Developmental and Liver Metabolite Changes Induced by TPhP Exposure in Brown Frog (Rana zhenhaiensis) Tadpoles
2021-04-02HongliangLUYingchaoHUChangqingXUWeiDANGandZhihuaLIN
Hongliang LU ,Yingchao HU ,Changqing XU ,Wei DANG and Zhihua LIN
1 Key Laboratory of Hangzhou City for Ecosystem Protection and Restoration,School of Life and Environmental Sciences,Hangzhou Normal University,Hangzhou 311121,Zhejiang,China
2 Medical School,Hangzhou Normal University,Hangzhou 311121,Zhejiang,China
3 College of Ecology,Lishui University,Lishui 323000,Zhejiang,China
Abstract As an organophosphorus compound that frequently detected in water samples,triphenyl phosphate(TPhP) has been showed to have multiple toxicological effects on aquatic species.However,no attention has been paid to its potential impact on non-model amphibian species.Here,tadpoles of the Zhenhai brown frog (Rana zhenhaiensis) were exposed to different concentrations of TPhP (0,0.02 and 0.1 mg/L) throughout the developmental period to assess physiological and metabolic impacts of TPhP exposure on amphibian larvae.After 30-day TPhP exposure,the developmental stage of tadpoles from the high-concentration treatment appeared to be more advanced than that from the other two treatments,but other measured traits (including body size,tail length and liver weight) did not differ among treatments.Metabolite profiles in tadpole livers based on liquid chromatographymass spectrometry (LC-MS) revealed a distinct metabolic disorder in exposed animals.Specifically,significant changes in various hepatic amino acids (such as glutamine,glutamate,valine and leucine) were observed.Overall,our results indicated that chronic TPhP exposure potentially caused developmental and hepatic physiological changes in R.zhenhaiensis tadpoles,although its impact on tadpole growth appeared to be minor.
Keywords larval development,liver metabolite,triphenyl phosphate exposure,Zhenhai brown frog
1.Introduction
Triphenyl phosphate (TPhP) is one of the most widely used organophosphorus additives as flame retardants or plasticizers in consumer products (Weiet al
.,2015).With the increase of its usage,TPhP and its primary metabolites have been frequently detected in various environments (Stapletonet al
.,2009;van der Veen and de Boer,2012;Tanet al
.,2016).Despite having a low water solubility (< 1.9 mg/L),TPhP can enter aquatic ecosystems through landfill leaching and runoff.In those detected surface water samples,the concentration of TPhP ranges from 2.6 ng/L to 14 μg/L (Andresenet al
.,2004;Greenet al
.,2008;Zhanget al
.,2018).Accordingly,it may be inevitable that the accumulation of TPhP and its metabolites occurs in aquatic organisms (Wanget al
.,2016,2017;Zhaoet al
.,2019).For example,fairly high detection frequencies of TPhP have been reported in different fish species,with the detected concentration in various tissue samples ranging from 6 μg/kg to 350 μg/kg lipid weight (Kimet al
