In-situ electro-deposition synthesis of MnOx-NiCo2O4 monolithic catalyst with rich phase interfaces
2021-04-02DongdongWangYingchaoDuXiaozeWangZhaxiCuoYunfaChen
Dongdong Wang,Yingchao Du,Xiaoze Wang,Zhaxi Cuo*,Yunfa Chen,d,*
a State Key Laboratory of Multi-Phase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
b University of Chinese Academy of Sciences, Beijing 100049, China
c Key Laboratory of Resource Chemistry and Ecoenviromental Protection in Tibetan Plateau, (Qinghai Nationalities University), State Ethnic Affairs Commission, School of Chemistry and Chemical Engineering, Xining 810007, China
d CAS Center for Excellence in Urban Atmospheric Environment, Xiamen 361021, China
ABSTRACTA hierarchically structured MnOx-NiCo2O4 monolithic catalyst with rich phase interfaces was designed by a simple, eco-friendly and time-saving in-situ electro-deposition method.The abundance of active oxygen species due to this rich phase interfaces contributed to the excellent benzene combustion performance of MnOx-NiCo2O4-2:2 sample,oxidizing about 90%of benzene(T90)at 198°C under 12000 h-1 gaseous hourly space velocity.This work shed new light on the design of excellent monolithic catalysts, which might pave the way for the industrialization of benzene combustion.
Keywords:Rich phase interfaces In-situ electro-deposition Active oxygen species Benzene combustion MnOx-NiCo2O4
In order to remove industrial volatile organic compounds(VOCs) more effectively, economically and practically, it is undergoing a transformation from traditional powder catalysts to monolithic catalysts with lower pressure drop and lower active phase loading mass [1-4].Aiming at increasing the activity and service life of monolithic catalysts by generating a stronger interphase anchorage force between the active phase and the support, it is an inevitable trend that in-situ growth strategy will replace the indirect synthesis strategy of combining the powder catalyst with support by coating or impregnating[4-6].However,there is still no significant progress in industrial application of monolithic catalysts,which is mainly due to the non-universal insitu synthesis strategy to limit the diversity of monolithic catalysts.Electro-deposition is a relatively inexpensive and rapid inorganic solid-state synthesis method that allows the deposition of metal layers on conductive substrates with complex structures.The salient features of electro-deposition are mainly reflected in the following aspects: electro-deposition process can be performed near room temperature from water-based electrolytes;regulation of product component can be realized by changing the electrolyte ratio or performing multistep electro-deposition;products can be scaled down to the deposition of a few atoms or up to large dimensions; kinetic parameters can be easily regulated by changing current density and thermodynamic parameters can be easily regulated by changing electrode potential[7,8].Therefore,in-situ electro-deposition strategy might shed new light on the enrichment of monolithic catalysts variety for the complete removal of VOCs.
The importance of active oxygen species, which are directly involved in VOCs combustion reactions, has been well demonstrated in previous work[9].At present,the widely used methods to enhance active oxygen species are to create intrinsic defects by regulating synthesis process or introducing doping elements.In fact, phase interfaces with higher energy states often produce abundant defects that can also induce a significant number of active oxygen species nearby[10].Therefore,it is an effective and feasible way to produce active oxygen species by preparing multiphase structure materials with abundant phase interfaces.
Herein,a series of MnOx-NiCo2O4monolithic catalysts with rich phase interfaces were designed and synthesized by a two-step insitu electro-deposition method.The special multistage structure,lamellar nickel-cobalt spinel phase grows vertically on the surface of manganese oxide which uniformly coated three-dimensional nickel foam skeleton, ensures the formation and full exposure of the phase interface.As expected, MnOx-NiCo2O4-2:2 monolithic catalyst reported in this work can completely transform 90%benzene at 198°C under 12000 h-1gaseous hourly space velocity.

Fig.1.SEM images of(a)MnOx-NiCo2O4-4:0,(b)MnOx-NiCo2O4-3:1,(c)MnOx-NiCo2O4-2:2,(d)MnOx-NiCo2O4-1:3 and(e)MnOx-NiCo2O4-0:4.(g)Element distribution of MnOx-NiCo2O4-2:2 sample.(f) N2 adsorption-desorption isotherm and (h) pore size distribution of these monolithic catalysts, respectively.
