Species Account of Anurans from the Western Slope of Mt.Kitanglad,Mindanao Island,Philippines
2021-04-02ElsaMayDelimaBARONMarkOliverMARINBenjoLOGRAMONTEandAlmaMOHAGAN
Elsa May Delima BARON ,Mark Oliver D.MARIN ,Benjo A.LOGRAMONTE and Alma B.MOHAGAN
1 Biology,Natural Sciences,and Math Division,Arts and Sciences Department,San Pedro College,Davao City 8000,Mindanao,Philippines
2 Biology Department,College of Arts and Sciences,Central Mindanao University,Musuan,Bukidnon 8714,Mindanao,Philippines
Abstract The Mt.Kitanglad Range is one of the country’s important key biodiversity sites;however,information about anuran diversity in this protected area remains depauperate.Herein we provided accounts of anuran species from high-elevation forests,in three sites of the western slope of Mt.Kitanglad range.The combined belt-transect sampling and microhabitat searches accounted for 13 species representing five families.The most represented family was Rhacophoridae with five representative species of the genus Philautus.Twelve out of the 13 species documented in the current survey are endemic.Four previously unaccounted species(Pelophryne brevipes,Pulchrana grandocula,Sanguirana mearnsi,and Philautus surrufus) were added and brought the total anurans known from Mt.Kitanglad to 26 species.Most of the species were also recorded in forested sites,suggestive of their lesser affinity to non-forested ecosystems.The additional species detected during our survey may also imply that full understanding of anuran diversity of Mt.Kitanglad remains far from complete.
Keywords key biodiversity area,Mindanao faunal region,Philippine endemic anurans,upper elevation forests,wildlife inventory in Mindanao
1.Introduction
Tropical forests,such as those found in the Philippines,are the most diverse ecosystems in the world attributed to the heterogenous ecological niches occurring on it (Gaston,2000;Schefferset al
.,2013) with anura as one of the faunal groups that has benefitted from it.Its diversification is attributed to the canopy cover,understory plants,and lush forest litter (Alcalaet al
.,2012;Angeliniet al
.,2011;Brownet al
.,2001).Although knowledge about anurans of the Philippines have increased dramatically in the last few decades,most studies and subsequent publications were conducted in several mountain ranges in Luzon (Binadayet al
.,2017;Brownet al
.,2000;Brownet al
.,2012;Cruzet al
.,2018;Diesmoset al
.,2004;McLeodet al
.,2011;Sileret al
.,2011;Sileret al
.,2012).Moreover,recent studies in 23 Mindanao,the second largest island in the country,provided preliminary knowledge on anuran diversity (Almeria and Nuñeza,2013;Baronet al
.,2019;Brunoet al
.,2017;Calo and Nuñeza,2015;Coriticoet al
.,2018;Dacaluset al
.,2017;Nunezaet al
.,2010;Plaza and Sanguila,2015;Supsupet al
.,2017;Warquezet al
.,2013),but is believed to be still very limited (Sanguilaet al
.,2016).Mt.Kitanglad Range Natural Park,one of the largest mountain ranges in Mindanao which is also considered as an ASEAN Heritage Park and a key biodiversity area was declared as a priority area under Proclamation 896 in 1996 and later as a protected area under the Republic Act 8978.It is an extensive mountain range with several peaks including Mt.Imbayao,Mt.Kaatoan,Mt.Nangkabulos,Mt.Dulangdulang,and Mt.Kitanglad.The range covers approximately 30 642 ha of mostly montane and mossy forests situated above 1000 meters (Mallariet al
.,2001).As a protected and key biodiversity area,extensive surveys have been done in Mt.Kitanglad in the past and in recent years.However,many of these surveys appear unpublished,thus limiting the accessible reports about anuran species found in Mt.Kitanglad.Moreover,published accounts (see Amoroso,2000;Beukema,2011;Heaney and Peterson,1992;Mohaganet al
.,2018) show an increasing number of anuran species.Unavailability of many anuran inventory reports,as well as the increasing species count,hamper deeper understanding of the actual anuran diversity in this important mountain range. Given the need for more information,the current study presents additional data on anurans,specifically in higher elevation forests of the western slope of Mt.Kitanglad.2.Methodology
2.1.Sampling Sites
Three different vegetation types in the western slope of Mt.Kitanglad,Barangay Lirongan,Municipality of Talakag,Province of Bukidnon,Mindanao Island,were surveyed from 3-9 January 2019 (Figures 1-3).This mountain ecosystem forms part of the Mt.Kitanglad Range Natural Park including Mt.Imbayao,Mt.Kaatoan,Mt.Nangkabulos,and Mt.Dulangdulang.The park contains large expanse of montane and mossy forests at elevations above 1400 masl while forest below this elevation is often a secondary growth type (Mallariet al
.,2001).The foothill of the mountain is surrounded by large tract of vegetable gardens maintained by the locals.Occasional drizzles in the early morning and late afternoon occur at the time of sampling.Weather during nocturnal searches were mostly humid,and with clear skies.Site 1
Philippines,Mindanao Island,Bukidnon,Municipality of Talakag,Barangay Lirongan-Mt.Kitanglad (83’46”,12449’15”,1483-1621 masl) is an agro-ecosystem with several plots of vegetables including potato (Solanum tuberosum
),cabbage(Brassica oleracea
),and carrots (Daucus carota
) planted on rotation and served as the primary economic produce of the locals.Alongside these vegetables,locals also plant legumes as primary cover crop.A creek was found on one side of the area but it was basically dried up at the time of sampling.Several small temporary pools,possibly collected in holes during occasional drizzles were visible in some spots near the creek.Piper adancum
was present on sides of the creek alongside with cogon grass,and several shrub plants.Thickets of bamboo stands were also present in the area.The area was fully exposed to the sun with few scattered stands of trees,and is the most humid among sites surveyed.Site 2
Philippines,Mindanao Island,Bukidnon,Municipality of Talakag,Barangay Lirongan-Mt.Kitanglad (84’15”,12449’16”;84’31”,12449’15”,1686-1729 masl) is a montane forest,approximately one-hour regular walk from the agro-ecosystem site.Several old growth trees were present in the area whose trunks were partly covered with moss.Several species ofHoya,
and plants of the ginger family were observed in the area.Bromeliads were also present.Leaf litter was approximately two centimeters thick.Unlike the first site,this area has more trees of old and secondary growth type.The canopy is moderately covered while the understory is filled with shrubs,epiphytes,and saplings of trees.Freshwater bodies were not evident in the area.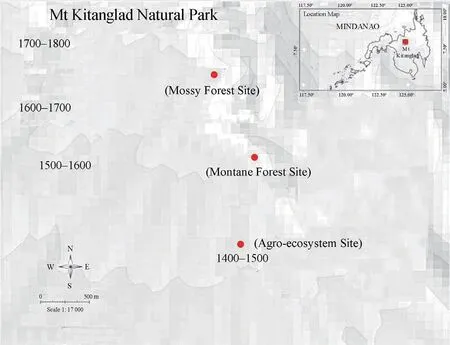
Figure 1 Map showing sites surveyed.Inset map shows location of Mt.Kitanglad in the southern part of the Philippines.