.,2011;Guoet al
.,2017;Liuet al
.,2018).Previous studies have showed that TPhP can implement multiple toxicological effects on aquatic organisms(McGeeet al
.,2013;Jaremaet al
.,2015;Duet al
.,2016;Honget al
.,2019).For example,in zebrafish (Danio rerio
),TPhP exposure may impact the development of embryos and larvae,interfere the expression of genes related to central nervous system development,disturb the metabolism of carbohydrate,lipid and amino acid,and cause DNA damage (Liuet al
.,2013a,2013b;Isaleset al
.,2015;Noyeset al
.,2015;Kimet al
.,2015;Duet al
.,2016;Wanget al
.,2017;Mitchellet al
.,2018;Shiet al
.,2018,2019;Zhanget al
.,2019).These studies are conducted in model aquatic species,such asD.rerio
(Isaleset al
.,2015;Duet al
.,2016;Liuet al
.,2016;Shiet al
.,2018),Oryzias latipes
(Liet al
.,2018) andDaphnia
magna
(Lin,2009;Scanlanet al
.,2015;Kovacevicet al
.,2018;Yuanet al
.,2018).However,data on toxicological effects of TPhP (or other organophosphorus esters) exposure in non-model wild species are still rather scarce.Additional toxicity studies on wild aquatic animals should be necessary to reveal the potential hazards associated with organophosphorus esters in aquatic systems.Untargeted metabolomics is a powerful tool for investigating physiological and metabolic upsets in wildlife due to environmental pollutant exposure (Oliveiraet al
.,2016).Liquid chromatography-mass spectrometry (LC-MS) based metabolomics can provide valuable information about metabolite changes induced by environmental pollutants,and becomes a primary choice in metabolomic analyses due to its high sensitivity and good repeatability (Luet al
.,2008;Fang and Gonzalez,2014).However,LC-MS has rarely been applied to explore the impacts of organophosphorus esters in aquatic species (Zhanget al
.,2019).Amphibian species show a high sensitivity to environmental pollutants,and are good model animals for evaluating adverse ecotoxicological effects of environmental pollutants (Kloas and Lutz,2006).Additionally,it has been widely recognized that the worldwide decline in amphibian species can be attributable to the extensive use of agricultural chemicals,including pesticides,herbicides and fungicides (Brühlet al
.,2013).Potential ecotoxicological impacts of other man-made chemicals are rarely evaluated in amphibians,so it remains unclear whether the use of them likewise accounts for amphibian decline.The Zhenhai brown frog (Rana zhenhaiensis
) is a species that commonly found in the central and southeast China,and has been used as a model organism for toxicological testing (Genget al
.,2010;Weiet al
.,2015).In the present study,we measured the size,developmental stage,and investigated the alterations in liver metabolites ofR.zhenhaiensis
