Hybrid vesicles of pillar[5]arene/silica: Host-guest complexation and application in pH-triggered release
2021-04-02ChenghoHouLijingLiuSiyngMengYueWuMeirnXieYongkuiShnPingngHePengfeiSunXiojunLio
Chengho Hou,Lijing Liu,Siyng Meng,Yue Wu,Meirn Xie,Yongkui Shn,Pingng He,Pengfei Sun,Xiojun Lio,,*
a School of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200241, China
b Key Laboratory for Organic Electronics and Information Displays, Institute of Advanced Materials (IAM), Nanjing University of Posts & Telecommunications,Nanjing 210023, China
ABSTRACT Integrating silica with organic nanoparticles can generate unique properties.Here pillar[5]arene/silica hybrid vesicles were constructed based on the amphiphilic and rigid properties of pillararenes,as well as the catalytic hydrolysis of tetraethoxysilane.Such vesicles exhibited the high strength of silica and unique molecular recognition of pillararenes,both of which could tune the pH-triggered release behavior.Furthermore, a rhodamine B derivative with hexyl group (RhB-C6) was synthesized, which can form a complex with the pillar[5]arene.Based on the host-guest interaction and high strength of silica, the hybrid vesicles could load more RhB-C6 and the rhodamine B was released more slowly compared with the organic vesicles.
Keywords:Pillararenes Hybrid materials Supramolecular chemistry Silica nanoparticles Host-guest interaction
Silica nanoparticles play an important role in producing functional hybrid materials due to their facile modification,nontoxic, and biocompatible properties, which arise from their stable structures [1-6].For example, Prof.Deng developed an approach to prepare core-shell structural magnetic mesoporous silica microspheres[7], which exhibited high loading capacity for enzyme immobilization and excellent biocatalysis efficiency.This strategy provided an effective method to fabricate functional hybrid materials with multicomponents and integrated properties.However,it was indispensable to add ammonia solution to catalyze the hydrolysis of tetraethoxysilane and two surfactants as cotemplates, which made the preparation procedure much complicated.Moreover, the recyclable property depended on the magnetic ingredient, which may limit its application.Hence, it is necessary to develop facile strategies to construct hybrid materials with stimulus-responsive properties combining other universal techniques.
Host-guest chemistry [8-12] has attracted increasing interest and been widely applied to provide supramolecular systems with stimuli-responsive properties,which derive from the noncovalent interaction.For instance,Prof.Sessler constructed a chemically and electrochemically responsive supramolecular ensemble by a calix[4]pyrrole derivative with phenyl C61 butyric acid [13].Prof.Harada reported a color changing hydrogel with stretching and self-healing properties based on the host-guest interactions between the side chains beta cyclodextrin and phenolpthalein[14].Therefore, host-guest interaction could enable the system responsivity and consequently provide new methods to prepare functional materials.
Pillararenes,as a new class of macrocyclic hosts,have attracted considerable attention for their essential applications in various fields since 2008 [15-27], especially in the formation of vesicles owing to their rigid structures.For instance, Prof.Wang reported the pH-responsive supramolecular vesicles on the basis of watersoluble pillar[6]arene and ferrocene derivative, which can be applied to load mitoxantrone [28].Prof.Huang reported the pillararene-based supra-amphiphilic polypseudorotaxane, which could form vesicles in water [29].So far, researches about pillararenes are mainly concerned about organic supramolecular systems [30-37].It is still a challenge to construct organicinorganic hybrid systems containing pillararenes to enrich the functionalities of the obtained materials, which were rarely studied [38-43].
In the present work we, for the first time, constructed a novel pillar[5]arene/silica hybrid vesicle by employing an amphiphilic pillar[5]arene AP5,which played both the roles of the template to self-assemble into vesicles and the role of catalyst to catalyze the hydrolysis of tetraethoxysilane(Fig.1).Such vesicles integrated the high strength of silica and unique molecular recognition of amphiphilic pillararenes in aqueous solution, which was hard to achieve.The coexistence of silica and pillararenes could tune the pH-triggered releasing behavior of rhodamine B effectively.
