BF3-promoted annulation of azonaphthalenes and ynamides for synthesis of benzo[e]indoles
2021-04-02ChangweiChenSunliangCui
Changwei Chen,Sunliang Cui*
College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China
ABSTRACT A novel BF3-promoted [3+2] annulation of azonaphthalenes and ynamides is described.This protocol provides a modular and efficient entry to functionalized amino benzo[e]indole derivatives smoothly.
Keywords:Azonaphthalenes Ynamides Annulation Benzo[e]indoles
Indole is a privileged structural motif of nitrogen containing heterocycles, and widely exists in many bioactive molecules like pharmaceuticals and agrochemicals[1-3].Among them,1-aminoindole derivatives exhibit important pharmacological properties,such as 5-HT2antagonists [4a], antioxidants [4b], diuretic agents[4c,d], ALDH1A1 inhibitors [4e -g], and potential therapeutic reagents for treatment of Alzheimer ’s disease [4h] (Fig.1).However, the synthetic methods are nearly limited to transitionmetal-mediated reactions [5].Therefore, the efficient synthetic method to diverse functionalized 1-aminoindoles has received great attention in organic synthesis and medicinal chemistry.
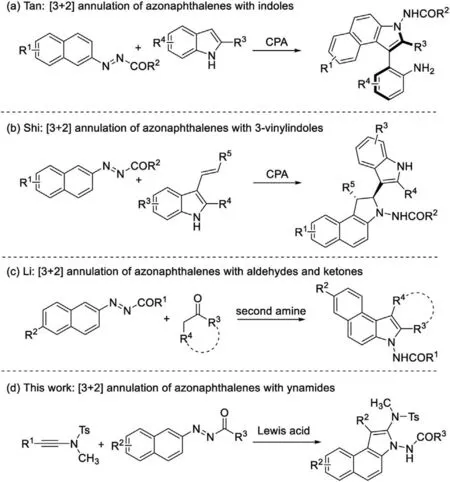
In the course of finding suitable synthons, we particularly focused on the azonaphthalene scaffolds [6].As we all know, azo group has been used as a directing group for many transformations viatransition-metal-catalyzed C--H activation[7].Meanwhile,the azo motif of azonaphthalenes could also work as an electronwithdrawing group to activate the aromatic rings for nucleophilic attack (Scheme 1).For example, Tan and co-workers reported a nice work of chiral phosphoric acid-catalyzed[3+2]annulation of azonaphthalenes with 2,3-disubstituted indoles to construct axially chiral benzo[e]indole derivatives [8] (Scheme 1a).Shi et al.reported another chiral phosphoric acid catalyzed [3+2]annulation of azonaphthalenes with 3-vinylindoles [9](Scheme 1b).Wang also developed a N-heterocyclic carbene catalyzed tandem dearomatization/rearomatization reaction of azonaphthalenes and α-chloroaldehydes for assembly of chiral dihydrocinnolinone derivatives [10].Recently, Li and co-workers have discovered a second-amine mediated [3+2] annulation of azonaphthalenes with aldehydes and ketones for synthesis of indole derivatives [11] (Scheme 1c).Therefore, azonaphthalenes are versatile building blocks for synthesis of nitrogen-containing heterocycles.
In continuation of our research in multicomponent reaction for heterocycle synthesis [12], we envisioned that ynamides might undergo cyclization with azonaphthalenes to deliver interesting heterocycles.Ynamides, a special type of alkynes, exihibit dual nucleophilic and electrophilic properties with carbon-carbon triple bonds attached to the nitrogen atom, which substitutes to electron-withdrawing groups [13].Owing to their unique activities,ynamides have become popular in organic synthesis and have been reported to act as flexible synthons in cyclization by Hsung,Liu,Ye,Huang and other groups[14].Our group also reported that ynamides could be used as C2 building blocks in cyclization with nitrile oxides [12f].Herein, we would like to report a [3+2]annulation of azonaphthalenes and ynamides for modular and efficient entry to structurally divergent N-substituted benzo[e]indoles (Scheme 1d).