tadpoles after exposure to different concentrations of TPhP throughout the larval developmental period to assess potential physiological and metabolic impacts of TPhP exposure in amphibians.2.Material and Methods
2.1.Chemical standards
TPhP (> 99% purity,CAS no.115-86-6) and chemical reagents required for sample processing,lipid analysis and LC-MS detection were purchased from the Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).Technical-grade TPhP was dissolved in dimethyl sulfoxide(DMSO) to create concentrated stock solutions of 0.2 and 1.0 mg/mL for the exposure experiment.Stock solutions were made weekly and refrigerated at 4 °C between water changes.2.2.Experimental animals
One clutch ofR.zhenhaiensis
eggs was collected from an ephemeral pond located in a suburb of Lishui (Zhejiang,China),and transported to the laboratory in Hangzhou normal university.Eggs were maintained in an aquarium (80 × 60 × 60 cm) filled with 30 cm depth of dechlorinated tap water until hatching,after which larvae were fedad libitum
with Sera mini bit fry food.Throughout the experiment,the aquarium containing frog eggs or tadpoles was placed in an artificial atmospheric phenomena simulator room set at 25 °C with a fluctuation of no more than ± 1 °C.Fluorescent lights in the room were switched on at 06:00 and off at 18:00 (a 12 h light:12 h dark photoperiod).2.3.Developmental bioassay
The exposure was commenced once the majority of animals had reached Gosner developmental stage (Gs) 26 (Gosner,1960).A total of 180 tadpoles were randomly selected and allocated to different aquaria each containing solvent control (DMSO),0.02 and 0.1 mg/L TPhP(6 replicate aquaria,60 tadpoles in each treatment group).The concentration of 0.02 mg/L is close to the maximum reported concentration of TPhP in surface water (Greenet al
.,2008),while the concentration of 0.1 mg/L is close to 1/10 median lethal concentration (LC50) for adult zebrafish (Wanget al
.,2016;Shiet al
.,2018).Each aquarium contained 4 L of dechlorinated tap water and housed 10R.zhenhaiensis
tadpoles,with gentle aeration to maintain oxygen levels.An equal volume of DMSO (for the control group) and concentrated stock solutions were respectively spiked to water for different treatments before tadpoles were transferred into aquaria.This resulted in maximal solvent concentrations of 0.01% DMSO in all treatment groups.Animals were fed Sera mini bit fry food twice daily.For feeding,we mixed the food with water and added three drops for each aquarium using a serological pipette,thereby ensuring equal rations amongst replicate aquaria.Full water changes were performed twice weekly to freshen holding water,remove faeces and food waste,and renew TPhP treatments.After 30-days of exposure the tadpoles were euthanized on ice.Snout-vent length (SVL;mm),tail length (TL;mm),body weight (BW;g) and developmental stage for each tadpole was quickly recorded,and then three tadpoles were randomly selected from each aquarium and dissected.Livers were excised and weighed (mg),flash frozen in liquid nitrogen,and stored at -80 °C until extraction of metabolites.Hepatic somatic index (HSI