The pillar[5]arene derivative AP5 with one side of hexyloxy groups and the other side of amino groups (Schemes S1-S4 in Supporting information) was synthesized similar to the literature[44] and characterized in Figs.S1-S6 (Supporting information).Compared with the amphiphilic pillararene with pentyl chains as reported,the alkyl chains were extended to six carbons in order to increase both the CH-π interaction with guest molecules[45]and the ratio of hydrophobic/hydrophilic of this supramolecular system, which is liable to form vesicles according to the rules reported by Prof.Eisenberg [46-48].As expected, owing to the amphiphilic property, vesicles with diameter of approximately 80 nm were formed when water was added into the ethanol solution of AP5 (ethanol: H2O=1:100) by TEM, SEM and DLS characterizations (Fig.2a and Figs.S7 and S8 in Supporting information) and ruptured vesicles were observed (Fig.S7c).The diameters of vesicles characterized by TEM and SEM were slightly smaller than that detected by DLS.This seems reasonable since TEM/SEM and DLS measured solid and swollen states [49].These organic vesicles were responsive to pH change arising from the amino groups.As shown in Fig.2b, a decrease of pH induced a considerable decrease in the diameter of the aggregates from 80 nm at pH 7 to 20 nm at pH 3,caused by the protonation of amino groups, which increased the hydrophilic part and consequently broke the hydrophilic/hydrophobic balance.Moreover, these organic vesicles were destroyed to irregular aggregates (Fig.2c)after ultrasonication for 10 min, revealing that they are brittle,which may limit their application.
In order to enhance the strength of these organic vesicles,hybridization technique was applied.In this work,hybrid vesicles with diameter of 110 nm(Fig.3a and Figs.S9 and S10 in Supporting information) were obtained when tetraethoxysilane (TEOS)aqueous solution was used instead of pure water,since the amino groups played not only the role of hydrophilic part to self-assemble into vesicles but also the role of catalyst to catalyze the hydrolysis of tetraethoxysilane (TEOS) to provide silica.The existence of Si(percentage:8.38)from energy dispersion spectrum(EDS,Fig.3b)and the coexistence of absorption band at 3338 cm-1assigned to the vibration of -OH stretching of SiO2as well as the bands at 1500 cm-1and 2930 cm-1ascribed to the phenyl plane bending and CH2stretching of AP5 from FT-IR spectra (Fig.S11 in Supporting information)demonstrated the successful preparation of hybrid vesicles (named AP5-SiO2for abbreviation).To our delight, they were not broken completely (Fig.3c) as the pure organic vesicles behaved (Fig.2b) upon the decrease of pH.Moreover,the vesicular morphology(Fig.3d)was maintained after ultrasonication for 10 min.All these experiments demonstrated that the existence of SiO2endowed the hybrid vesicles with relatively high strength.
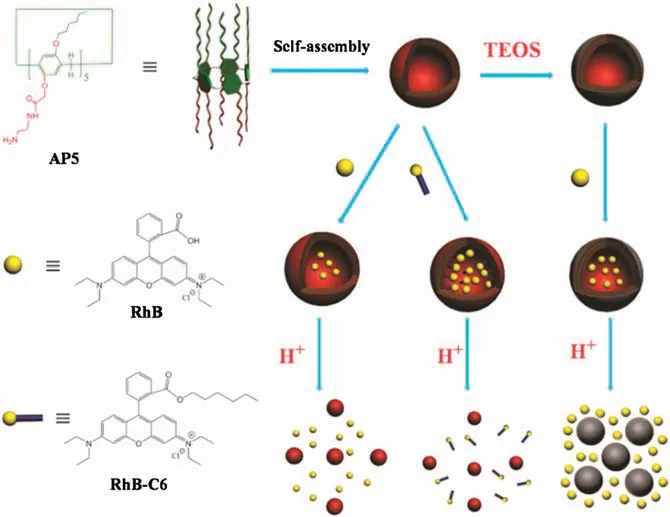
Fig.1.Schematic representation of preparation of pillar[5]arene/silica hybrid vesicles and the pH-triggered release.

Fig.2.TEM images of aggregates of AP5 at pH 7 (a); pH 3 (b) and after ultrasonication for 10 min (c) in the concentration of 3.0×10-4 mol/L.
