Native conjugation between proteins and [60]fullerene derivatives using SpyTag as a reactive handle
2021-04-02GungzhongYinJingjingWeiYuShoWenHoWuLinjieXuWenBinZhng
Gungzhong Yin,Jingjing Wei,Yu Sho,Wen-Ho Wu,Linjie Xu,Wen-Bin Zhng,*
a Beijing National Laboratory for Molecular Sciences, Key Laboratory of Polymer Chemistry and Physics of the Ministry of Education, College of Chemistry and Molecular Engineering, Center for Soft Matter Science and Engineering, Peking University, Beijing 100871, China
b College of Chemical and Environmental Engineering, Anyang Institute of Technology, Anyang 455000, China
ABSTRACT Herein, we report the facile conjugation between proteins and water-soluble [60]fullerene derivatives(DC60)under native conditions using SpyTag as a reactive handle.Water-soluble[60]fullerene derivatives were first prepared via sequential Bingel-Hirsch reaction and“clicked”with SpyTag to give DC60-SpyTag for native conjugation with proteins by the highly efficient SpyTag-SpyCatcher chemistry.The bioconjugation was confirmed by MALDI-TOF MS spectra and SDS-PAGE analysis.The TEM and UVvis spectroscopic study further revealed that the DC60 could alter the optical performance and induce aggregation of the target proteins.It thus provides a general and robust method for modifying proteins with C60 derivatives and could potentially be adapted for native conjugation between proteins and other nonbiological motifs as well.
Keywords:SpyTag SpyCatcher Fullerene Protein Bioconjugate
Bioconjugation [1] plays an essential role in life science and drug development because it provides opportunity to combine the complementary advantages of biomolecules with drugs [2,3],polymers[4-6],nanoparticles[7],and other compounds of distinct composition and function[8].As such, they have become a major component for many biochemical and pharmaceutical applications including protein stabilization[4,9],drug delivery[10,11]and gene therapy [12].To date, most bioconjugates have been produced using only a handful of chemistries that can proceed efficiently and selectively under physiological conditions.The complexity of biomacromolecules imposes stringent requirements on the reaction conditions that it can tolerate.Successful examples include thiol-Michael addition [13], thiol-ene reaction [14], and tyrosinase-mediated bioconjugation[15].The concept of bioorthogonal reactions further expands this toolbox.Such reactions include strain-promoted azide-alkyne cycloaddition [16], Diels-Alder reaction [3,17] and Staudinger reaction [18].Highly reactive foreign functional groups are usually installed on biomolecules by metabolic engineering or site-selective labelling with unnatural monomers, typically with low protein yields, which limits application on a larger scale.
Recently, genetically encoded peptide-protein chemistry, such as SpyTag-SpyCatcher reaction[19,20],has emerged as a new way to do chemistry with proteins within the biological reactivity space[21,22].These reactive pairs are based entirely on natural amino acids and the reaction is compatible with physiological conditions,obviating the need for additional chemical reagents or catalysts[23].Moreover,it is genetically encodable and can be programmed directly into the protein’s sequence.The reactivity is insensitive to the location of reactive sequences in the chain.The highly selective,rapid and irreversible conjugation has made it very attractive for a variety of applications,such as protein topology engineering[24],making nanoparticle conjugates [25], engineering architecturally sophisticated supramolecular catalytic systems [26] and so on.Although this is an ideal reaction for making bioconjugates,there has been only a few reports on its use to conjugate with nonbiological compounds [27].Since SpyTag is a 13-amino acid peptide and can be prepared by solid phase peptide synthesis,we envisioned that it could serve as a bridge for native conjugation between proteins and other synthetic compounds.The introduction of SpyTag shall provide a reactive handle for subsequent conjugation under native conditions.
Fullerenes are fascinating carbon nanostructures[28].Although pristine[60]fullerene(C60)showed potential biological activities,it is essentially insoluble in aqueous media and thus unfavorable for application in biological environments[29].To extend its scope of application, it is important to find water soluble C60derivatives that are readily synthesized and can be easily attached onto biomacromolecules.For example, fullerenols [C60(OH)x] display a large range of nanomedicine applications owing to their enhanced water solubility and various biological effects, including photosensitizing property, anti-oxidative stress, and scavenging of free radicals [30-32].Despite their potential application as antiangiogenic agents, they are difficult to be further functionalized.It has been demonstrated that hydroxyl-or carboxylic acid-functionalized C60with precisely defined structures can serve as a hydrophilic molecular nanoparticle to drive the assembly of the conjugate.In this case,the hydrophilic nature is not revealed until deprotection after conjugation.It would be highly desirable to develop hydrophilic fullerene building blocks for making bioconjugates under native conditions.