We commenced our study by investigating ynamide (1a) and azonaphthalene(2a)in the presence of 4 Å molecular sieve(MS)in DCM under argon, and BF3-Et2O (20 mol%) was used as catalyst(Table 1).However, no new product was observed when the reaction was performed at ambient temperature (entry 1).To our delight, a new product 3a was detected and isolated in 36% yield while lowering the temperature to -20°C(entry 2).The standard characterization including1H NMR,13C NMR and mass spectroscopy identified 3a as a benzo[e]indole compound.And we further confirmed the structure of 3a by single-crystal X-ray diffraction analysis(CCDC:1987024)(Fig.2).For its structural details,see the Supporting Information.In addition, various Lewis acids such as CPA,AgOTf,Sc(OTf)3,Cu(OTf)2,Zn(OTf)2and FeCl3were also tested instead of BF3-Et2O.These reactions were carried out in DCE at 80°C, but they were all found ineffective (entries 3-8), and only Sc(OTf)3and Zn(OTf)2could lead to trace amount of 3a.Gratifyingly,decreasing the temperature to -78°C could facilitate the reaction to improve the yield to 60% (entry 9).When the amount of BF3-Et2O was increased to 1 equiv.,the yield of 3a could be improved to 65% (entry 10), while using 2 equiv.of BF3-Et2O could further improve the yield to 90%(entry 11).The screening of solvents showed that toluene and CH3CN were also effective,albeit with slightly lower yields (80% and 84%, entries 12 and 13).
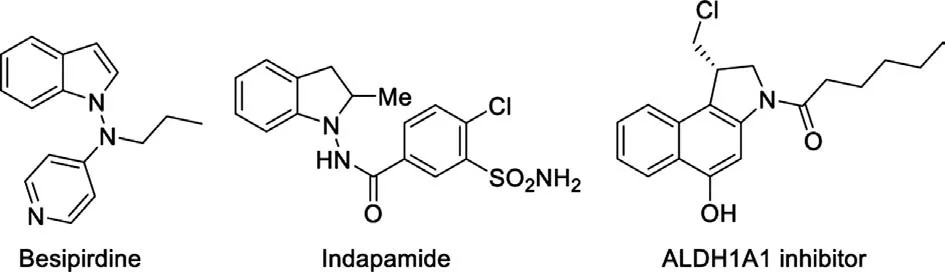
Fig.1.Selected bioactive molecules containing 1-aminoindole unit.
Scheme 1.Annulation of azonaphthalene derivative.
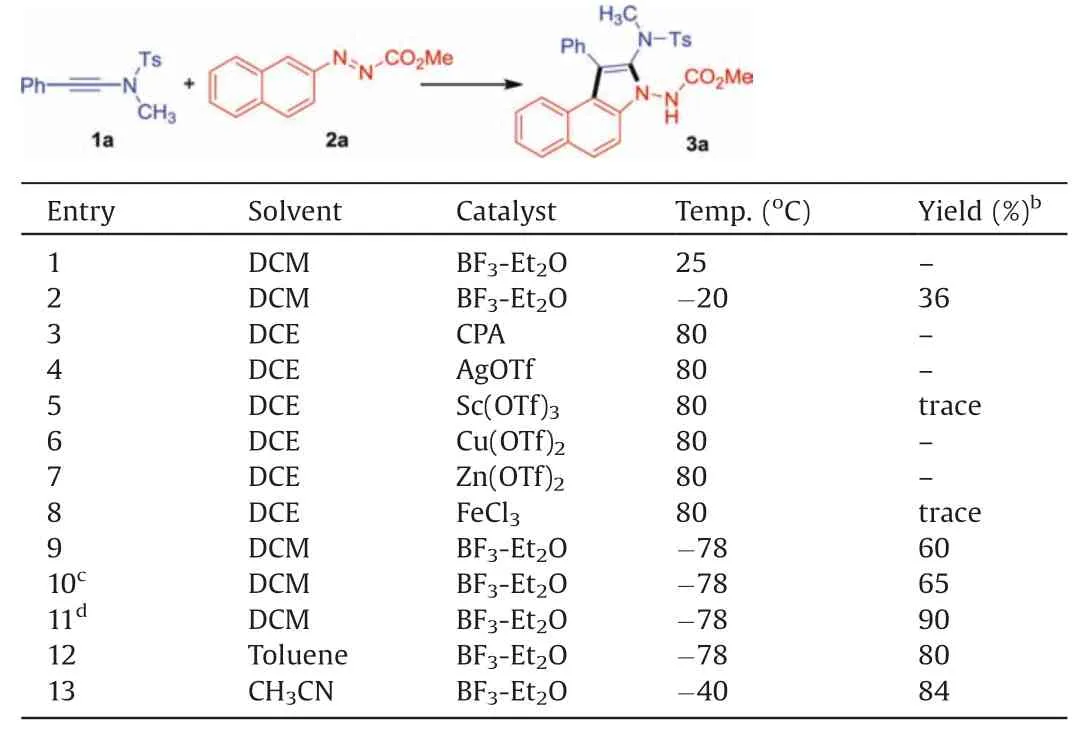
Table 1 Optimization of reaction conditions.a

Fig.2.Single crystal X-ray diffraction of 3a.