In the three-electrode system of this experiment, nickel foam was directly selected as the working electrode, and counter electrode and reference electrode were platinum sheet and saturated calomel electrode (SCE), respectively.The electrodeposition process lasts two minutes and involves two successive steps: anodic electro-deposition with manganese acetate as electrolyte and cathode electro-deposition with cobalt nitrate and nickel nitrate mixture (Co(NO3)2:Ni(NO3)2=2:1) as electrolyte.The loading ratio of manganese oxide and nickel-cobalt spinel was adjusted by changing the reaction time of the two electrodeposition steps, a series of catalysts were thus synthesized and named as MnOx-NiCo2O4-m:4-m (m=0, 1, 2, 3, 4).At last, all samples were calcined at 573 K for 4 h.
The powder X-ray diffraction (XRD) data used to define the catalyst phase were obtained by Panalytical X'Pert PRO MPD.Scanning electron microscopy (SEM, JEOL JSM-6700 F) with an energy dispersive X-ray (EDX) spectroscopy can not only analyze the microscopic morphology of the catalysts, but also calculate the loading ratio of manganese oxide and nickel cobalt spinel phases in different samples.The specific surface area and pore size distribution of the monolithic catalysts were analyzed on the Quantachrome NOVA 3200e equipment by using Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) methods,respectively.Raman spectra of all catalysts were collected by Renishaw inVia-Reflex Raman Spectrometer.To evaluate the redox activity of the monolithic catalysts,H2temperature-programmedreduction (H2-TPR) tests were performed on Quantachrome chemBET pulsar TPR/TPD chemisorption analyzer.The details of catalysts evaluation are in Supporting information.
Figs.1a-e show the microscopic morphology of the catalysts,and the huge difference in morphology between these samples indicates that the loading ratio of manganese oxide and nickelcobalt spinel does affect the structure of monolithic catalysts.The monolithic catalyst of single manganese oxide (Fig.1a) shows crisscrossing small worm-like projections, however, the monolithic catalyst of single nickel-cobalt spinel (Fig.1e) appears an irregular grid structure constructed by a series of nearly perpendicularly oriented lamellae, which greatly expands its specific surface area and gas channel.Hence, in the specific surface area and pore size distribution results shown in Figs.1f and h and Table 1, single nickel-cobalt spinel sample possesses larger hysteresis area and wider pore size distribution than single manganese oxide sample.As shown in Fig.1b,MnOx-NiCo2O4-3:1 inherits the small particle size of manganese oxide and the growth orientation of nickel-cobalt spinel, exhibits a vertically orientedlamellar structure with inadequate growth and dense distribution,and its specific surface area has been improved to some extent.For MnOx-NiCo2O4-2:2 sample(Fig.1c),lamellae are well developed in vertical direction and the spaces between the lamellae are abundant.Naturally, it has the largest specific surface area and the widest pore distribution of these monolithic catalysts,moreover, its specific surface area of the active phase component is up to 201.5 m2/g.According to Fig.1d, the growth direction of MnOx-NiCo2O4-1:3 sample is chaotic, which is manifested as three-dimensional disordered stacking of lamellae structures.The SEM element distribution mapping of MnOx-NiCo2O4-2:2 sample is shown in Fig.1g, the uniform distribution of the four elements indicates that nickel-cobalt spinel grows indiscriminatingly on the surface of manganese oxide.In order to evaluate the actual element mole ratios of these multi-stage catalysts,ICP results were shown in Table S1 (Supporting information).The actual ratio of manganese and cobalt is basically the same as the theoretical value, while the higher nickel content may be due to the partial stripping of the carrier (nickel foam).

Table 1 Active phase loading ratio, physical characterization and catalytic performance of as-prepared samples.
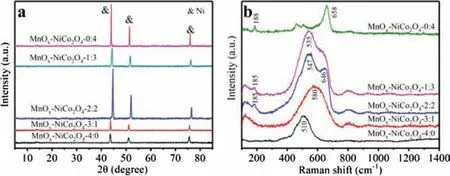
Fig.2.(a) XRD patterns and (b) Raman spectra of all these samples.