Figure 2 Arboreal microhabitat in the montane forest site in the western slope of Mt.Kitanglad.Photo by E.M.D.BARON.

Figure 3 Mossy forest site sampled in the western slope of Mt.Kitanglad.Photo by E.M.D.BARON.
Site 3
Philippines,Mindanao Island,Bukidnon,Municipality of Talakag,Barangay Lirongan-Mt.Kitanglad (84’42”,12449’13”;84’50”,12449’17”,1706-1815 masl) is a mossy forest reachable by approximately one hour regular walk from the montane forest site.Vegetations,especially the tree trunks,were thickly covered with moss.Several epiphytic plants includingAsplenium
species were abundant in the area.Leaf litter was approximately three to four centimeters thick.There are more trees in this site,and tree girth was lesser than those found in the second site.Canopy was more covered than Site 2 but understory had less saplings.Freshwater bodies were still not evident.2.2.Sampling Technique
Anurans were documented using belt-transect sampling,covering 100-meter long by 10-meter wide transect and coupled with microhabitat searches (Heyeret al
.,1994).Sampling was done from 0900 H-1400 H (diurnal search) and 1800 H-2200 H (nocturnal search).Individuals of anurans were hand caught whenever encountered,and placed in specimen bags.Representative individuals of species were photographed prior to standard specimen preservation processing while other individuals were released.Additional notes,such as microhabitats where frogs were first encountered and associated activities,were also recorded.The identification was based on morphological characters described by Alcala and Brown (1998),Brown and Alcala (1994),and Inger (1954),but supplemental descriptions from recent publications on new species and taxonomic redescriptions were also consulted.Voucher specimens are currently deposited in the Zoology Museum of Central Mindanao University.3.Results
A total of 13 anurans species representing five families were documented in the western slope of Mt.Kitanglad (Table 1).The most represented family was Rhacophoridae with five representative species of the genusPhilautus
(P.acutirostris
,P.leitensis
,P.
cf.surdus
,P.surrufus
,andP.worcesteri
).Twelve out of the 13 species documented in the current survey are endemic.OnlyRhinella marina
,is the non-endemic and invasive species accounted.One individual of the relatively cryptic rhacophoridP.surrufus
,originally documented from Mt.Malindang,Dapitan Peak (Brown and Alcala,1994),and known from northwest and central mountains of Mindanao (Diesmoset al
.,2015;Frost,2020) was accounted during the survey.This species together withPelophryne brevipes
,Hylarana grandocula
,andSanguirana mearnsi
,was the fourth additional species known to occur in Mt.Kitanglad.Accounts for each species reported in this current survey is provided with notes on their ecology and distribution.Species Account
Bufonidae
Boulenger,1887
Müller’s Stream Toad
Remarks:
Endemic.A.muelleri
(Figure 4) has a relatively smooth back due to lesser tubercles differentiating it from its close congener,A.mcgregori
(Inger,1954).It is known to occur in eastern and central Mindanao (Sanguilaet al
.,2011).Although majority of the samples was encountered in stream banks or in streams with free and fast moving current (Sanguilaet al
.,2016),samples of the current study were found on low-lying vegetation approximately 3 kilometers away from a stream,and on moss adhering to tree trunks in the mossy sampling area.Some males were heard vocalizing at the time of nocturnal search.Samples from Mt.Kitanglad also have a somewhat uniform dark coloration on its dorsum.
Distribution:
mountains of central and western Mindanao,and Dinagat Island (Diesmoset al
.,2015;Frost,2020)Specimens:
