Behavioral and Neurogenomic Responses to Acoustic and Visual Sexual Cues are Correlated in Female Torrent Frogs
2021-04-02LonghuiZHAOJichaoWANGYanlinCAIJianghongRANStevenBRAUTHYezhongTANGandJianguoCUI
Longhui ZHAO ,Jichao WANG ,Yanlin CAI ,Jianghong RAN ,Steven E.BRAUTH ,Yezhong TANG and Jianguo CUI*
1 Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,Sichuan,China
2 Key Laboratory of Bio-Resource and Eco-Environment of Ministry of Education,College of Life Sciences,Sichuan University,Chengdu 610041,Sichuan,China
3 University of the Chinese Academy of Sciences,Beijing 100049,China
4 Key Laboratory for Tropical Plant and Animal Ecology of Ministry of Education,College of Life Sciences,Hainan Normal University,Haikou 571158,Hainan,China
5 Department of Psychology,University of Maryland,College Park,Maryland 27042,USA
Abstract Diverse animal species use multimodal communication signals to coordinate reproductive behavior.Despite active research in this field,the brain mechanisms underlying multimodal communication remain poorly understood.Similar to humans and many mammalian species,anurans often produce auditory signals accompanied by conspicuous visual cues (e.g.,vocal sac inflation).In this study,we used video playbacks to determine the role of vocal-sac inflation in little torrent frogs (Amolops torrentis).Then we exposed females to blank,visual,auditory,and audiovisual stimuli and analyzed whole brain tissue gene expression changes using RNAseq.The results showed that both auditory cues (i.e.,male advertisement calls) and visual cues were attractive to female frogs,although auditory cues were more attractive than visual cues.Females preferred simultaneous bimodal cues to unimodal cues.The hierarchical clustering of differentially expressed genes showed a close relationship between neurogenomic states and momentarily expressed sexual signals.We also found that the Gene Ontology terms and KEGG pathways involved in energy metabolism were mostly increased in blank contrast versus visual,acoustic,or audiovisual stimuli,indicating that brain energy use may play an important role in response to these stimuli.In sum,behavioral and neurogenomic responses to acoustic and visual cues are correlated in female little torrent frogs.
Keywords energy metabolism,multimodal communication,little torrent frogs,neurogenomic states,sexual traits
1.Introduction
Studies elucidating the mechanisms of social behavior,such as mate choice and resource competition,are of key importance in ecology and evolutionary biology (Tothet al.,
2010).While progress has already been made,the development of molecular techniques promises to provide unprecedented opportunities to determine how behavioral patterns and processes are governed(Alvarezet al.,
2015).Increasingly,studies have used sophisticated methods to explore the regulation of specific phenotypes using genome-wide approaches.Although the genome has been viewed in the past as a passive agent in controlling adult brain function (Donget al.,
2009),widespread measurements of gene expression in different experimental systems have clearly revealed that behavioral activity,perceptual experience,and changing social conditions can result in rapid gene expression changes in the brain (Clayton,
2000;Robinsonet al.,
2008).In certain environments,animal behavior may evolve through changes in specific gene regulation in the brain (Bell and Robinson,
2011),yet we still know little about the relationship between brain gene expression and social behavior (Donget al.,
2009;Zayed and Robinson,
2012).Multimodal communication has received widespread attention in the study of animal behavior.Although many animals seem to communicate primarily with signals in a single modality (Ryanet al.,
2018),an increasing amount of studies have indicated that multimodal communication is more ubiquitous (Partan and Marler,
1999;Hebets and Papaj,
2004;Partan,
2013;Starnbergeret al.,
2014b).A well-known human example is the McGurk effect,in which visual cues associated with the facial gestures involved in speech production have a profound impact on speech perception (McGurk and MacDonald,
1976;Driver,
1996).The perception of stimuli across sensory modalities can improve selective attention,signal detection,learning,and memory in humans as well as other animal groups (Bahricket al.,
2004;Halfwerket al.,
2019).Though it has been the subject of much research,determining how the brain integrates signals derived from multiple sensory modalities remains challenging.Dynamic genome analysis,which is based on new gene expression and sequencing methods,has provided an excellent opportunity to uncover potential mechanisms involving multimodal communication behavior (Partan,
2013).These methods have already been applied to the study of the genetic basis of acoustic communication in songbirds (Lovellet al.,
2008;Balakrishnanet al.,
2012;Balakrishnanet al.,
2013;Balakrishnanet al.,
2014;Frankl-Vilcheset al.,
2015),although,as yet,the regulatory architecture of the neurogenomic states regulating complex behaviors is not well understood.Several single-gene studies,however,provide a foundation for using genomic techniques to address questions about how the brain processes multimodal signaling (Partan,
2013).Frogs are excellent model systems for the experimental investigation of multimodal communication (Starnbergeret al.,