In this communication, we report the design, synthesis, and characterization of C60-protein bioconjugates using SpyTag-Spy-Catcher chemistry (Fig.1), which provides a general and robust method for modifying proteins with C60derivatives.It is expected that the C60would strongly affect the self-assembly behaviour of proteins and the optical properties of the bioconjugate.
The synthetic strategy was divided into two steps: (1) the installation of SpyTag onto C60under non-native conditions and(2)the native conjugation between SpyTag-bearing C60derivatives and SpyCatcher-bearing proteins.The first step could be accomplished by using Cu(I)-catalyzed alkyne-azide cycloaddition between a water-soluble, azide or alkyne-functionalized C60and cognate functional SpyTag.The synthesis of the C60derivatives are shown in Scheme S1 (Supporting information).It began with the mono-functionalization of C60by stoichiometry-controlled Bingel-Hirsch reaction to install a TMS-protected alkyne group (compound 2a)or chloro group which can be easily converted to azide functionality(compound 2b).To bring in potential water solubility,multiple protected hydroxyl groups were introduced by an additional step of Bingel-Hirsch reaction to get the [1:5] adducts(compounds 4a and 4b).Then, after functional group conversion and deprotection,two“clickable”C60derivatives were obtained in decent yields (compounds 6a, DC60-alkyne and 6b, DC60-N3).The synthetic procedures and detailed characterizations could be found in supporting information (Figs.S1-S9 in Supporting informaiton).Notably,before deprotection of the hydroxyl groups,the compounds are highly soluble in common organic solvents.The structures of these[60]fullerene derivatives were proven by1H NMR,13C NMR,IR and MALDI-TOF MS spectra.After deprotection,DC60-alkyne and DC60-N3can be easily dissolved in methanol and water.
The “click” reaction between DC60-alkyne and azide endcapped SpyTag (N3-SpyTag) was performed in water with CuSO4/sodium ascorbate as the catalyst at 25°C to give the DC60-SpyTag.The sample was dialyzed to remove the catalyst followed by freeze drying to yield a light brown-red fluffy solid.Notably, two equivalents of DC60-alkyne were added to ensure complete consumption of N3-SpyTag during the click reaction.Excess DC60-alkyne was easily removed by fast protein liquid chromatography (FPLC) during the purification process.The success of click reaction was demonstrated by the complete disappearance of the azide absorption at 2108 cm-1in the FTIR spectrum of the product(Fig.2A).Correspondingly,a series of new peaks emerge and can be clearly assigned to the presence of new functional groups,such as 1741 cm-1for C=O and 1250 cm-1for the-(CO)-O-groups in DC60.The DC60-SpyTag is fully soluble in water,which shall facilitate the attachment of DC60to any SpyCatcher-containing proteins in water using SpyTag-SpyCatcher chemistry.
The reaction between DC60-SpyTag and SpyCatcher was performed in PBS buffer at 25°C as the model native conjugation reaction.After 12 h, the solution was purified by FPLC to remove excess DC60-SpyTag.After desalting,the solution was freeze-dried to yield light brown solid for further characterization.The DC60-SpyTag/SpyCatcher conjugate has much better solubility in water than pure DC60-alkyne.The successful preparation of DC60-SpyTag/SpyCatcher conjugate was convincingly demonstrated in MALDITOF MS spectrum where only one single major distribution was observed at the m/z value of 18547 Da matching well with the calculated mass of 18567 Da for the DC60-SpyTag/SpyCatcher conjugate (Fig.2B).As compared to the mass of the SpyCatcher,there is an apparent shift of molecular weight of ~3916 Da,which is close to the calculated value of 3940 Da for one DC60-SpyTag motif within experimental error.