EMD1045,EMD1046,EMD1048,EMD1049Peters,1867
Zamboanga Flathead Toad
Remarks:
Endemic.P.brevipes
(Figure 5) can be distinguished from other Philippine bufonids by the noticeable short first finger and an hourglass-like pattern on its back.Pelophryne brevipes
does not have a white line that passes from behind the eye towards the back,as if forming a dorso-lateral line which is present on its close congener,P.lighti
(Inger,1954).Only two individuals were encountered in the current survey,possibly due to its diminutive size and its cunning way of adhering to same colored foliage of shrub plants.Samples initially appeared as mere speck on top of foliage.Such ability to camouflage could also be one reason why the species was not accounted in prior anuran inventories in Mt.Kitanglad.Distribution:
Basilan and Mindanao (Diesmoset al
.,2015;Frost,2020)Specimens:
EMD1005 and 1006Linnaeus,1758
Cane Toad
Remarks:
Introduced.R.marina
(Figure 6)is easily identified by its stocky body and prominent parotid glands.This frog was encountered in the agro-ecosystem site near vegetable plots.Our observation is consistent with previous reports of the occurrence of this species in plantations,rice paddies,and buildup areas (Alcala and Custodio,1995;Delima,Ates,and Ibañez,2006;Diesmoset al
.,2006;Sanguilaet al
.,2016;).No specimens were collected.Distribution:
East of Andes throughout Amazonian and Guianan South America,introduced in Florida,Fiji,Antilles,Hawaii,Taiwan,New Guinea,Solomon Islands,Australia,other Pacific islands,and several major islands of the Philippines (Piliet al
.,2019;Frost,2020)Dicroglossidae
Stejneger,1910
Mindanao Fanged Frog
Remarks:
Endemic.L.magnus
(Figure 7) is easily differentiated from other MindanaoLimnonectes
species by its large and stocky body,a huge head,and the axial region of its dorsum that is almost devoid of asperities or tubercles giving it a smooth look(Inger,1954;Sileret al
.,2009).Most of theL.magnus
individuals were encountered in aquatic microhabitats with a few individuals observed on forest litter away from water bodies.Samples from the agro-ecosystem site were collected near water pools along the dried creek.This species appears to be one of the favored exotic food among locals and is intentionally collected in streams and creeks.The species also appears abundant in the area during rainy season.
Figure 4 Ansonia muelleri (Boulenger,1887) sampled from the mossy forest.Photo by E.M.D.BARON.

Figure 5 Pelophyrne brevipes (Peters,1867) sampled from the mossy forest.Photo by E.M.D.BARON.
Distribution:
Mindanao,Biliran,Camiguin Sur,Basilan,Bohol,Dinagat,Samar,and Leyte Islands of the Philippines (Diesmoset al
.,2015;Frost,2020)Specimens:
EMD984 and 985Megophryidae
Brown,Siler,Diesmos,and Alcala,2010
Remarks:
Endemic.L.lumadorum
’s (Figure 8) gray dorsal coloration with minute dermal asperities,a pair of glands appearing as“tits”on the axial region on its ventral side together with the absence of dermal projection in its eyes,can be used to identify this species from other gray-colored species (Brownet al
.,2009).Although this species was recorded in all three sites in Mt.Kitanglad,only a single specimen was collected.The darkcolored dorsum allowed it to blend well in the canopy-covered forests,especially in the forest floor,making it difficult to spot.However,during nocturnal searches when humidity was low,males were heard vocalizing loudly.Distribution:
southern portions of Mindanao,Basilan,Dinagat but absent in other smaller islands associated with the Mindanao Faunal Region (Diesmoset al
.,2015;Frost,2020)
Figure 6 Rhinella marina (Linnaeus,1758) documented in the agro-ecosystem.Photo by E.M.D.BARON.