;[liver weight/BW]×100) was calculated from morphometric measurements.2.4.Sample preparation
The livers of three tadpoles from the same aquarium were mixed,and therefore a total of 18 liver samples (6 replicates in each treatment group) were used for metabolomic profiling.Liver sample was transferred into a 2 ml polypropylene centrifuge tube,in which 1000 μL prechilled methanol (methanol :molecular grade water=4 :1 v/v),60 μL 2-chlorphenylalanine (8 mg/L,used as the internal standard solution) and five steel balls were added.Samples were ground using a SCIENTZ-48 high flux organization grinding apparatus for 1 min at 70 Hz (Ningbo Scientz Biotechnology Co.,Ningbo,China),ultra-sonicated using a KW-100TDV ultrasound machine (Kunshan Ultrasonic Instruments Co.,Kunshan,China) for 30 min at 20 °C,and then incubated on the ice for 30 min.The samples were vortexed and centrifuged(14 000 rpm) for 10 min at 4 °C,the supernatant (800 μL) was transferred into a new centrifuge tube and then blow-dried using a 53050 Eppendorf concentratorplus (Eppendorf AG,Hamburg,Germany).The residue was dissolved with 400 μL methanol aqueous solution (methanol :water=1:1) at 4 °C,and then filtrated through 0.22 μm filtering membrane before LCMS detection.2.5.Instrumental conditions and LC-MS spectra processing
A Thermo Ultimate 3000 (Thermo,San Jose,CA,USA) platform equipped with a Waters Acquity UPLCHSS T3 column (2.1 mm × 150 mm,1.8 μm) (Waters Acquity,Milford,MA,USA)that maintained at 40 °C was used for the separation.Each sample (2 μL) was loaded in the column with a flow rate of 250 μL/min.The gradient elution of chromatographic separation used a mixture of acetonitrile and water with 0.1% formic acid(v/v) for the positive ion mode,and a mixture of acetonitrile,ammonium formate and water for the negative ion mode as mobile phases.The linear elution program of acetonitrile was as follows:from 0 to 1 min,2% formic acid-acetonitrile(or acetonitrile);from 1 to 9 min,increased to 50% formic acidacetonitrile (or acetonitrile);from 9 to 12 min,increased to 98%formic acid-acetonitrile (or acetonitrile) and maintained at this level until 13.5 min;from 13.5 to 14 min,decreased to 2% formic acid-acetonitrile (or acetonitrile) and maintained at this level until 17 min.