2014b;Bee,
2015;Stangeet al.,
2017).Anuran acoustic signals can be readily synthesized and,in some species,male sexual displays incorporate visual cues that can be used as stimuli in playback experiments (Tayloret al.,
2008;Starnbergeret al.,
2014a).Notably,in most anuran species,male vocalizations are accompanied by synchronous inflation of the vocal sac.Vocal sac inflation may act as a secondary cue as opposed to a signal or a signal component.Although the evolved function of the vocal sac is to cycle air during calling (Paulyet al.,
2006),many studies have indicated that its role in mating is to facilitate detection and localization through movement and coloration(Rosenthalet al.,
2004;Tayloret al.,
2008;Preiningeret al.,
2013a;Taylor and Ryan,
2013).Thus,vocal sac visual cues could act on both female mate choice and male-male interactions(Starnbergeret al.,
2014b).For instance,in Kottigehar dancing frogs (Micrixalus kottigeharensis
),a pulsating vocal sac induces more agonistic behaviors than unimodal acoustic stimuli(Preiningeret al.,
2013b).In this study,we focused on the little torrent frog (Amolops torrentis
),a species endemic to Hainan island that lives along mountain streams and calls during the day and at night during the breeding season.Males of this species prefer to call from stones with the same background color as the frog’s body,which differs distinctively from the white color of the vocal sac.Thus,it is likely that visual cues associated with vocal sac inflation could serve as visible signals capable of increasing communication effectiveness in little torrent frogs in a noisy stream environment.In this study,we first employed video playbacks in behavioral experiments in order to compare the sexual attractiveness of visual cues associated with male vocal sac movement with or without accompanying acoustic call stimuli.Then we presented females with blank,visual,acoustic,or audiovisual stimuli and subsequently collected brain tissues to obtain whole transcriptomes using RNA-seq.For the first time,we assessed whether neurogenomic states correlate with audio-visual behavior in order to identify potential molecular response mechanisms.In view of the limited research background and gene characterization in little torrent frogs,we focused on statistical analyses designed to identify broad functional expression patterns related to groups of genes and on the characterization of whole-genome expression.2.Materials and Methods
2.1.Signal design
Male little torrent frogs produce acoustic signals during both day and night.The time of day does not influence signal properties.During the breeding season,we synchronously recorded videos and sounds during daylight hours using a Nikon camera (D800) fixed on a tripod,connected to a directional microphone (Sennheiser ME66 with K6 power module) at the Mt.Diaoluo Nature Reserve (18.44°N and 109.52°E),Hainan Province,China.We chose a male calling at the site with uniform illumination,not in direct sunlight,with no nearby calling conspecific individuals.We obtained a video recording of the calling male and a video recording of the calling environment with the frog excluded.We used the two videos to create three base stimuli that were subsequently edited in Adobe Premiere Pro CS6.The base stimuli were (1) an 18 s video with a calling male in which the vocal sac inflation and the acoustics were both present,(2) a 9 s video with a male not calling and no vocal sac inflation,and (3) an 18 s video with a blank screen on which the frog and the acoustics were both absent.The videos with different duration were designed to ensure that all stimulus pairs had equal intervals (see below).The audio files were edited in Adobe Audition 3.0 after being separated from the video tracks,and subsequently resynchronized using Adobe Premiere Pro CS6 in order to create five stimuli for the present study.These stimuli were (1)a 9 s stimulus with a silent frog;(2) a 9 s stimulus with vocal sac inflation but no sound;(3) an 18 s stimulus with vocal sac inflation but no sound;(4) an 18 s stimulus with a call but no vocal sac inflation;and (5) an 18 s stimulus with vocal sac inflation accompanied by a call.Analysis of the time-frequency domain characteristics showed that all call parameters fell within the natural range (Zhaoet al.,
2017b).We conducted four two-choice tests with the abovedescribed stimuli.The first experiment involved a 9 s stimulus with vocal sac inflation versus a 9 s stimulus with a silent frog,in order to assess whether vocal sac inflation acts as a visual signal.The second experiment involved an 18 s stimulus with a call versus an 18 s stimulus with vocal sac inflation,in order to compare the relative attractiveness of acoustic and visual signals.The third and fourth experiments involved an 18 s stimulus with both vocal sac inflation and the accompanying call versus an 18 s stimulus with only a call and versus an 18 s stimulus with only vocal sac inflation,respectively.The two experiments were used to determine whether acoustic and visual cues jointly enhance the attractiveness of the stimulus in the other modality.The interval between two stimuli was set at 9 s for all stimulus pairs.Consequently,the first experiment had a different time unit than the other three experiments (9 s and18 s,respectively).
