1,3-butadienes: A computational study
- Recent advances in the improvement of g-C3N4 based photocatalytic materials
- In-situ electro-deposition synthesis of MnOx-NiCo2O4 monolithic catalyst with rich phase interfaces
- Aconapelsulfonines A and B, seco C20-diterpenoid alkaloids deriving via Criegee rearrangements of napelline skeleton from Aconitum carmichaelii
