Visible-light-induced three-component reaction of quinoxalin-2(1H)-ones, alkenes and CF3SO2Na leading to 3-trifluoroalkylated quinoxalin-2(1H)-ones
2021-04-02MengYufenLvQishunLiuRuishengLiuXiohuiZhoWeiWei
N Meng,Yufen Lv,Qishun Liu,Ruisheng Liu,Xiohui Zho**,Wei Wei,*
a School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, China
b Qinghai Provincial Key Laboratory of Tibetan Medicine Research and Key Laboratory of Tibetan Medicine Research, Northwest Institute of Plateau Biology,Chinese Academy of Sciences, Qinghai 810008, China
ABSTRACTA facile and metal-free visible-light-enabled three-component reaction of quinoxalin-2(1H)-ones,alkenes and CF3SO2Na has been developed under air at room temperature.This photocatalytic tandem reaction using 4CzIPN as the photocatalyst and air as the green oxidant, provides a mild and environmentally friendly approach to access a series of 3-trifluoroalkylated quinoxalin-2(1H)-ones.
Keywords:Metal-free Visible-light-catalysis Three-component reaction Quinoxalin-2(1H)-ones
As an important structural motif, quinoxalin-2(1H)-one is frequently found in a substantial number of natural products and biologically active molecules, which exhibited a broad range of biological properties including anti-histaminic, antibacterial,antiplasmodial, antiviral, and anticancer activities [1].In particular, 3-functionlized quinoxalin-2-ones have aroused great synthetic interest due to their remarkable and interesting pharmacological properties [2].Consequently, a number of efficient protocols have been exploited for the construction of 3-substituted quinoxalin-2-ones via C3-H functionalizations of quinoxalin-2(1H)-ones.Through these strategies, a series of functionalities such as aryl [3], acyl [4], alkoxyl [5], amino [6],oxyalkyl [7], cyano [8], phosphoryl [9] and sulfenyl [10] groups could be smoothly introduced into this framework.On the other hand,the incorporation of 3-trifluoroalkyl group has an important affection of the physical and pharmacological properties of organic molecules (i.e., electronegativity, lipophilicity, metabolic stability and bioavailability) [11].As such, the efficient synthetic methods for the preparation of 3-trifluoroalkylated quinoxalin-2(1H)-ones are greatly demanded.In general, the oxidative C3-H trifluoroalkylations of quinoxalin-2(1H)-ones with trifluoroalkylated reagents have been accomplished in the presence of stoichiometric quantities of oxidants such as PhI(OAc)2, PhI(TFA)2, or K2S2O8[12].Nevertheless, most of these reactions are limited to the use of inert gases, excess oxidants or additives,which would lead to the generation of excess amounts of wastes.
Recently, visible-light-initiated photoredox catalysis has elicited considerable interests of chemists because it can offer particularly appealing approach to construct various valuable compounds with the advantages of operational simplicity, mild conditions and environmental friendliness[13,14].In 2019,Xia and Jin realized visible-light-mediated methods for the synthesis of 3-trifluoromethylated quinoxalin-2(1H)-ones through C3-H trifluoromethylation of quinoxalin-2(1H)-ones with CF3SO2Na [15].The same year, Studer described a DBU mediated per-fluoroalkylation of alkenes with per-fluoroalkyl iodides and quinoxalin-2(1H)-ones under visible-light irradiation, in which only one trifluoromethylation example using gaseous CF3I as trifluoromethylated reagent was reported [16].In searching for mild and general method to construct more diverse 3-trifluoroalkylated quinoxalin-2(1H)-ones, herein, we wish to report a facile and efficient visiblelight-induced three-component reaction of quinoxalin-2(1H)-ones,alkenes and CF3SO2Na leading to various 3-trifluoroalkylated quinoxalin-2(1H)-ones at room temperature (Scheme 1).The present photocatalytic tandem reaction, which utilizes 4CzIPN as the photocatalyst and air as the green oxidant, offers a mild and environmentally friendly protocol to access a number of 3-trifluoroalkylated quinoxalin-2(1H)-ones in moderate to good yields.