2.2.Playback experiments
We performed the behavioral experiments at field research bases in the Mt.Diaoluo Nature Reserve.Amplexed male and female frogs are generally found in rock crevices or holes in the stream.In this study,gravid females were collected (between 2000 and 2200 h) from the stream and nearby shrubs near the laboratory.Prior to the experiment,females were placed in darkness for at least one hour to allow their eyes to adapt to the dark experimental conditions (Stangeet al.,
2017).After testing,we measured their snout-vent length and body mass and returned them to the stream on the same day they were collected.We conducted playback experiments in a sound-attenuating phonotaxis chamber.Females were tested in a corridor (1.3 m × 1.5 m) constructed with foam walls.At each side near the corner,an LCD monitor (Philips 17S4LSB) and a speaker(JBLCLIP+BLK,JBL) were coupled to broadcast sound and video,respectively (Figure 1).For each monitor,an area (14 cm× 11 cm) at the bottom left or bottom right was used to present the video stimulus,thereby assuring that the apparent body size of the video frog was equivalent to that of a live frog.Each speaker was fixed alongside the playback area of a coupled monitor,thereby making the distance between the center of the speaker and the male frog in the video equal to 12 cm.A previous anuran study showed that a male frog and a speaker are not perceived as separate objects if they are located within 12 cm of each other (Narinset al.,
2005).In front of the screens,we marked a position 1 m from the two video playback areas as the initial female placement point for each playback trial.The distance between the two video playback areas also was 1 m,resulting in a 60° angle between monitors with respect to the marked position.This allowed females to easily see the vocal sac and body of the male on both screens (Tayloret al.,
2008;Tayloret al.,
2017).We observed female behavior on a monitor using a video system with an infrared light source.
Figure 1 Schematic of the acoustic and visual playback arena.The blank rectangle (14 cm × 11 cm) on each screen represents the area of presenting video stimuli during two-choice test.The 12 cm is the distance between the center of the speaker and the male frog in the video.The image of frog represents the initial female placement point for each playback trial.
For each female,calls were played at 75 dB SPL (1 m from the speaker),which is near the auditory threshold of the call frequency range (Zhaoet al.,
2017a),and videos were adjusted to a dim condition (1 lux on the screen;measured by TES 1399 Light Meter Pro.),which was approximately equal to the crepuscular light level at the stream in the frog’s natural environment,in order to best simulate conditions in which visual and acoustic integration might normally occur (Rowe,
1999;McDonaldet al.,
2000).Prior to each trial,we used a light tight box to restrain the females at the marked position.We elevated the box and freed them so that they could choose between alternative stimulus pairs while the stimulus pairs were broadcast antiphonally from each side.A choice was recorded if the female approached a speaker-monitor combination within 5 cm.We only scored females who were responsive in each of the four experiments.We considered a female as lacking motivation if she failed to make a choice within 10 min.For each frog,we stochastically presented all stimulus pairs in order to avoid potential partial side effects.None of the females were re-used for multiple experiments.Female choice data were analyzed with the two-tailed exact binomial test using R version 3.2.5.2.3.Brain sample collection
Females were collected on the morning of the day (between 1000 and 1200 h) that they were tested.These frogs were not the same set of individuals used in the playback experiments.Prior to the test,each female was isolated for at least eight hours in a dark soundproof chamber in order to bring about a decline in mRNA expression that might have been induced at the site of the breeding choruses(Burmeisteret al.,
2008).For each frog,a speaker and a video screen were coupled to present one stimulus consisting of either a blank contrast,a visual signal,an acoustic signal,or an audiovisual signal for 30 min.All animals were randomly assigned to different treatment groups.The arena,sound,and lighting conditions were the same as described for the previous playback experiments.During the playbacks,females were confined to a cylindrical cage (diameter=7 cm,height=10 cm)fixed 1 m from the male in the video.Each frog was alone in the cage,and frogs in the cage were able to hear the calls and see the visual display stimuli.After the playbacks,the speaker and video screen were immediately turned off,and the frogs remained in the dark for 30 min.Previous studies have shown that immediate early gene (IEG) mRNA accumulation in the frog brain reaches the highest level after 30 min following exposure to a continuous stimulus for 30 min (Burmeisteret al.,
2008).Ambient temperatures were maintained at 23-25°C during the experiment.In order to avoid bias caused by differing female activity levels,we only sampled individuals that did not move around but were motivated and faced the monitor during the entire experiment.After the experiments,the females were euthanized with an overdose of MS-222 solution (0.3%),and whole brain tissue was quickly collected.In addition to the four groups presented with different stimuli (n
=3 samples per group),we collected samples from frogs with dark treatment alone as a further control (n
=3 samples).The dissection implements were treated with Surface RNase Erasol (BioTake Corporation,China) according to the manufacturer’s instructions,and all operations were conducted on ice.Samples were preserved in RNA Later (Sigma-Aldrich) and stored at -20°C.2.4.RNA extraction,sequencing,and
assembly
Total RNA was isolated using TRIzolreagent (Invitrogen,CA,USA) according to the manufacturer’s protocols.RNA degradation and contamination were analyzed using 1%agarose gels.RNA purity,RNA concentration,and RNA integrity (RIN scores ranged from 9.0 to 9.6) were tested using the NanoPhotometerspectrophotometer (IMPLEN,CA,USA),QubitRNA Assay Kit in Qubit2.0 Flurometer (Life Technologies,CA,USA),and RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies,CA,USA),respectively.The cDNA libraries were constructed using the NEBNextUltraRNA Library Prep Kit for Illumina(NEB,USA) according to the manufacturer’s instructions.All libraries were sequenced on an Illumina Hiseq X-ten platform(San Diego,CA,USA).Prior to assembly,we obtained clean reads by removing low-quality raw reads,reads with an adapter,and reads with poly-N,and calculated their Q20,GC content,and the number of sequences.The clean data and expression profiling data were deposited in NCBI’s Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE122947).We completed transcriptome assembly based on clean reads using Trinity software with min_kmer_cov set to two by default (Grabherret al.,
2011).Clean reads from all samples were used to build the reference transcriptome in order to avoid biasing results toward different samples.2.5.Gene function annotation and differential expression analyses To obtain comprehensive function information,we annotated unigenes against seven databases including the Swiss-Prot,Protein family (Pfam),NCBI non-redundant protein sequences (NCBI-NR),NCBI nucleotide sequences (NCBI-NT),Gene Ontology (GO),euKaryotic Ortholog Groups (KOG),and the Kyoto Encyclopedia of Genes and Genomes (KEGG)databases.The Swiss-Prot and NR annotations were performed using diamond (v0.8.22) with an e-value ≤ 10and the KOG annotation with an e-value ≤ 10.The Pfam,NT,GO,and the KEGG annotations were performed using hmmscan (HMMER 3.0) with an e-value ≤ 0.01,NCBI blast (v2.2.28+) with an e-value≤ 10,blast2go (b2g4pipe_v2.5) with an e-value ≤ 10,and KAAS (r140224) with an e-value ≤ 10,respectively.