The electrospray ionization with multistage tandem mass spectrometry (ESI-MSn) was executed on the Thermo Q Exactive Focus mass spectrometer (Thermo,San Jose,CA,USA)with the spray voltage of 3.8 kV and -2.5 kV in positive and negative modes,respectively.The instrumental parameters were set as follows:sheath gas,45 arbitrary units;auxiliary gas,15 arbitrary units;and capillary temperature,325 °C.The Orbitrap analyzer scanned over a mass range from m/z 81 to m/z 1000 for full scan at a mass resolution of 70 000.Data dependent acquisition for high-resolution tandem mass spectrometer (MS/MS) experiments were performed with high-energy collisional dissociation (HCD) scan.The normalized collision energy was 30 eV.Dynamic exclusion was implemented to remove some unnecessary information in MS/MS spectra.
Thermo raw files were converted into mzXML format using ProteoWizard v3.0.8789 (with peak-picking turned on),and subsequently the converted files were imported to the XCMS software in R v3.3.2 for peak identification,filtration and alignment of metabolite peaks (Tautenhahnet al
.,2012).The parameters were set as follows:bw=2,ppm=15,peakwidth=10,20,mzwid=0.01,mzdiff=0.01,method=centWave.2.6.Data analysis
We used Statistica 8.0 for windows(StatSoft,Tulsa,USA) to analyze the measured phenotype data.Assumptions of normality and homogeneity of variance were tested using Kolmogorov-Smirnov tests,and Bartlett's test,respectively.Differences in developmental stage,SVL,TL,BW andHSI
between treatment groups were compared using one-way analysis of variance (ANOVA) or nonparametric Kruskal-Wallis test (for developmental stage).Post-hoc comparison were performed with Tukey’s multiple comparisons test.Normalized LC-MS data were analyzed using the MetaboAnalyst web-based platform (Chonget al
.,2018).After data were mean-centred and scaled to unit variance,both the principal component analysis (PCA,supervised) and partial least squares discriminant analysis (PLS-DA,unsupervised)were conducted to compare the metabolic variations between groups.Metabolites were identified by searching the mass spectra against available databases [i.e.,Human Metabolome Database (HMDB),MassBank Database].One-way ANOVAs were performed to compare average spectral areas for identified metabolites.3.Results
No death occurred in any treatment groups throughout the exposure duration.Mixed model ANOVA with the aquarium identity as the random factor showed no significant batch effect on all measured traits of tadpoles (P
> 0.05),so this factor was excluded in subsequent analyses.Tadpoles from 0.1 mg/L-treated group had more advanced developmental stages than those from the other two groups (Kruskal-Wallis test,H
=24.26,P
< 0.001) (Figure 1).However,there were no significant differences in other traits measured in this study (SVL,F
=0.18,P
=0.832;TL,F
=2.20,P
=0.114;BW,F
=0.22,P
=0.804;HSI
,F
=0.42,P