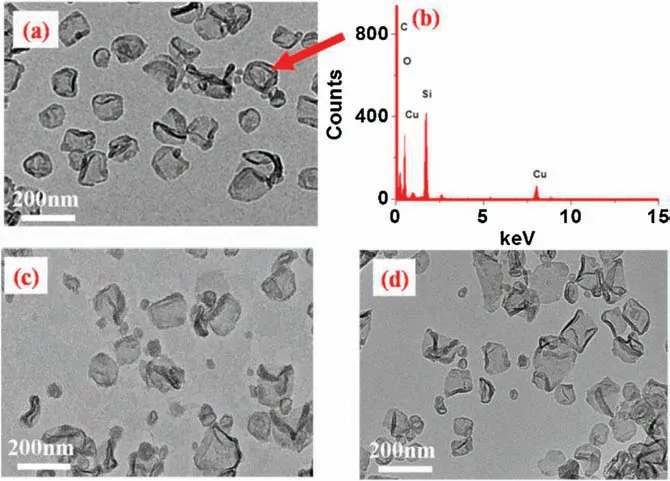
Fig.3.TEM images of aggregates of AP5-SiO2 at pH 7 (a); pH 3 (c); and after ultrasonication for 10 min (d) in the concentration of 3.0×10-4 mol/L.(b) EDS of sample (a).
This pH-responsive vesicle could be potentially applied in controlled release and selective drug delivery in tissues, for instance, infected and tumor tissues always behave a lower pH than the normal tissues [44,50].With this in mind, rhodamine B(RhB) was selected as a model guest to investigate the process of controlled release.It was firstly encapsulated in the organic and hybrid vesicles separately,followed by dialysis to remove the free RhB.The absorption peak of RhB at 550 nm decreased gradually and did not change until 48 h(Fig.S12 in Supporting information),revealing the completement of encapsulation, which was verified by the increasement of fluorescence intensity ( Figs.4a and d, red lines).It was noteworthy that the hybrid vesicles could encapsulate much more RhB than the organic ones as evidenced by fluorescence microscope images ( Figs.4b and e) and UV-vis spectra (Fig.S12 in Supporting information).Furthermore,decreasing the pH value to 3 lead to the release of abundant RhB due to the collapse of vesicles triggered by the disturbance of hydrophilic/hydrophobic balance, as demonstrated by the increasement of fluorescence intensity( Figs.4a and d,blue lines),which was in good agreement with the literatures [44,51,52].On the other hand, the absorption peak of RhB at 550 nm decreased along with the proceeding of dialysis under acidic condition(Fig.S13 in Supporting information),revealing that RhB remained in the aggregates became little and little,which can be observed in fluorescence microscope images ( Figs.4c and f).Compared with the pure organic vesicles, the hybrid vesicles showed weaker fluorescence intensity (Fig.4d, blue line) and slower decrease of UV-vis absorption intensity (10% for hybrid vesicles and 27% for organic vesicles(Fig.S13),revealing that RhB in hybrid vesicles was released more slowly and less than that in organic ones.Therefore,the existence of SiO2could tune the speed of RhB release.
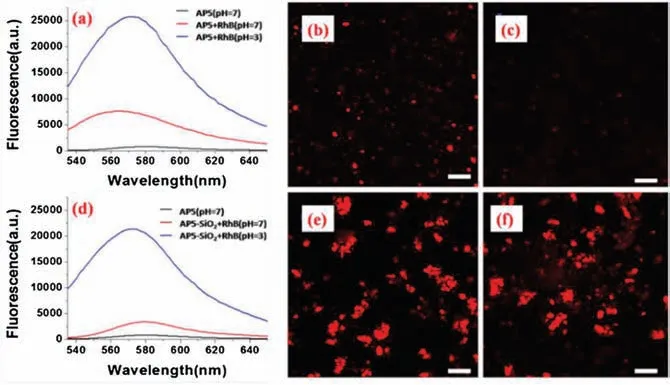
Fig.4.Fluorescence emission spectra of RhB (λex =530 nm) encapsulated in a solution of AP5 (a) and AP5-SiO2 (d) in the concentration of 3.0×10-4 mol/L at different pH values.Fluorescence microscope images of RhB encapsulatedin a solution of AP5(b,c)and AP5-SiO2(e,f)(3.0×10-4 mol/L)at pH 7(b,e)and pH 3(c,f) after dialysis.The scale bar is 10 μm.
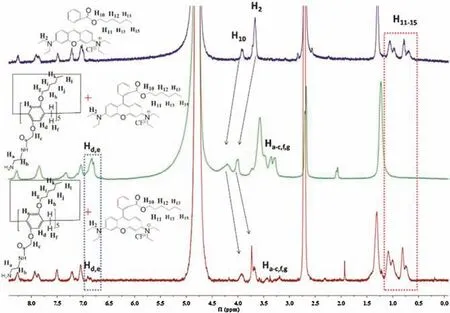
Fig.5.1H NMR spectra(500 MHz,D2O:DMSO=5:1,298 K)of RhB-C6(1.00 mmol/L)(blue line); AP5 (1.20 mmol/L) with RhB-C6 (1.00 mmol/L) (green line) and upon addition of aqueous DCl solution to (b) (red line).