We regarded the assembled transcriptome as a reference and mapped clean reads of each sample to the reference transcriptome using RSEM software (Li and Dewey,
2011).The software calculated the number of read counts for each gene.Gene expression levels were calculated using the fragments per kb per million reads (FPKM).The differentially expressed genes(DEGs) that were evoked by different stimuli were analyzed using the data of read count.The analysis was completed with the DESeq R package (1.10.1) (Anders and Huber,
2010).The resultingP
-values were adjusted using the Benjamini and Hochberg method for controlling the false discovery rate(Storey and Tibshirani,
2003).An adjustedP
< 0.05 was assigned as differentially expressed levels for the DEGs in response to various stimuli.The number of DEGs was compared with a two-tailed exact binomial test.3.Results
3.1.Female behavioral responses to different sexual displays
In present study,video animations evoked female responses efficiently,and most individuals jumped on the viewing screen directly when making choices.Our previous study indicated that male calls (acoustic cues) were an important sexual display inA.torrentis
(Zhaoet al.,
2017a).Females significantly preferred stimuli with vocal sac inflation over those without inflation(12/3;Table 1),indicating that vocal sacs are also a visual cue.However,females significantly preferred calls to inflated vocal sacs (12/3;Table 1).When we presented females with vocal sac inflation accompanied by calls (audiovisual cue) paired with an alternative of vocal sacs only or calls only,females showed a significant preference for the complex multisensory components (12/3;Table 1).In sum,our results suggest that the attractiveness hierarchy of sexual displays is as follows,from least attractive to most attractive:blank contrast (in the absence of acoustic and visual cue),visual cues,and acoustic cues and audiovisual cues.3.2.Neurogenomic and behavioral responses to different stimuli are closely correlated
Sequencing andde novo
assembly results are included in Table S1.We analyzed DEGs in whole female brains to explore the potential influences of specific sexual displays onA.torrentis
neurogenomic states.A total of 808 DEGs were detected from all whole brain tissues in response to different types of stimulation.Hierarchical clustering has been used to determine whether brain gene expression patterns track behavior in other species (Chandrasekaranet al.,
2011).We therefore employed this method to reveal brain transcriptional profiles of total DEGs across all video playback samples,and thus to determine transcriptome responses to different behavioral categories.Hierarchical cluster analysis indicated a close relationship between brain gene expression and behavior (Figure 2).Overall,the transcriptional profiles of samples from the same treatment groups were more similar to one another than to those from different treatment groups.All samples from the same behavioral condition were gathered in a distinct cluster,in addition to a blank contrast and an acoustic stimulus.Moreover,the cluster of samples exposed to the audiovisual stimulus condition lay between the visual stimulus cluster and the acoustic stimulus cluster.We compared the number of DEGs evoked by multimodal cues versus blank contrast and evoked by multimodal cues versus unimodal (acoustic or visual) cues.A total of 362 DEGs were obtained in the audiovisual versus blank group,while only 169 and 67 DEGs were identified in the audiovisual versus acoustic group and the audiovisual versus visual group,respectively (Table 2).The binomial test showed that the number of DEGs found for audiovisual cues versus blank contrast was significantly higher than for audiovisual cues versus acoustic cues (P
< 2.2 × 10) and for audiovisual cues versus visual cues (P
< 2.2 × 10).These results indicated that the number of DEGs related to behavioral condition differences,and that comparison between approximate signals can produce fewer DEGs.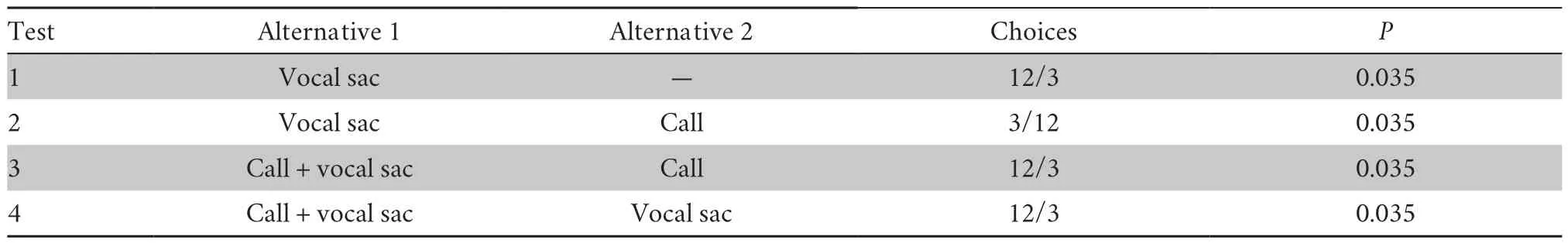
Table 1 Summary of video playback experiments for A.torrentis.