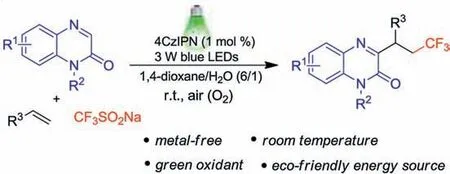
Scheme 1.Visible-light-induced three-component reaction of quinoxalin-2(1H)-ones, alkenes and CF3SO2Na.
Initially,we chose the model reaction of 1-methylquinoxalin-2(1H)-one (1a), allylbenzene (2a) and CF3SO2Na (3) to screen the reaction conditions.The product 4a was isolated in 30%yield when the model reaction was conducted in CH3CN/H2O (6/1) by employing of rose rengal (2 mol%) as the photocatalyst under the irradiation of 3 W blue LED lamps at room temperature(Table 1, entry 1).Then, various photocatalysts were investigated to enhance the product yield(Table 1,entries 2-7).To our delight,the yield of product 4a could be increased to 56% when 4CzIPN(1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene) was used as photocatalyst(Table 1,entry 6).Other photocatalysts such as Na2-eosin Y, eosin B, rhodamine B, acridine red, or 9-fluorenone only gave lower reaction efficiency.Next,the screening of the loading of photocatalyst found that 1 mol%of 4CzIPN was the best choice,and the increase of photocatalyst loading would lead to the lower yields (Table 1, entries 8-10).No transformation was observed in the absence of 4CzIPN(Table 1,entry 11).Furthermore,a series of mixed solvents were examined.The highest yield was obtained when reaction was carried out in 1,4-dioxane/H2O (6/1) (Table 1,entry 16).No product was detected when H2O was used as the solvent (Table 1, entry 24).The desired product was obtained in 62% or 59% yield when the reaction was performed under the irradiation of green or white LEDs (Table 1, entries 25 and 26).Finally, no transformation was observed when the reaction was conducted without visible-light irradiation (Table 1, entry 27).
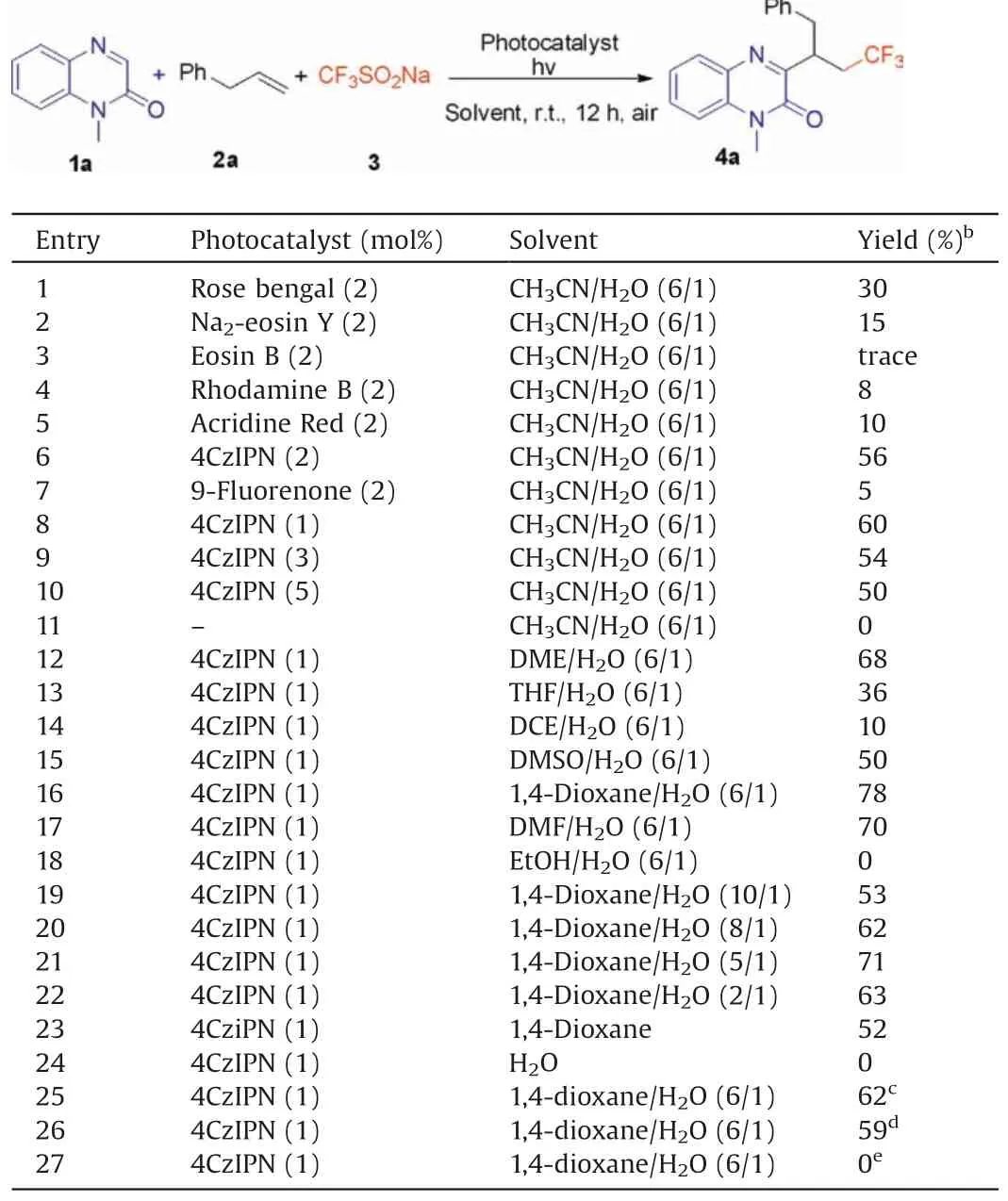
Table 1 Screening of the reaction conditions.a