Pillararenes can form comlexes with alkyl chains in organic phase due to the hydrophobic and CH-π interactions [53-55].However, rare studies regarding the host-guest behavior of pillararenes with alkyl chains performed in water were reported[56-58].In this work, a rhodamine B derivative with hexyl group was synthesized (RhB-C6, Scheme S5 (Supporting information),characterization in Fig.S14 in Supporting information) as a new model guest molecule to investigate the complexation with AP5,further the encapsulation and controlled release behaviors.1H NMR characterization of RhB-C6 (1.00 mmol/L) (Fig.5, blue line),RhB-C6 (1.00 mmol/L) with AP5 (1.20 mmol/L) (Fig.5, green line)and RhB-C6 (1.00 mmol/L) with AP5 (1.20 mmol/L) upon addition of aqueous solution of DCl(Fig.5,red line)were carried out in the mixture solution of D2O/DMSO(v/v=5:1).It was noteworthy that NMR signal of AP5 in such mixture solution was too low to be detected due to its poor solubility.However,the addition of AP5 to the solution of RhB-C6 causes remarkable changes, that is, the signals corresponding to protons H11-15of RhB-C6 disappeared,whereas the signals of protons H2,10shifted downfield and broadened, owing to the shielding effect of the electron-rich cavities of AP5.Meanwhile, other protons of RhB-C6 had no obvious change.All these phenomena provided the critical evidence that the alkyl chain formed complexes with AP5 with the protons H11-15in the cavity of AP5 and other protons out of the cavity, in good agreement with the literatures reported [56-61].However, upon addition of aqueous DCl solution, all the proton signals of RhB recovered to their original values and proton signals of AP5 decreased dramatically, indicating that RhB was excluded from the cavities of AP5.Therefore,the complexation of RhB with AP5 was responsive to pH value,similar to the literatures reported[62,63].
The binding behavior of AP5 with RhB-C6 may affect the encapsulation and releasing processes of RhB-C6, which was further investigated (Fig.S15 in Supporting information).To load RhB-C6,aqueous solution of RhB-C6 instead of RhB was added into the solution of AP5, followed by dialysis.Then HCl was added to release RhB-C6.Compared with RhB, the amount of RhB-C6 released was less since the fluorescence intensity of RhB-C6 with AP5 at pH 3 is lower than that of RhB,and the RhB-C6 remained in the aggregates was much more, due to the host-guest inclusion complexation between AP5 and RhB-C6, which hindered RhB-C6 from leaving the cavities of AP5 quickly.
In conclusion, we have successfully developed a template method to prepare hybrid vesicles composed of amphiphilic pillar[5]arene and silica by skillfully utilizing the amphiphilic and rigid properties of pillararenes,as well as the catalytic hydrolysis of TEOS.Such vesicles can load rhodamine B and exhibited pHtriggered release behavior by reducing the pH of the solution.The introduction of silica into the vesicles can not only greatly improve the strength of the composite, but also improve the loading capability and tune the speed of release.Most importantly,for the first time,a rhodamine B derivative with hexyl group was selected as a new guest molecule to investigate the host-guest complexation between AP5 and RhB-C6,which can also tune the process of release.One can envision that it may be helpful for the fabrication of functional materials and definitely bring about many promising applications, such as sensors, nanodevice, drug delivery and controlled release.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.21774033)and the open research fund of Key Laboratory for Organic Electronics and Information Displays.
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.11.030.
杂志排行
Chinese Chemical Letters的其它文章
- Diverse synthesis of the C ring fragment of bryostatins via Zn/Cu-promoted conjugate addition of α-hydroxy iodide with enone
- Directly conversion the biomass-waste to Si/C composite anode materials for advanced lithium ion batteries
- Mechanism and selectivity of copper-catalyzed borocyanation of 1-aryl-1,3-butadienes: A computational study
- Recent advances in the improvement of g-C3N4 based photocatalytic materials
- In-situ electro-deposition synthesis of MnOx-NiCo2O4 monolithic catalyst with rich phase interfaces
- Aconapelsulfonines A and B, seco C20-diterpenoid alkaloids deriving via Criegee rearrangements of napelline skeleton from Aconitum carmichaelii