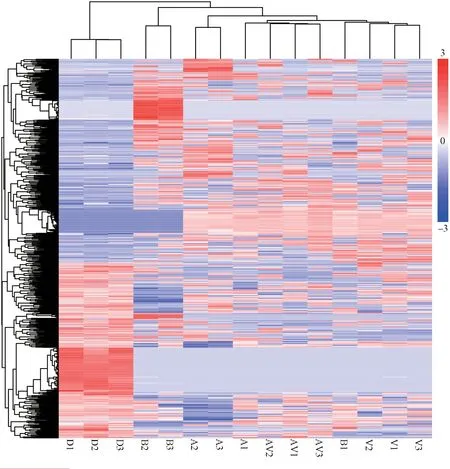
Figure 2 Hierarchical cluster analysis on global gene expression patterns reveals a close relationship between neural genomic responses and different communication behaviors.Colors from blue (low expression) to red (high expression) represent the relative expression quantity of DEGs.The cluster relationships represent the similarity between different samples and DEGs.Apart from A1and B1,the analysis shows five clusters corresponding to dark contrast,blank contrast,visual,auditory,and audiovisual stimuli.D1-D3,samples from dark contrast;B1-B3,samples from blank contrast;V1-V3,samples from visual stimuli;A1-A3,samples from auditory stimuli,AV1-AV3,samples from audiovisual stimuli.
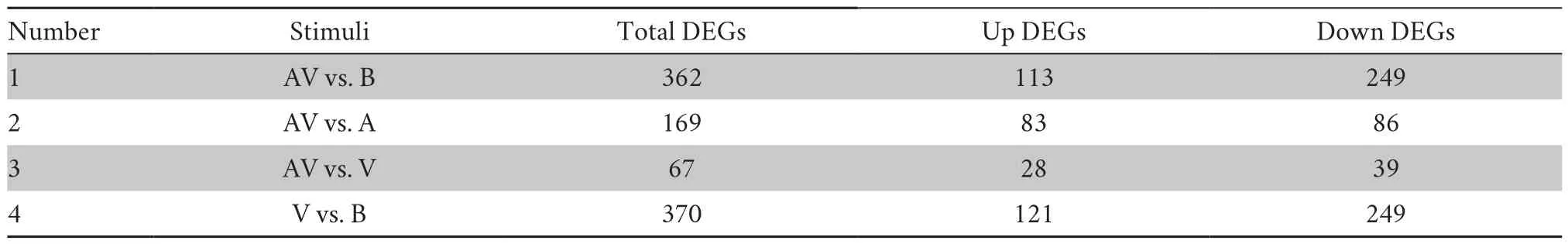
Table 2 Summary of DEGs in response to different behavioral categories.
3.3.Differentially expressed genes in response to different stimuli
To detect potential genes involved in the response to cues associated with different behavioral conditions,we compared whole brain gene expression among the multimodal/unimodal versus blank stimuli conditions and between the multimodal versus unimodal stimuli conditions.A total of 370 DEGs (121 up-regulated and 249 down-regulated genes) were identified from the visual versus blank treatments,and a total of 388 DEGs (180 up-regulated and 208 down-regulated genes)were obtained from the acoustic versus blank treatments.These results were not significantly different from those associated with the audiovisual versus blank contrast treatments (362 DEGs:113 up-regulated and 249 down-regulated genes)(binomial test:P
> 0.05;Table 2).A total 169 DEGs (83 upregulated and 86 down-regulated genes) were detected from the audiovisual versus acoustic treatments,while only 67 DEGs (28 up-regulated and 39 down-regulated genes) were found for the audiovisual versus visual treatments (Table 2).To obtain information about the functions of the DEGs,we conducted GO analysis focused on three main categories including biological processes,molecular functions,and cellular components.We identified many significantly enriched GO terms in the blank contrast versus visual cue,acoustic cue,or audiovisual cue comparisons (Table S2).In these comparisons,the up-regulated genes involved in energy metabolism were found to be significantly enriched (Table S2).The most significantly expressed down-regulated genes were involved in various stimulus responses and in lipid or sterol metabolism.In the multimodal versus unimodal stimuli comparison,however,none of the significant GO terms were found to mediate multimodal cue integration specifically (Table S2).Expression and gene function of the top 10 DEGs annotated in the GO database were then analyzed by adjusting theP
-value.We found that most significant DEGs were involved in energy metabolism,sterol and lipid metabolism,transcription and translation,and ion binding and transport in different comparisons (i.e.,blank contrast versus visual cues/acoustic cues/audiovisual cues) (Table 3).Specifically,all genes involved in energy metabolism were found to be significantly upregulated in these comparisons (Table 3).Consistent with GO analysis,the most significantly enriched pathways when compared against KEGG databases were all related to energy generation among all DEGs associated with the response to visual,acoustic,or audiovisual stimuli (Figures 3A-C).Possibly because the samples were from brain tissue,the next most significantly enriched pathways after energy metabolism were all related to neurodegenerative diseases such as Parkinson’s disease (Figures 3A-C).In the multimodal versus unimodal stimuli comparison,however,we obtained few significantly enriched pathways (Figures 3D and E).