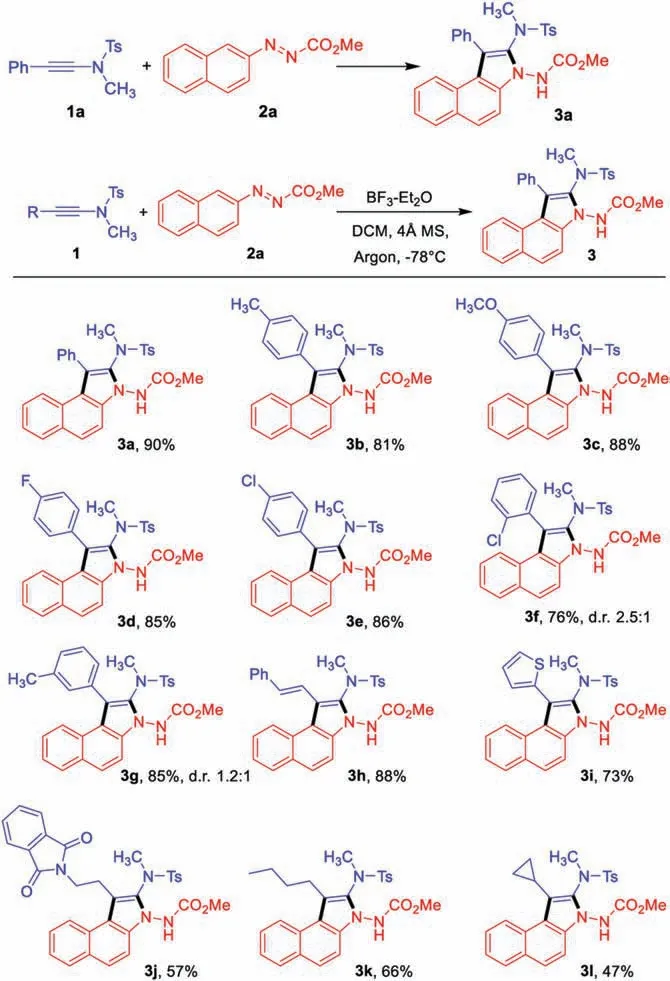
Scheme 2.Substrate scope of ynamides.Reaction conditions: 1 (0.3 mmol),(0.2 mmol),DCM(2 mL),4 Å MS(50 mg),argon,then BF3-Et2O(2.0 equiv.), -78°C,2 h.Yield refers to isolated product.For experimental procedure and characterization data of products, see the Supporting information.
With the optimal reaction conditions in hand, we next examined the substrate scope of ynamides.As shown in Scheme 2,ynamides with electron-donating groups on the para-position of the aromatic ring could well engage in this process with azonaphthalene 2a to deliver the corresponding products in excellent yields,such as methyl and methoxy(3b and 3c).On the other hand, ynamides with electron-withdrawing groups like fluoro and chloro,were also tolerated to give the desired products 3d and 3e.In addition, ortho-and meta-substituents were applicable as well and did not show significant difference in yields compared to para-substituents (3f and 3 g).It should be noted that the reaction of o-chloro and m-methyl substitution at the phenyl ring of the ynamides furnished the products with dr values ranging from 2.5:1 to 1.2:1, probably due to the steric hindrance of the phenyl ring with the naphthalene ring.Moreover,the styrene containing ynamide was also compatible in this protocol to deliver 3 h.Heterocyclic ynamide could also participate well in this process to afford 3i in good yield.Furthermore, when alkyl-substituted ynamides were employed,including N-protected amine moiety, butyl and cyclopropyl, the products could be obtained in moderate yields (3j-3l).