Under the optimal reaction conditions, the substrate scope of this metal-free three-component reaction of quinoxalin-2(1H)-ones, alkenes and CF3SO2Na was investigated (Scheme 2).In general, a number of quinoxalin-2(1H)-ones bearing electrondonating or electron-withdrawing groups on the phenyl ring were well compatible in this procedure, giving access to the corresponding products in moderate to good yields (4b-4f).Furthermore, various N-protecting groups including N-ethyl, N-propyl,N-esteryl, N-2-oxo-2-phenylethyl and N-aromatic groups (i.e.,4-methylphenyl and phenyl groups) were well tolerated in this reaction to give the desired products 4g-4l in good yields.It is worth mention that N-free protected quinoxalinone was also well suitable for this transformation,generating the product 4m in 61%yield.Next,the scope of different alkenes was examined.A series of 3-aryl substituted allylbenzenes, 3-acyloxy and 3-phthalamido substituted propylenes were all concerted to the desired products 4n-4r in moderate yields.It was found that cycloalkene such as cyclohexene also took part in this reaction to afford the desired product 4s in 34% yield.Finally, a series of aromatic alkenes that containing both electron-rich and electron-poor groups were evaluated, and the results demonstrated that the corresponding products 4t-4x could be obtained in moderate to good yields.
Several experiments were carried out to get insights into the reaction mechanism.When radical scavenger TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy)was added in this three-component reaction system, the model reaction was completely suppressed and TEMPO-trapped complex TEMPO-CF3was detected by LC-MS(Scheme 3a).This result indicated that a radical process might be involved in this reaction.Furthermore, when the model reaction was performed under nitrogen atmosphere,only a trace amount of product 4a was detected,suggesting that air(O2)is important for promoting this transformation (Scheme 3b).In addition, the On/Off light-illumination experiments were also carried out under the standard conditions, and the result demonstrated that the continuous irradiation of visible-light is essential for the present procedure (Fig.1).