4.Discussion
In anurans,vocal sac movement has traditionally been regarded as a by-product of call production.However,an increasing number of studies have found that stimuli associated with the vocal sac can provide a basis for composite signaling during communication (Starnbergeret al.,
2014a).For example,agonistic male interactions in both diurnal dart-poison frogs(Allobates femoralis
) and Kottigehar dancing frogs (Micrixalus kottigeharensis
) are only provoked by conspecific calls synchronized with vocal sac movement (Narinset al.,
2003;Narinset al.,
2005;Starnbergeret al.,
2014a).In the Krefft’s river frog (Phrynobatrachus kref ftii
),male-male agonistic behaviors can be induced by the dynamic visual signal of vocal sac inflation in addition to calls (Starnbergeret al.,
2014a).Nocturnal anuran species can also communicate with visual cues associated with the vocal sac.For example,vocal sac inflation and coloration have been shown to influence female choice in a few nocturnal frog species (Rosenthalet al.,
2004;Tayloret al.,
2008;Gomezet al.,
2009;Richardsonet al.,
2009).Thus,the roles played by vocal sac traits in social behavior are diverse in anuran groups.Many conditions can favor the evolution of multimodal communication systems.Stream noise is an important environmental factor for torrent frogs because animal communication sounds can be masked by high background noise.Studies of multimodal communication have also emphasized the importance of determining if individual signal components are redundant (i.e.,conveying the same information) or nonredundant (i.e.,conveying different information) (Partan and Marler,
2005).In the present study,female little torrent frogs preferred temporally overlapping bimodal signals to unimodal signals,suggesting that the interaction between acoustic and visual cues can increase communication efficiency in noisy stream environments.However,both visual vocal sac inflation signals and advertisement call acoustic signals alone were sufficient for mate attraction.Our results therefore indicate that vocal sac inflation transmits at least some of the same information as male advertisement calls for sexual selection.In addition,the little torrent frog is a territorial species,and males often perform additional visual signals such as foot-flagging displays.More study is needed to reveal the extent to which vocal sac and other visual cues combined with call stimulation affect malemale competition and female mate choice in this species.Many investigators have used microarrays or RNAseq(i.e.,transcriptomics) to measure brain gene expression related to various behavioral traits.An excellent example comes from studies of honey bees (Apis mellifera