=0.657) (Figure 1).The PCA revealed that the first two principal components contributed 32.6% (positive ion mode) and 36.6% (negative ion mode) of the variation of metabolite data in the positive and negative ion modes,respectively.The PCA model revealed clear variation in metabolomics among different treatment groups along the first principal component (PC1).The loadings of the principal components revealed no overwhelming contribution of any single metabolite to the principal components.PLSDA revealed a greater separation between groups than PCA(both in positive and negative ion modes,Q> 0.80,Figure 2).The liver samples of tadpoles from different groups could be distinguished along various latent variables (LVs) (both in positive and negative ion modes,one-way ANOVA,P
< 0.01).Compared with the control group,1133 metabolites in the 0.02 mg/L TPhP-treated group,and 827 metabolites in the 0.1 mg/L TPhP-treated group changed remarkably (thresholds of≥ 1.2-fold change,P
≤ 0.05).By searching and comparing the information from available databases,a total of 61 metabolites were identified to be associated with TPhP exposure and exhibited significant variation between groups.The volcano plots of several differentially expressed metabolites were showed in Figure 3.These metabolites were primarily involved in amino acid metabolism.For example,some amino acids(such as glutamine,proline,leucine,valine etc.) were increased somewhat under the 0.02 mg/L TPhP exposure,and decreased under the 0.1 mg/L TPhP exposure (Figure 4).The levels of creatine and creatinine appeared to decrease with increasing TPhP concentration (Figure 4).4.Discussion
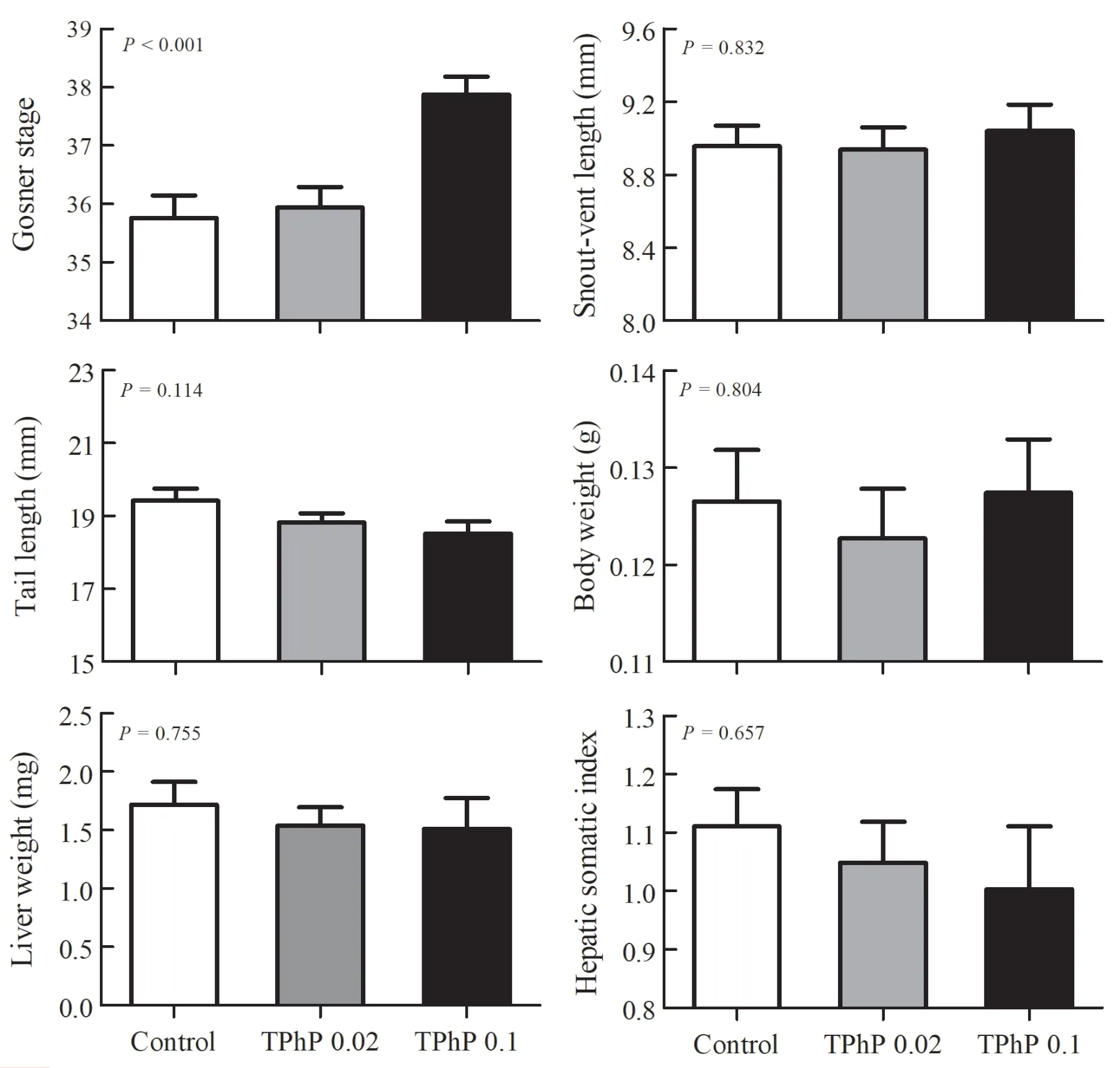
Figure 1 Mean values (+SE) for developmental stage,body parameters (snout-vent length,tail length,and body weight),liver weight and hepatic somatic index of Zhenhai brown frog (Rana zhenhaiensis) tadpoles exposed to control,0.02 and 0.1 mg/L TPhP.
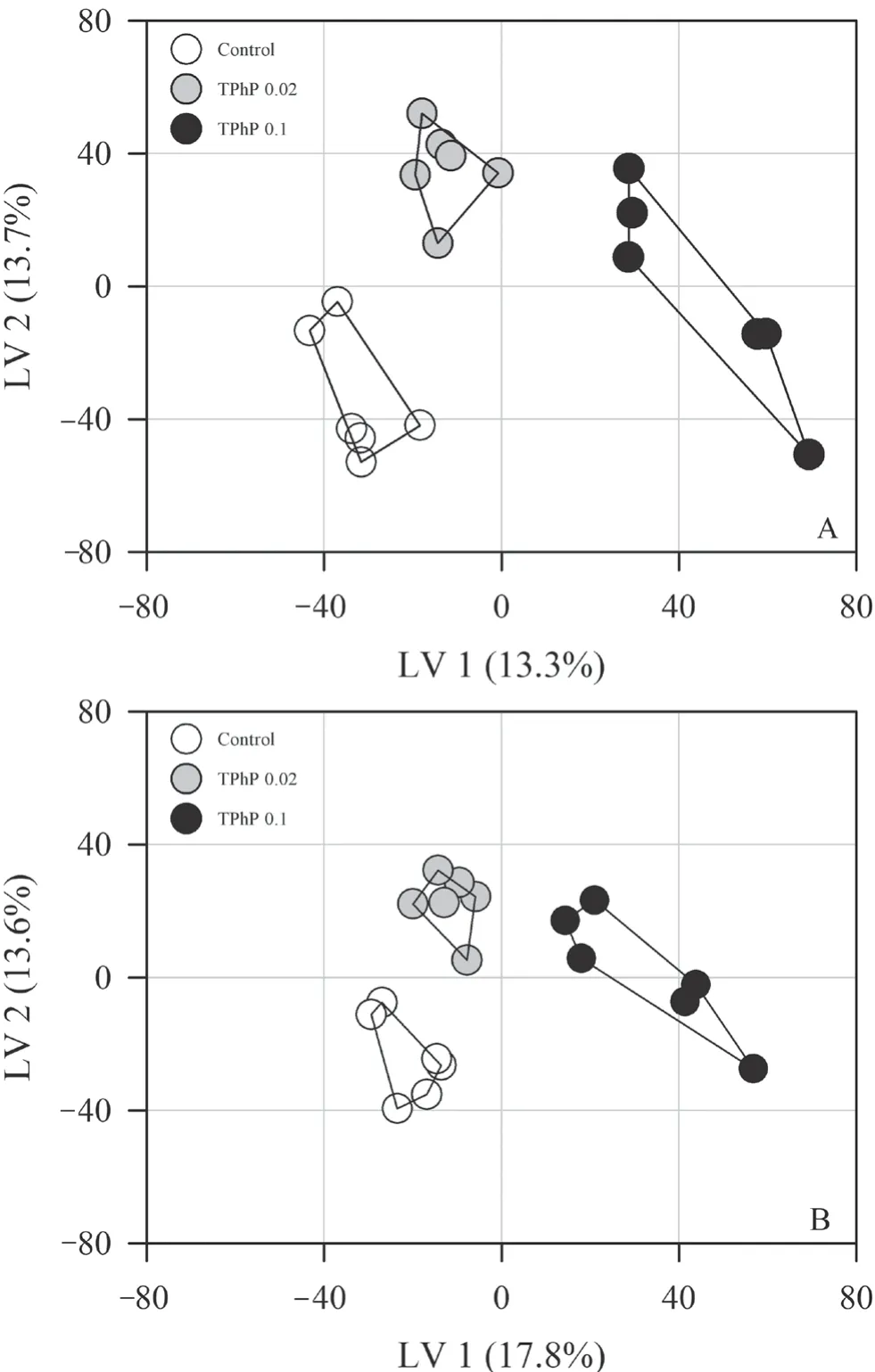
Figure 2 Score plots (A:positive ion mode;B:negative ion mode)from PLS-DA analysis showing separation of treatment groups.
In this study,several physiological changes due to TPhP exposure were investigated inR.zhenhaiensis
tadpoles.TPhPinduced developmental and metabolic disorders have been observed in other species,including cladocerans,fish,rats (Alamet al
.,2010,2012;Duet al
.,2016;Yuanet al
.,2018;Kovacevicet al
.,2019;Zhanget al
.,2019).However,the toxicological outcome of these studies conducted on different species appeared to show some discrepancy.Despite no significant inter-group differences in body size (SVL and BW),the developmental stage of tadpoles exposed to 0.1 mg/L TPhP was more advanced than those of control and 0.02 mg/L TPhP-treated tadpoles.High-concentration TPhP exposure might accelerate tadpole development and metamorphosis,but did not affect somatic growth rate,which probably made them to become shortstatured individuals.Taken in this sense,the impact of TPhP exposure on tadpole growth and development would be deleterious.In the present study,we did not determine the postmetamorphic survival and growth of froglets,and therefore were unable to evaluate the effects of TPhP exposure on their fitness.However,the time of metamorphosis is believed to be a fitness-related trait in frogs and have a long-lasting effect in subsequent life stages (Rose,2005).It might be predictable that the fitness of high-concentration TPhP treated froglets ofR.zhenhaiensis