The XRD patterns of these monolithic catalysts were shown in Fig.2.Unfortunately,due to the extremely low loading of the active phase (5% ± 0.2%), only the diffraction peaks of nickel foam were detected in the XRD patterns.According to the previous report(PDF 01-070-0989),diffraction peaks at 44.6°,52.0°and 76.6°can be attributed to (111), (200) and (220) crystal faces of metallic nickel, respectively [11].Raman spectrum is also a powerful tool for determining the phase structure on the premise of defining elements, especially some metallic oxides with low crystallinity,therefore, Raman spectra of these monolithic catalysts were detected and shown in Fig.2 [12-14].The characteristic peaks of spinel structures were detected in single nickel-cobalt spinel sample, 658 cm-1peak can be attributed to cobalt-oxygen bond vibrations at octahedron sites (CoO6) and 188 cm-1peak can be attributed to cobalt-oxygen bond vibrations at tetrahedron sites(CoO4) [15].The monolithic catalyst of single manganese oxide shows unique characteristic peak at 510 cm-1, which is the deformation modes of Mn-O-Mn chains in the MnO2phase [16].For MnOx-NiCo2O4-3:1 catalyst, a wide peak which can be attributed to the stretching vibration of Mn-O bond appeared at 580 cm-1, and no characteristic peak of nickel-cobalt spinel was detected,which indicated that its growth was not sufficient,which was consistent with SEM results [17,18].Of course, both manganese oxide and nickel-cobalt spinel characteristic peaks were detected in MnOx-NiCo2O4-2:2 and MnOx-NiCo2O4-1:3 samples.It is worth mentioning that the nickel-cobalt spinel characteristic peak of MnOx-NiCo2O4-2:2 catalyst has an obvious red shift,indicating that the cobalt-oxygen bond weakens, which is very conducive to the transformation of cobalt ions between bivalent and trivalent, thus accelerating the catalytic oxidation process.
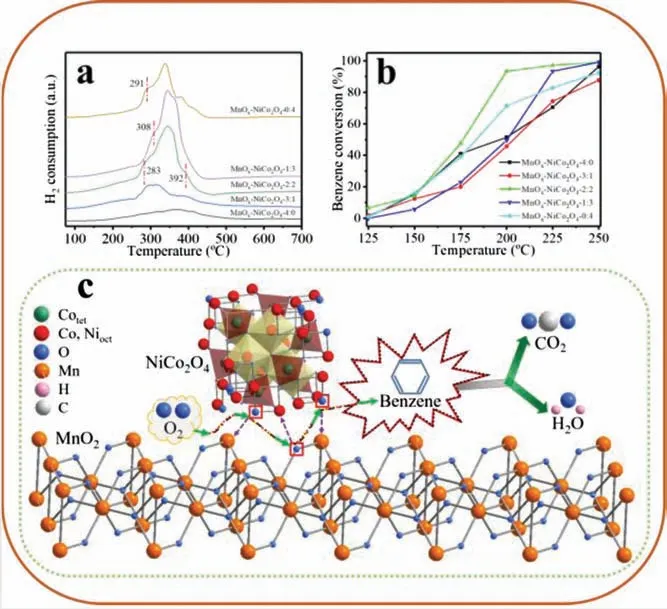
Fig.3.(a) H2-TPR patterns, (b) benzene conversions over prepared catalysts at 12,000 h-1.(c) Schematic diagram of reaction mechanism.
Fig.3a exhibits the H2-TPR patterns of these monolithic catalysts, which may clarify the role of manganese oxide and nickel-cobalt spinel loading ratio for catalysts reducibility.The oxidation of hydrogen continued in single manganese oxide monolithic catalyst over a wide temperature range,which reflects a low crystallinity and/or rich defects characteristic of MnOxactive phase.However,single nickel-cobalt spinel monolithic catalyst has two characteristic peaks with an area ratio of approximately 1:3,which also conforms to the reduction process of spinel material,namely the phase transition from NiCo2O4to NiCoO2, and from NiCoO2to metal phase respectively [19].It is worth mentioning that MnOx-NiCo2O4-2:2 sample shows the first characteristic peak at the lowest temperature of 283°C,which can be attributed to the proper ratio of manganese oxide and nickel-cobalt spinel phase resulting in a rich phase interface.In Fig.3b,100 ppm benzene was selected to evaluate the VOCs removal capability of the monolithic catalyst at a gaseous hourly space velocity (GHSV) of 12,000 h-1.The benzene catalytic activity of the prepared catalysts is basically consistent with H2-TPR reducibility.MnOx-NiCo2O4-2:2 sample completely catalyzed 50% and 90% of benzene to H2O and CO2at 176°C and 198°C, respectively.As can be seen from Fig.S2(Supporting information), MnOx-NiCo2O4-2:2 sample possesses excellent long-term stability,degradation ratio of benzene was still as high as 95%after 48 h test.The carbon balance of all the samples was greater than 96% during the reaction above T90temperature,indicating that the by-products could be ignored.A detailed analysis of the reactivity sequence of MnOx-NiCo2O4catalysts for T10, T50and T90were exhibited in Supporting information.Furthermore,we have calculated the reaction rate of each sample at 150°C (benzene conversion of all the samples is between 5%-20%),198°C(T90of MnOx-NiCo2O4-2:2 sample)and drawn the Arrhenius plots for the oxidation of benzene over these catalysts,all