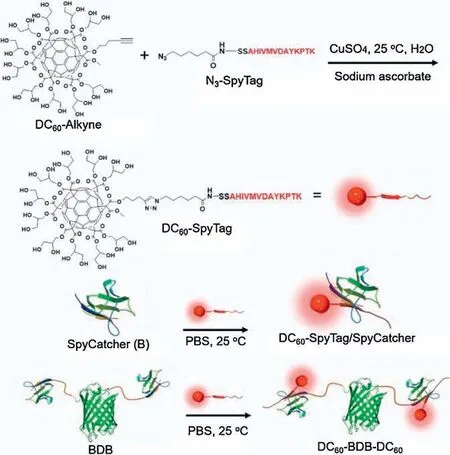
Fig.1.Synthesis of C60-protein bioconjugates by “click” reaction and SpyTag-SpyCatcher chemistry.
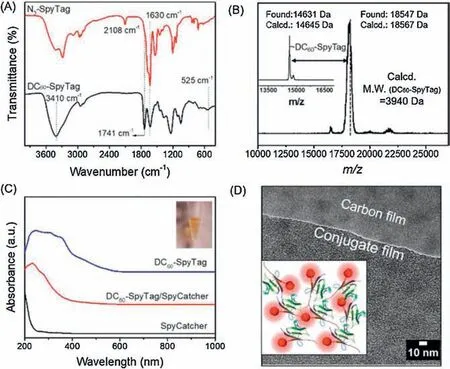
Fig.2.(A)FT-IR spectra of DC60-SpyTag and N3-SpyTag.(B)MALDI-TOF MS spectra of SpyCatcher(inset)and DC60-SpyTag/SpyCatcher conjugate.(C)UV-vis spectra of DC60-SpyTag, DC60-SpyTag/SpyCatcher conjugate (the inset is the solution of the conjugate at the concentration of 1 mg/mL) and SpyCatcher.(D) TEM of DC60-SpyTag/SpyCatcher conjugate with concentration of 10 mg/mL in water.
The UV-vis absorption characteristics of DC60-SpyTag, Spy-Catcher and DC60-SpyTag/SpyCatcher conjugate in water at the same concentration of 1.0 mg/mL were depicted in Fig.2C.It showed typical absorption maxima in UV-vis spectroscopy between 200 nm to 600 nm for DC60-SpyTag.The specific absorbing peak of SpyCatcher was at ~200 nm.The onset of the peak of DC60-SpyTag/SpyCatcher conjugate was calculated to be~400 nm.The image (Fig.2C inset) shows the orange brown solution of DC60-SpyTag/SpyCatcher conjugate purified by FPLC.All these show that DC60moiety imparts the optical properties to the protein.TEM shows the nano-membrane of DC60-SpyTag/Spy-Catcher conjugate with isotropic distribution of DC60dots (dark phase,Fig.2D).More pictures of the morphology could be found in Fig.S10 (Supporting information).There was no obvious ordered phase in the membrane.Hence,we proposed a random molecular distribution to account for the observed morphology as illustrated in the inset of Fig.2D.