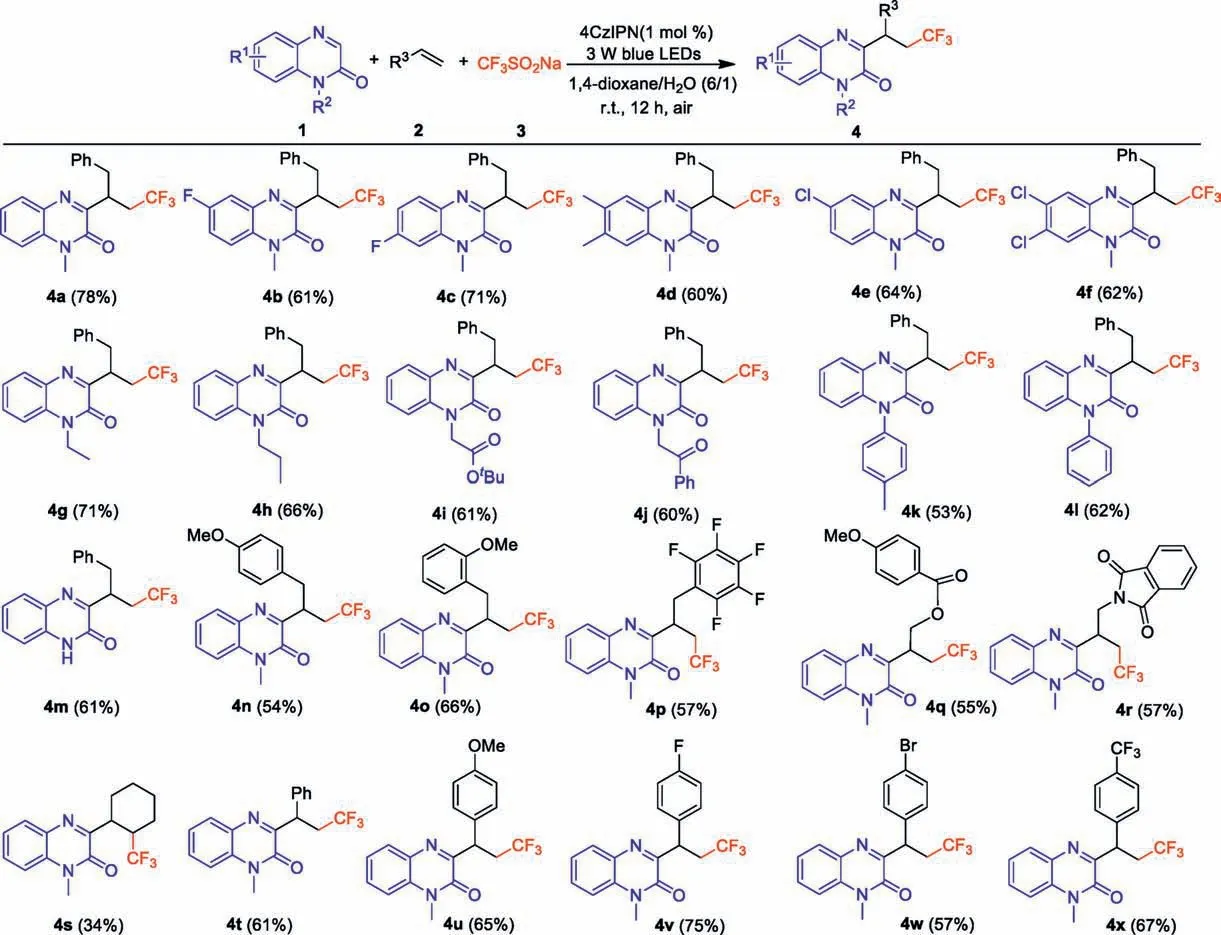
Scheme 2.Substrate scope.Reaction condition:1(0.1 mmol),2(0.2 mmol),3(0.2 mmol),4CzIPN(1 mol%),1,4-dioxane/H2O(6/1)(2 mL),3 W blue LED lamps,r.t.,air,12 h.Isolated yields based on 1.
Moreover,fluorescence quenching(Stern-Volmer)experiments were conducted to prove an energy transfer process between 4CzIPN and substrates under visible-light irradiation.As shown in Figs.2 and 3, when the concentration of 1-methylquinoxalin-2(1H)-one (1a) was gradually increased, the emission intensity of excited 4CzIPN was found to be diminished.In contrast,no obvious phenomenon was observed when 4CzIPN was separately mixed with allylbenzene (2a) or CF3SO2Na (3).These results indicated that an electron transfer process should occur between excited 4CzIPN and 1-methylquinoxalin-2(1H)-one(1a)in the presence of light.