Furthermore, the generality of azonaphthalenes were also investigated.As shown in Scheme 3, a variety of azonaphthalene derivatives were amenable to this [3+2] annulation.When substituents R ′ were alkoxyl groups, the corresponding products could be obtained in high yields (4a-4c).The reaction was applicable to azonaphthalene with benzoyl group to afford 4d in moderate yields.The electronic nature and the substituents of the aromatic rings did not show significant effects on the reactivity(4f-4 h), except for the bromo-substituted azonaphthalene with slightly lower yield (4e).
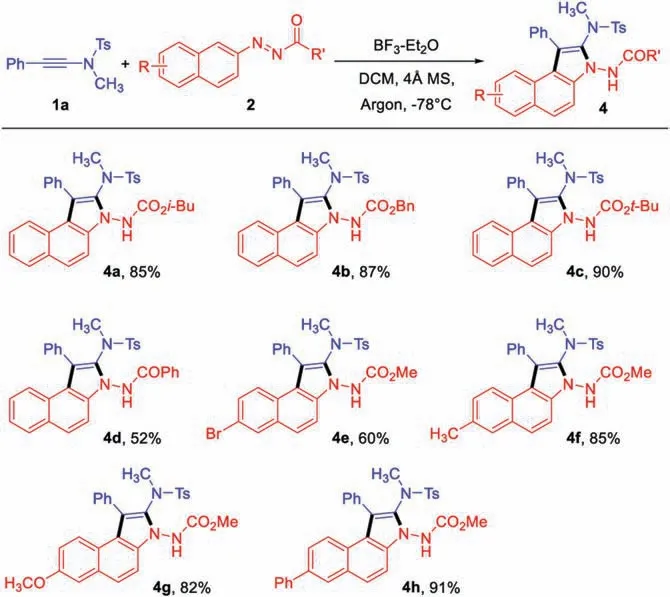
Scheme 3.Substrate scope of azonaphthalenes.Reaction conditions: 1a(0.3 mmol), 2 (0.2 mmol), DCM (2 mL), 4 Å MS (50 mg), argon, then BF-Et2O (2.0 equiv.), -78°C,2 h.Yield refers to isolated product.For experimental procedure and characterization data of products, see Supporting information.
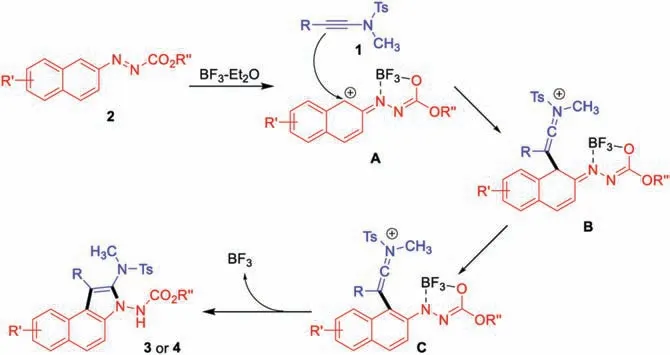
Scheme 4.Proposed mechanism.
On the basis of these results and literatures[8,11,12],a plausible reaction mechanism was proposed in Scheme 4.Initially, the azonaphthalene 2 could be activated by BF3-Et2O to deliver species A,which is added by electron rich ynamides to give intermediate B.The following isomerization furnishs the annulation products 3 or 4.And the released BF3might coordinate with the amino group and equivalent amount of BF3-Et2O is necessary.
In conclusion, a novel [3+2] annulation of azonaphthalenes and ynamides has been developed to establish N-substituted benzo[e]indole derivatives in good to excellent yield.This process features a broad substrate scope and high efficiency.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (No.21971222).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.010.
杂志排行
Chinese Chemical Letters的其它文章
- Diverse synthesis of the C ring fragment of bryostatins via Zn/Cu-promoted conjugate addition of α-hydroxy iodide with enone
- Directly conversion the biomass-waste to Si/C composite anode materials for advanced lithium ion batteries
- Mechanism and selectivity of copper-catalyzed borocyanation of 1-aryl-1,3-butadienes: A computational study
- Recent advances in the improvement of g-C3N4 based photocatalytic materials
- In-situ electro-deposition synthesis of MnOx-NiCo2O4 monolithic catalyst with rich phase interfaces
- Aconapelsulfonines A and B, seco C20-diterpenoid alkaloids deriving via Criegee rearrangements of napelline skeleton from Aconitum carmichaelii