was reduced.In fact,early metamorphosis has been showed to occur in some frog tadpoles under exposure to environmental pollutants (such as gold nanoparticles),and is regarded as an escape response to environmental stress(Fonget al
.,2016).A reduced body size due to 0.5 mg/L TPhP exposure has been reported in a species of cladocerans,Daphnia magna
(Yuanet al
.,2018).In fact,the concentration of TPhP that detected in natural water bodies rarely exceeds 1.0 μg/L.Exposure to TPhP at environmentally relevant concentrations has been showed to have limited impacts on survival and growth in various aquatic organisms (Liuet al
.,2013a;Isaleset al
.,2015;Yuanet al
.,2018;Shiet al
.,2018;Zhanget al
.,2019).Additionally,liver weight andHSI
ofR.zhenhaiensis
tadpoles was not significantly altered under different TPhP treatments in this study,also suggesting that hepatic injury associated with TPhP exposure would be minor.Compared with biochemical and molecular endpoints,the general indicators that used to evaluate animal body condition(e.g.,body size,HSI
) may be less sensitive.Here,untargeted metabolites in hepatic tissues were explored using LC-MS analysis.The samples from different treatment groups could be clearly differentiated in multivariate analyses (both PCA and PLS-DA),indicating that hepatic metabolic profile could detect TPhP-related metabolic perturbations.Consistent with the findings in TPhP-exposed zebrafish,D.rerio
(Duet al
.,2016;Zhanget al
.,2019),the changes in liver metabolites of TPhPexposedR.zhenhaiensis
tadpoles appeared to be dose-dependent.Some identified liver metabolites were showed to be increased under 0.02 mg/L TPhP exposure,and decreased under 0.1 mg/L TPhP exposure (Figure 4).Similarly,such a dose-response relationship was also found in several metabolites,although a majority of them were decreased with increasing concentration of TPhP exposure,in the previous study onD.rerio
(Zhanget al
.,2019).Unfortunately,our concentration treatments of TPhP only included 0.02 and 0.1 mg/L,which might be higher than that in natural water bodies.Whether a hormesis effect occurred at environmentally relevant concentration levels of TPhP exposure need to be confirmed in future studies.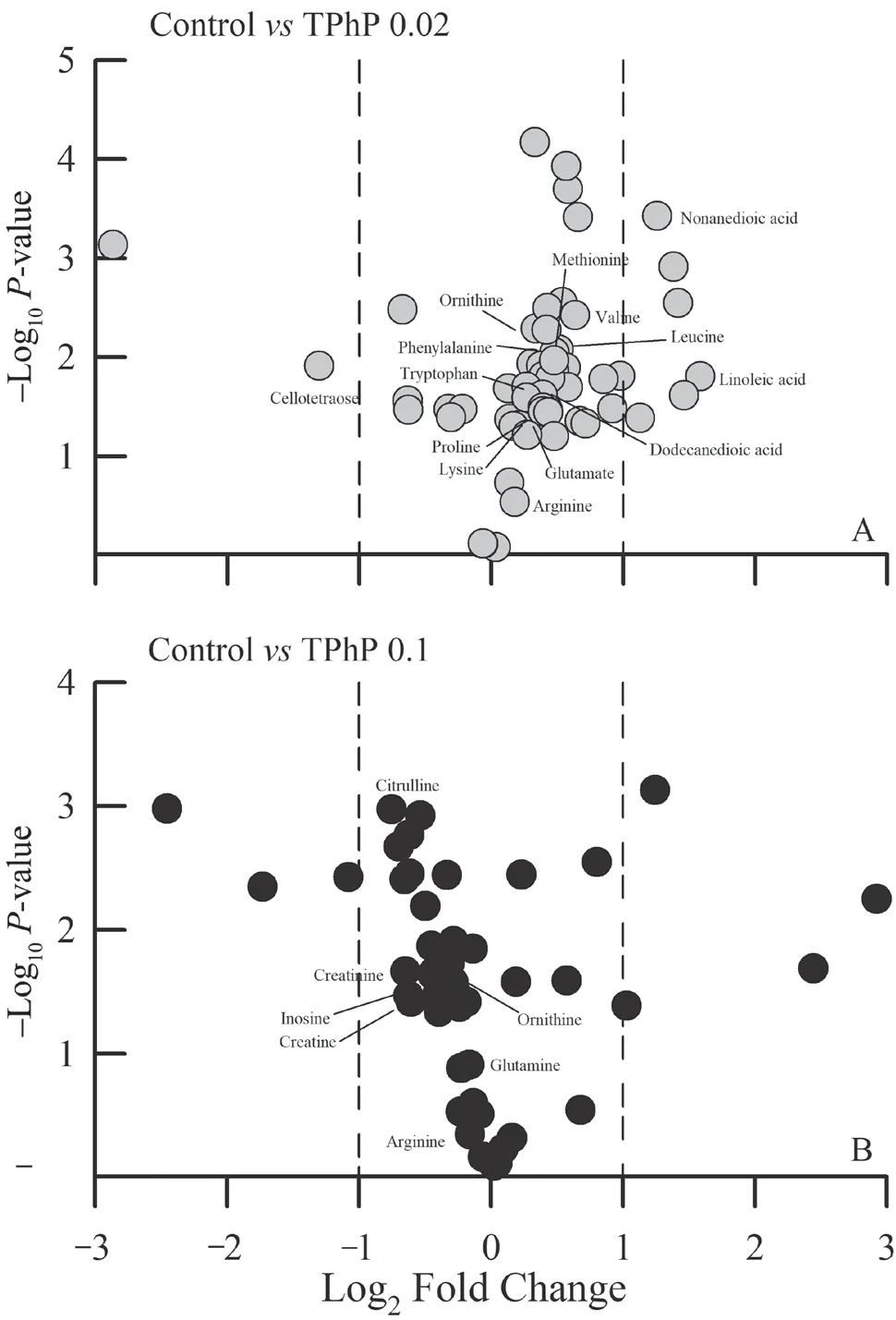
Figure 3 Volcano plots showing several differential liver metabolites between the control and 0.02 mg/L TPhP-treated group (A),and between the control and 0.1 mg/L TPhP-treated group (B) of Zhenhai brown frog (Rana zhenhaiensis) tadpoles.