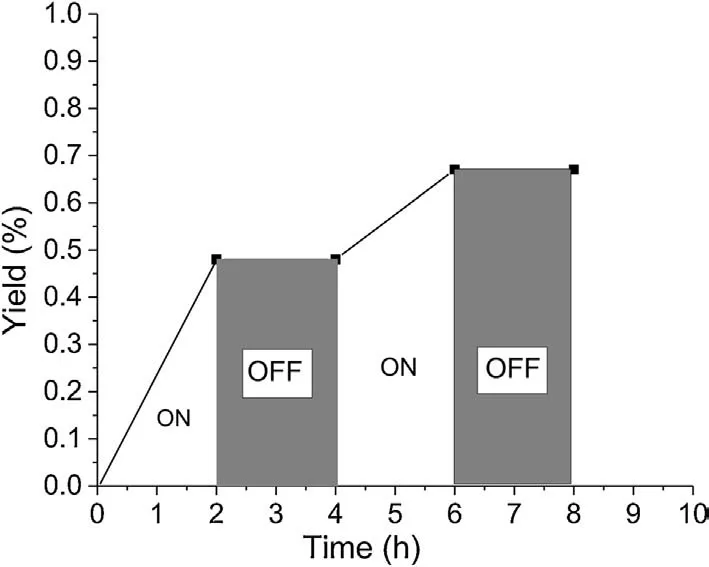
Fig.1.On/off experiments.
On the basis of the above results and previous reports[15-18],a possible reaction pathway was proposed as shown in Scheme 4.Firstly, the excited state 4CzIPN* was generated from 4CzIPN under visible-light irradiation [17a,b].Then, a single-electron transfer (SET) from quinoxalin-2(1H)-one 1 to the excited state of 4CzIPN*gave 4CzIPN·-and radical cation 5.The oxidation of 4CzIPN·-by dioxygen produced the ground state 4CzIPN and O2·-[10,17b].Meanwhile, CF3SO2Na (3) was also oxidized by O2(air)generating O-centered radical 6[18].Radical 6 underwent a spontaneous loss of SO2to give CF3radical 7.Subsequently,the addition of CF3radical 7 to alkene 2 formed alkyl radical intermediate 8, which attacked the radical cation 5 afforded a nitrogen cation intermediate 9.Finally, the desired product 4 was produced by the deprotonation of intermediate 9.
In summary, we have demonstrated a facile and efficient method for the synthesis of 3-trifluoroalkylated quinoxalin-2(1 H)-ones via visible-light-promoted three-component reaction of quinoxalin-2(1H)-ones, alkenes and CF3SO2Na at room temperature.Through this methodology, various 3-trifluoroalkylated quinoxalin-2(1H)-ones could be obtained in moderate to good yields from simple and readily available materials in the absence of any external strong oxidant.The strategy is useful in synthetic organic chemistry due to its advantages of metal-free,operational simplicity,mild conditions,green oxidant,and eco-friendly energy source.Further synthetic application and mechanistic investigation is ongoing in our group.
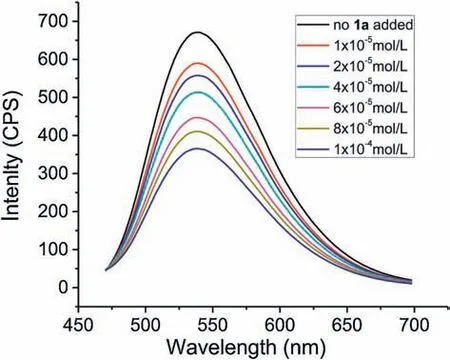
Fig.2.Quenching of 4CzIPN fluorescence emission in the presence of 1a.