these results were presented in Table S1 and Fig.1.In combination with the structural analysis and catalytic performance results, a schematic diagram of benzene reaction mechanism with the participation of MnOx-NiCo2O4monolithic catalyst is presented in Fig.3c.It is well known that active oxygen species play an important role in thermocatalytic VOCs removal, and its content directly affects the reaction rate of benzene combustion[9,20].In addition,compared with bulk phase,the phase interface is often in a higher energy state due to abundant structural defects and interphase forces,thus generating active oxygen species with a higher probability.In order to deepening the understanding and demonstration of these defects,X-ray photoelectron spectra(XPS)and electron paramagnetic resonance(EPR)was tested and shown in Figs.S3 and S4 (Supporting information).MnOx-NiCo2O4-2:2 sample indeed possesses a symmetrical peak at g=2.01,however,other samples cannot detect this characteristic peak of oxygen radical,so it indicated that the rich phase interfaces can escalatory oxygen vacancies.Therefore,benzene combustion reaction occurs mainly at the interface of manganese oxide and nickel-cobalt spinel,a complete redox cycle consists of the following two steps:lattice oxygen bonds at the phase interface form active oxygen species and leave oxygen vacancies, active oxygen species participate in benzene combustion reaction to produce carbon dioxide and water;oxygen molecules occupy the oxygen vacancies left,and the lattice oxygen at the phase interface is re-formed[21].Hence,MnOx-NiCo2O4-2:2 sample possesses lowest T90of benzene combustion due to the presence of a vertically oriented spinel phase that allowed the phase interface to be fully exposed.This also explains the phenomenon that dense-growing MnOx-NiCo2O4-3:1 sample and three-dimensional disorderly stacked MnOx-NiCo2O4-1:3 sample exhibited lower benzene catalytic activity than the single-phase monolithic catalysts due to the lack of exposed phase interfaces.
In this work,a series of MnOx-NiCo2O4monolithic catalysts with great morphological differences have been prepared by using a twostep electro-deposition strategy and felicitously adjusting the loading ratio of manganese oxide and nickel-cobalt spinel.The manganese oxide phase uniformly covering the nickel foam surface andthenickel-cobaltspinelphasegrowingalmostverticallyresulted in an abundant phase interfaces of MnOx-NiCo2O4-2:2 sample.This richphaseinterfaceproducedabundantactiveoxygenspecieswhich is essential for benzene combustion.Therefore,MnOx-NiCo2O4-2:2 monolithic catalyst possesses an excellent catalytic activity, and completely oxidizing 90% of benzene at 198°C under a gaseous hourly space velocity of 12000 h-1.In conclusion, we have successfully prepared MnOx-NiCo2O4-NF monolithic catalysts with abundant phase interfaces through a simple electro-deposition method, which not only provides a feasible path for designing materials by electro-deposition but also enriches the in-situ synthesis strategy of monolithic catalysts.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This research described above was financially supported by National Key R&D Program of China (Nos.2017YFC0211503,2016YFC0207100),the NationalNaturalScienceFoundationofChina(Nos.21401200,51672273)and the Open Research Fund of State Key LaboratoryofMulti-phaseComplex Systems(No.MPCS-2017-D-06).
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.11.012.
杂志排行
Chinese Chemical Letters的其它文章
- A biomass based photonic crystal made of “konjac tofu”
- Hydrothermal-assisted grinding route for WS2 quantum dots (QDs)from nanosheets with preferable tribological performance
- Superiority of poly(L-lactic acid) microspheres as dermal fillers
- Zwitterionic comb-like lipid polymers encapsulating linalool for increasing the fragrance retention time
- Construction of a nano-rectangular Zn-Nd complex with near-infrared luminescent response towards metal ions
- Synthesis and structure of Au19Ag4(S-Adm)15 nanocluster:Polymorphs and optical properties