To examine the generality of the approach, we designed a bifunctional protein containing multi-domains, namely, Spy-Catcher-ELP-Dronpa-ELP-SpyCatcher (BDB).Elastin-like protein(ELP) is an intrinsically disordered protein [33] and Dronpa is a photo-responsive green fluorescent protein [34].This construct was adapted from BCB in our previous publication in the layer-bylayer assembly of an entirely protein-based thin film [35].By simply mixing BDB with DC60-SpyTag in PBS at room temperature,we introduced the DC60moiety to both ends of the protein and obtained a novel bioconjugate denoted as DC60-BDB-DC60(Fig.1).The successful synthesis of the conjugate was demonstrated directly by its lower mobility and slightly higher position in SDSPAGE image (Fig.3A).The elution profile on liquid chromatogram(LC) was essentially the same as the BDB precursor, indicating relatively minor changes to the overall size of the molecule(Fig.3B).The MALDI-TOF MS spectra further confirm the precise attachment of two DC60motifs(Fig.3C).The molecular weight of the reactant BDB was found at ~70888 Da, which is close to the calculated value of 70,907 Da(taking into consideration the loss of one water molecule and two hydrogen upon chromophore maturation [36]).After successful bi-functionalization, the mass of DC60-BDB-DC60shifted to ~78357 Da in MALDI-TOF MS spectrum, which is close to the expected molecular weight of 78,751 Da within experimental error at this high molecular weight range(≤0.5%).Moreover,TEM were also used to observe the direct phase separation in DC60-BDB-DC60.The freeze-dried samples were dispersed in water by ultrasonication and spotted on Formvar/Carbon 200 mesh grids to prepare the TEM sample.The flexible chains of the ELP give sufficient mobility to the two DC60ends and facilitate their self-aggregation.As shown in Fig.3D,the dark phase is assigned to DC60-rich phase.The average diameter of the dark phase is ~7.95 nm(Fig.3E).The illustration of molecular aggregation is depicted in Fig.3F.We also noticed that once lyophilized, the sample could hardly be dissolved in water again.This could probably be explained by the physical crosslinking as a result of DC60aggregation(Fig.3D inset and Fig.S11 in supporting information).Hence, the introduction of C60into proteins indeed brings in intriguing optical properties and selfassembly behaviors.In addition, according to the literature [37],the DC60could target specific protein structure with implications in functional biomolecular communication.It indicates that the conjugate may enhance or regulate specific protein-protein interactions involving protein/fullerenol recognition.Such conjugates may be useful for making drugs with better cell penetration and antibacterial capability.
In summary, we report a water-soluble C60derivative bearing a reactive SpyTag (DC60-SpyTag) for facile conjugation with proteins.The water solubility was imparted to C60by multiple hydroxyl groups.The SpyTag was installed on C60by CuAAC click chemistry and served as the reactive handle to couple with any SpyCatcher-bearing proteins.The efficient,cell-compatible SpyTag-SpyCatcher reaction enables the native conjugation between nonbiological motifs and protein molecules.The convenient synthesis of water-soluble DC60-SpyTag offers a versatile and efficient reagent for site-specific modification of proteins under physiological conditions.It could help preserve the native functions of a protein during bioconjugation, which is particularly important for those proteins whose structures and functions are sensitive to non-physiological conditions.The number of DC60on the protein could be controlled by the predetermined number of SpyCatcher units in the protein design.We believe that the method offers a general and ready access to multifunctional protein bioconjugates containing exotic nonbiological components including, but not limited to C60.
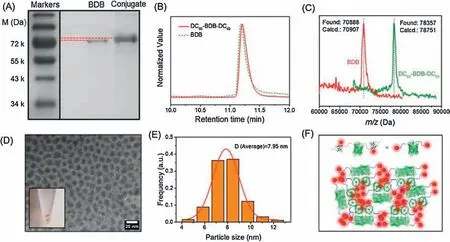
Fig.3.(A)SDS-PAGE analysis of BDB and DC60-BDB-DC60.(B)Liquid chromatogram(reversed-phase column)curves of BDB and DC60-BDB-DC60.(C)MALDI-TOF MS Spectra of BDB and DC60-BDB-DC60.(D)TEM image of DC60-BDB-DC60.(E)Size distribution of DC60 aggregates.(F)Illustration of the aggregated structure of DC60-BDB-DC60 with DC60 domains in red color and protein domains in green color.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China(Nos.21925102,21991132 and 21674003).This work was also supported by Beijing National Laboratory for Molecular Sciences (No.BNLMS-CXXM-202006)and Clinical Medicine Plus X Project of Peking University,Fundamental Research Funds for the Central Universities.
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.04.034.
杂志排行
Chinese Chemical Letters的其它文章
- Diverse synthesis of the C ring fragment of bryostatins via Zn/Cu-promoted conjugate addition of α-hydroxy iodide with enone
- Directly conversion the biomass-waste to Si/C composite anode materials for advanced lithium ion batteries
- Mechanism and selectivity of copper-catalyzed borocyanation of 1-aryl-1,3-butadienes: A computational study
- Recent advances in the improvement of g-C3N4 based photocatalytic materials
- In-situ electro-deposition synthesis of MnOx-NiCo2O4 monolithic catalyst with rich phase interfaces
- Aconapelsulfonines A and B, seco C20-diterpenoid alkaloids deriving via Criegee rearrangements of napelline skeleton from Aconitum carmichaelii