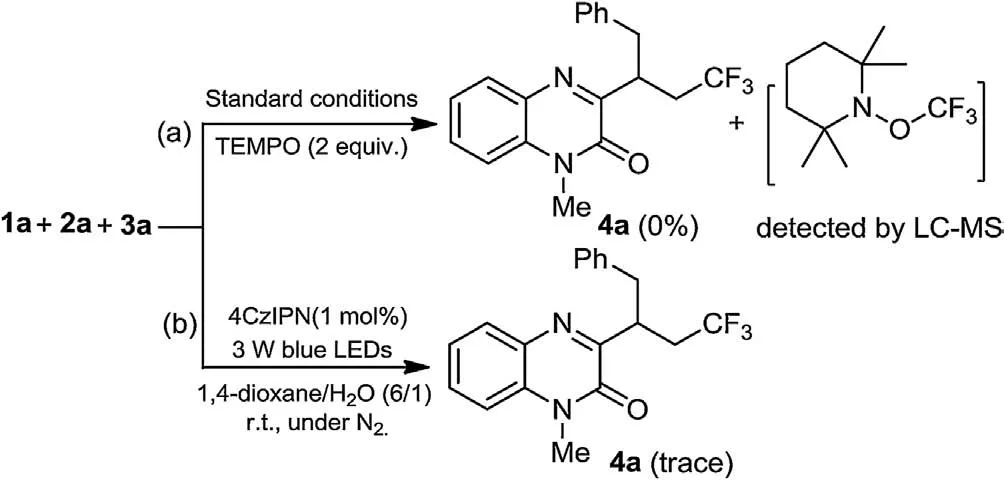
Scheme 3.Control experiments.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by Youth Innovation and Technology Project of Shandong Province (No.2019KJC021), the International Cooperation Project of Qinghai Province (No.2018-HZ-815), the Natural Science Foundation of Shandong Province (No.ZR2018MB009), the Qinghai key laboratory of Tibetan medicine research(No.2017-ZJ-Y11)and CAS“Light of West China”Program 2018.
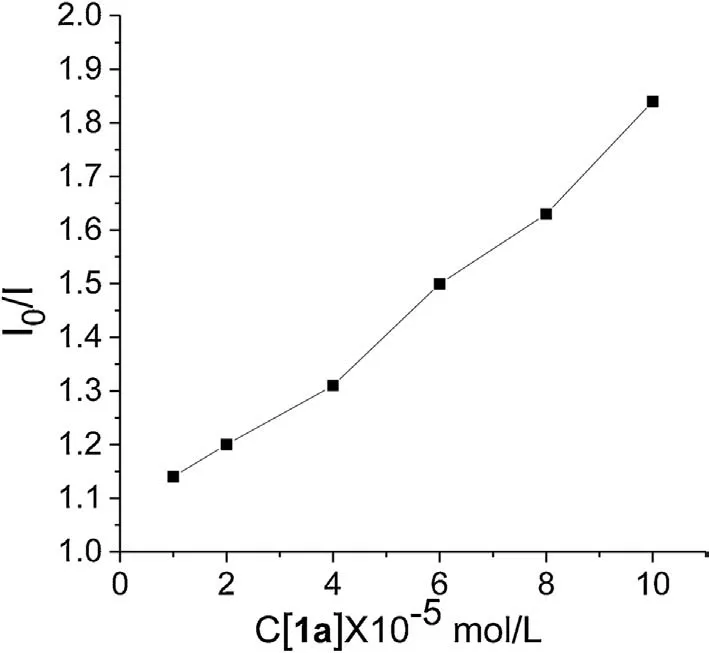
Fig.3.Stern-volmer plots.
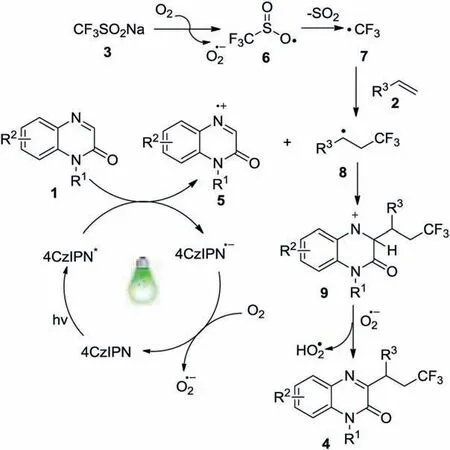
Scheme 4.Possible reaction pathway.
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.11.034.
杂志排行
Chinese Chemical Letters的其它文章
- Diverse synthesis of the C ring fragment of bryostatins via Zn/Cu-promoted conjugate addition of α-hydroxy iodide with enone
- Directly conversion the biomass-waste to Si/C composite anode materials for advanced lithium ion batteries
- Mechanism and selectivity of copper-catalyzed borocyanation of 1-aryl-1,3-butadienes: A computational study
- Recent advances in the improvement of g-C3N4 based photocatalytic materials
- In-situ electro-deposition synthesis of MnOx-NiCo2O4 monolithic catalyst with rich phase interfaces
- Aconapelsulfonines A and B, seco C20-diterpenoid alkaloids deriving via Criegee rearrangements of napelline skeleton from Aconitum carmichaelii