),whose social role plasticity is mediated by brain gene expression over multiple timescales (Zayed and Robinson,
2012).In the field of acoustic communication,neurogenomic states have been used in songbird species to link brain gene expression with seasonal singing behavior (Frankl-Vilcheset al.,
2015) and song response habituation (Donget al.,
2009).Previous studies,however,have focused primarily on animal behaviors that are maintained for reasonably sustained periods.It is therefore unclear whether brain transcriptomics are sensitive enough to reflect the momentary processes associated with multimodal communication in real time.Although this field would seem to have potential,it remains to be seen if these methods can reveal the genetic bases of complex behaviors (Partan,
2013).In this study,we asked whether neural genomic responses track female behavioral responses to complex communication signals after controlling for experimental conditions and animals’ reproductive states.Interestingly,hierarchical cluster analysis suggested a strong association between neurogenomic states and the stimuli to which the animal was exposed.Meanwhile,the number of DEGs was consistent with the behavioral condition difference hierarchy;that is,fewer DEGs were produced between the two leastdifferentiated behavioral conditions.These results indicate that multimodal communication behaviors may be related to brain transcriptional profiles in anurans,which is similar to reports on the relationship between whole brain gene expression and complex behavior in social insects (Zayed and Robinson,
2012)as well as túngara frogs (Engystomops pustulosus
) (Hokeet al.,
2007).When animals were transferred from a dark,quiet environment to the playback setup,the frame and running water in the blank contrast (in the absence of acoustic and visual cue) may have provided visual or acoustic information.We therefore included the dark group as a further contrast in this study.However,animals can behave differently just sitting in the dark compared to any of the other four conditions.In order to assess whether the dark effect would significantly change the clustering result,we also analyzed the data with the dark group excluded.Consequently,the clustering results of four conditions (Figure S1) were consistent with results of the five conditions analyzed together (Figure 2).Thus,the results should be compelling and conclusive.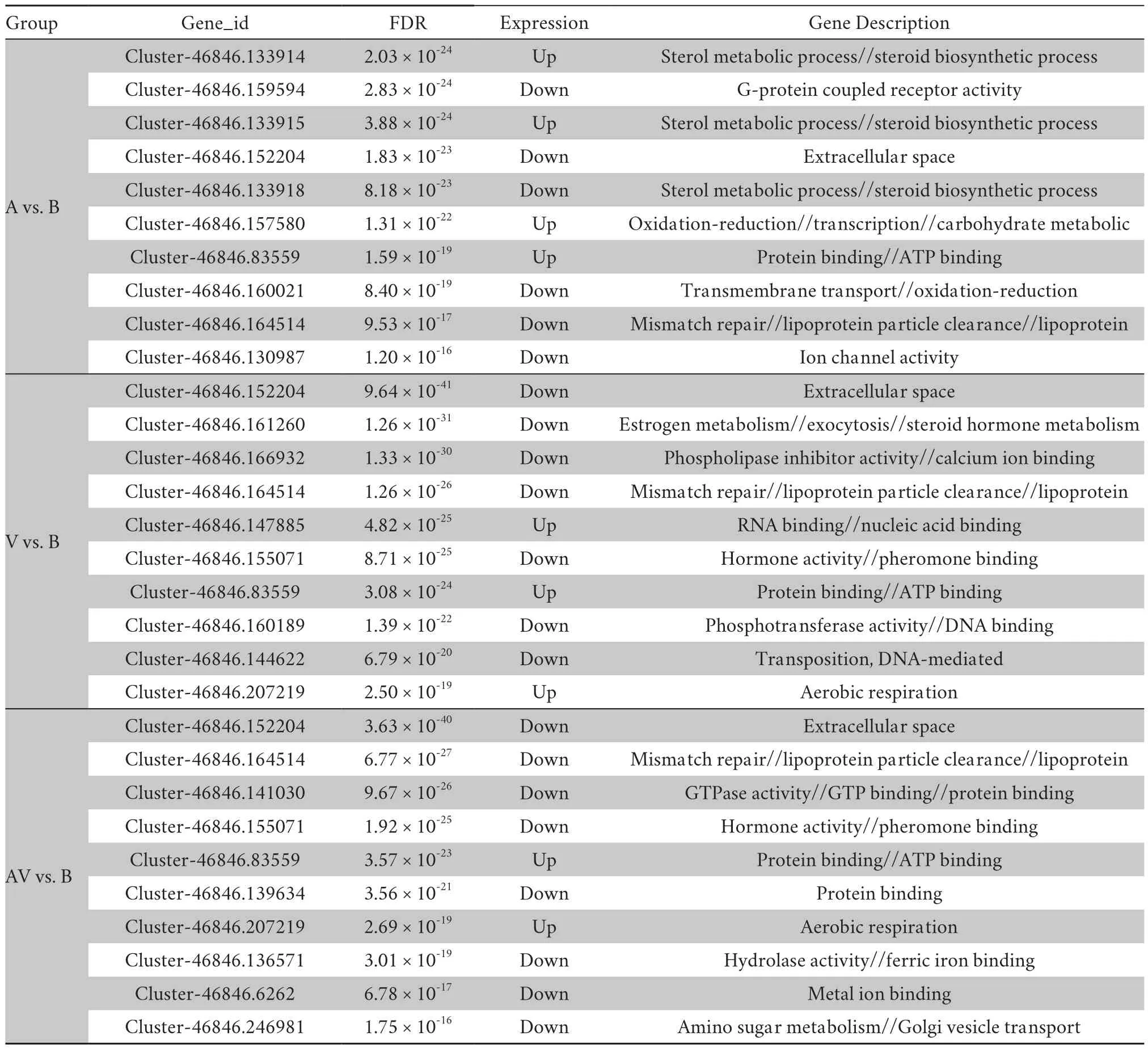
Table 3 Top 10 DEGs in response to different behavioral categories.