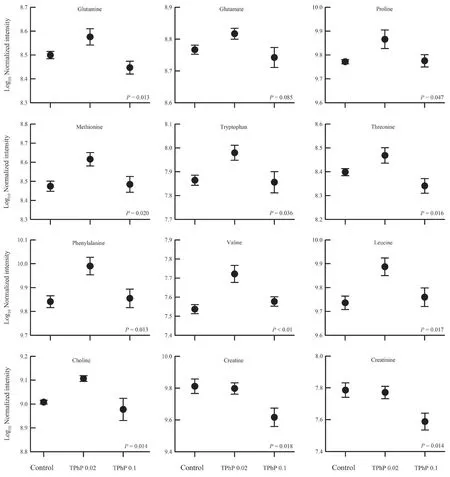
Figure 4 Mean values (± SE) for several identified liver metabolites of Zhenhai brown frog (Rana zhenhaiensis) tadpoles exposed to control,0.02 and 0.1 mg/L TPhP.
A number of amino acids were changed after TPhP exposure,indicating TPhP induced metabolic disorders in amino acids.For example,glutamate and glutamine play important roles in cell metabolism (Newsholmeet al
.,2003;Brosnan and Brosnan,2009).A noticeable increase at lowdose TPhP exposure but decrease at high-dose TPhP exposure in hepatic glutamine and glutamate (despite no statistical significance) level possibly reflected a disruption of amino acid metabolism in liver.The trend of changes in amino acids could be divergent between different studies.For example,hepatic glutamine and glutamate level decreases at relatively high concentrations (0.05 and 0.3 mg/L) of TPhP exposure in a study onD.rerio
(Duet al
.,2016),but increases at low concentrations (≤ 1 μg/L) in another study (Zhanget al
.,2019),while glutamine level increases but glutamate level decreases due to TPhP exposure inDaphnia magna
(Scanlanet al
.,2015).Additionally,significant increases in the levels of branch-chain amino acids (BCAAs),such as valine and leucine,in livers of 0.02 mg/L TPhP-treated tadpoles might reflect an inhibition of BCAA catabolism under relatively low concentrations of TPhP exposure.Many carbohydrates,lipids and fatty acids in the livers ofD.rerio
are showed to be changed significantly after TPhP exposure (Duet al
.,2016;Zhanget al
.,2019).However,such carbohydrate and lipid metabolism disturbance induced by TPhP exposure was not apparent in this study.In summary,several physiological and metabolite changes were showed in this study.These results might reflect toxic effects of TPhP on the development and liver physiology ofR.zhenhaiensis
tadpoles.Although no detectable effect on larval growth ofR.zhenhaiensis
was found,potential consequences of chronic TPhP exposure still need to investigate in further studies.For example,BCAAs play important roles in muscle protein synthesis,and in the regulation of carbohydrate metabolism (Zhanget al
.,2017).Abnormally elevated BCAA level might be associated with the occurrence of some diseases,such as obesity (Thomaset al
.,2013);while reduced BCAA level might result in an increase in the incidence of external deformity (Zhanget al
.,2019).Accordingly,some additional fitness-related variables (such as deformity,mortality of larvae)and correlation with metabolite changes induced by TPhP exposure should be determined in further studies.Acknowledgements
The experimental procedures complied with the current laws on animal welfare and research in China and were approved by the Animal Care and Ethics Committee of Hangzhou Normal University.This study was supported by a grant from the Natural Science Foundation of Zhejiang Province (LY15C030006).杂志排行
Asian Herpetological Research的其它文章
- Calls of the Large Odorous Frog (Odorrana graminea) Show Nonlinear and Individual Characteristics
- Nonlinear Phenomena Conveying Body Size Information and Improving Attractiveness of the Courtship Calls in the Males of Odorrana tormota
- The Influence of Environmental Factors on the Behavior and Mortality Risk of Polypedates megacephalus Tadpoles
- Behavioral and Neurogenomic Responses to Acoustic and Visual Sexual Cues are Correlated in Female Torrent Frogs
- Species Account of Anurans from the Western Slope of Mt.Kitanglad,Mindanao Island,Philippines
- Species Diversity,Distribution,and Microhabitats of Anurans on Mt.Kalo-Kalo of the Mt.Kalatungan Range Natural Park,Bukidnon,Philippines