,
2013).For instance,gene expression may be determined by behavior as well as by environmental stimulation.Research on gene expression changes needs to be conducted under strictly controlled internal and external conditions.In this study,female reproductive states and all experimental conditions were consistently controlled.Moreover,the playback and dark treatment times were designed according to several gene expression researches on anurans.Thus,our results not only demonstrate that the most widespread transcriptome technology (i.e.,RNA-seq) can be a powerful tool for measuring brain gene expression in response to complex stimuli,but may also improve the experimental design of future research on multimodal communication.A previous study on birdsong indicates that after song exposure,down-regulated genes outnumber the increasing ones in the brain (Donget al.,
2009).Interestingly,the same result was obtained when little torrent frogs were exposed to visual,acoustic,or audiovisual stimuli as compared with a blank contrast stimulus,as well as when frogs were exposed to an audiovisual versus a visual or acoustic stimulus (Table 2).Thus,these findings reveal a function of gene expression suppression in the brain.It is possible that such a mechanism is highly conserved due to its existence in birds and frogs as well as in the responses to different stimuli.At present,however,we know little about the mechanism of such gene suppression in the brain (Donget al.,
2009).It is possible that the suppression of gene expression is a homeostatic response evoked by an increase in signaling activity (Chewet al.,
1995;Striplinget al.,
1997).Brain energy metabolism has a close relationship with animal behavioral phenotypes (Rittschof and Schirmeier,
2018).However,we have limited knowledge on how energy metabolism is linked to the neural mechanisms,which ultimately give rise to these behavioral phenotypes (Raichle,
2015) due to the complexity and challenges of brain function exploration.In the little torrent frog,we found that brain energy consumption was linked to differential stimulus exposure.The functional classes of the up-regulated genes we identified showed that GO terms associated with energy metabolism were mostly enriched in the brain when females were presented with visual,acoustic,or audiovisual sexual stimuli (Table S2).Moreover,the analysis based on the top 10 DEGs showed a similar result;that is,genes associated with energy availability were all found to be up-regulated when females processed these stimuli (Table 3).These results are consistent with the idea that the female brain utilizes amounts of energy for processing different types of sexual signals.Further support for this idea is provided by the KEGG annotation in which the most significantly enriched cellular metabolic pathways were cardiac muscle contraction and oxidative phosphorylation.In male Zebra finches (Taeniopygia guttata
),the majority of nuclear genes associated with mitochondrial energetics change significantly in the process of song response habituation (Donget al.,
2009).In the little torrent frog,we suggest that female preferences for acoustic or visual cues may be accompanied by rapid changes in energy metabolism.Several neuronal activity-dependent molecular mechanisms have been proposed by which external stimuli trigger a neurogenomic shift (Wolf and Linden,
2012;Cardosoet al.,
2015).One possible mechanism depends on the activation,such as by phosphorylation,of pre-existing proteins that subsequently regulate IEGs or the expression of other response genes,or act on the MAPK or other intracellular signaling pathways.IEGs are a set of activity-dependent genes that respond rapidly to various stimuli and have been commonly used to explore neuronal activity in the vertebrate brain (Terleph and Tremere,
2006).Many researchers use immunocytochemistry orin situ
hybridization procedures to explore sensorydriven IEG expression in the brains of songbirds and frogs evoked by acoustic or visual stimuli.In zebra finches,visual information (i.e.,colored lights) can influence gene responses to song stimulation (Baileyet al.,
2002;Kruseet al.,
2004),while pairing visual cues with song stimulation does not increaseegr-1
expression in higher-order auditory telencephalic regions including the caudal medial mesopallium (CMM) and caudal medial nidopallium (NCM) (Aveyet al.,
2005).This study was a good starting point for gene function related to multimodal communication behavior.More research is needed to examine whether the neurons in brain areas involved in processing audiovisual multimodal signals increase the expression of IEGs.5.Conclusions
In sum,visual and auditory cues conveyed some of the same information related to mate-choice and in combination increased the sexual attractiveness of one another in little torrent frogs.Sequencing data of whole brain tissue showed different neural genomic responses in females exposed to different communication behaviors,suggesting that the brain transcriptome can be used to track audiovisual behavioral preferences as has been demonstrated for behavioral plasticity in some social insects.Based on these results,we analyzed energy metabolism which has been reported to regulate acoustic and visual communication in other animal species.GO and KEGG annotation revealed a significant energy metabolism response when females were exposed to visual,acoustic,or audiovisual stimuli as compared with a blank contrast stimulus,but not when comparing an audiovisual versus a visual or acoustic stimulus.These findings suggest that behavioral and neurogenomic responses to acoustic and visual sexual cues are correlated in anurans.Brain activities such as energy use are often temporally and spatially dynamic.Future studies on these dynamic processes would provide further insights into multimodal sensory mechanisms.
Acknowledgments
We are grateful to Tongliang WANG and Xiaoqian SUN for their help during the experiments.This work was supported by the National Natural Science Foundation of China (31772464 and 31572275),the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2012274),and the“Light of West China”Program of the Chinese Academy of Sciences.All subjects were collected from Mt.Diaoluo Nature Reserve in China with the consent of the management office.All experiments complied with the Animal Care and Use Committee of the Chengdu Institute of Biology,Chinese Academy of Sciences(CIB2017050004).Appendix

Figure S1 Hierarchical cluster analysis on DEGs shows that the transcriptional profiles of samples from same treatment group were more similar than those from different treatment group.

Table S1 Summary of A.torrentis brain transcriptomes in all treatments.
Table S2 GO annotations of differentially expressed genes in different comparisons.
The table is available at the website https://github.com/woxinfei/2020/blob/woxinfei-patch-1/Zhao%20et%20al_table%20S2.xlsx
杂志排行
Asian Herpetological Research的其它文章
- An Integrative Taxonomy of Amphibians of Nepal:An Updated Status and Distribution
- A New Species of the Gekko japonicus Group (Squamata:Gekkonidae) from Southwest China
- Genetic Diversity and Population Structure of the Oriental Garden Lizard,Calotes versicolor Daudin,1802 (Squamata:Agamidae) along the Mekong River in Thailand and Lao PDR
- Species Diversity,Distribution,and Microhabitats of Anurans on Mt.Kalo-Kalo of the Mt.Kalatungan Range Natural Park,Bukidnon,Philippines
- Species Account of Anurans from the Western Slope of Mt.Kitanglad,Mindanao Island,Philippines
- The Influence of Environmental Factors on the Behavior and Mortality Risk of Polypedates megacephalus Tadpoles