Figure 7 Limnonectes magnus (Stejneger,1910) sampled from the agro-ecosystem and montane sites.Photo by E.M.D.BARON.
Specimen:
EMD1024Taylor,1920
Remarks:
Endemic.M.stejnegeri
(Figure 9)is easily distinguished by the presence of a pair of prominent gland of the axial region on its ventral side and the presence of dermal projections on top of its eyelids (Inger,1954;Sanguilaet al
.,2016).This species is also found to be successfully creeping into the forest floor and blending well on the dominant coloration of the environment where the samples were initially encountered.Such observation is substantiated by the dominant coloration of the specimens that is similar to the color of the forest litter where it is initially encountered.Two individuals were observed,but not collected.
Figure 8 Leptobrachium lumadorum (Brown,Siler,Diesmos,and Alcala,2010) sampled in all sites.Photo by E.M.D.BARON.

Figure 9 Megophrys stejnegeri (Taylor,1920) encountered on forest floor of higher elevation forests.Photo by E.M.D.BARON.
Distribution:
Biliran,Bohol,Leyte,Samar,Dinagat,Basilan,and Mindano (Diesmoset al
.,2015;Frost,2020)Ranidae
Taylor,1920
Big-eyed Frog
Remarks:
Endemic.P.grandocula
(Figure 10) can be distinguished from other Philippine ranids by having a strictly brown to dark brown dorsal coloration and a complete dorsolateral line or coloration (Brown and Guttman,2002).Some samples were collected in temporary pools near the dried creek,while majority were initially found perched on low lying vegetation on the creek bank. This observation is consistent with previous accounts of the preferred aquatic microhabitat of this species (Delima,Diesmos,and Ibañez,2007;Plaza and Sanguila,2015;Sanguilaet al
.,2016).Despite being widespread across all elevations in several localities in Mindanao (Sanguilaet al
.,2016),this species was not listed in previous surveys conducted in Mt.Kitanglad.Distribution:
islands of Biliran,Bohol,Samar,Leyte,Camiguin,Dinagat,Mindanao (Diesmoset al
.,2015;Frost,2020)Specimens:
EMD 982,EMD1022,EMD1023Stejneger,1905
Cabilian Frog
Remarks:
Endemic.S.mearnsi
(Figure 11) possesses a metallic bright green dorsal coloration with bright yellow dorsolateral folds,and with no transverse tibial bars (Diesmoset al
.,2015;Brownet al
.,2017).Sanguirana mearnsi
individuals were found on aquatic microhabitats,but one was found on top of a tree branch on the creek bank while the other sample was found near a pool of water.Although observed to inhabit riparian microhabitats,this species appears to be less common during dry seasons.This species was also not accounted in previous anuran inventories conducted in Mt.Kitanglad possibly because of its greenish coloration easily mistaken as leaf and the low vocalization of males (Sanguilaet al
,2016).Distribution:
Samar,Leyte,Mindanao (Diesmoset al
.,2015;Frost,2020)Specimens:
EMD1021,EMD1025Rhacophoridae
Peters,1867
Philippine Bubble-nest Frog

Figure 10 Pulchrana grandocula (Taylor,1920) sampled in the agro-ecosystem.Photo by E.M.D.BARON.

Figure 11 Sanguirana mearnsi (Stejneger,1905) sampled in the agro-ecosystem.Photo by E.M.D.BARON.
Remarks:
Endemic.P.acutirostris
(Figure 12) is easily distinguished from other currently recognizedPhilautus
species present in Mindanao because of its distinctly pointed snout tip,nearly smooth dorsum which is almost devoid with asperities,and a relatively short snout to vent length (Brown and Alcala,1994).Samples were encountered in both montane and mossy forests of Mt.Kitanglad.Individuals encountered were either perched on leaves,twigs,and dry branches;partially hidden on moss adhering to tree trunks or branches;while others were found on leaf litter on the ground.The vertical stratification of this species ranged from zero to two meters above ground.Dorsal coloration was also highly variable from shades of yellow,reddish-orange,and brown to tan.This is also the most encountered among thePhilautus
species recorded for this survey.Distribution:
Bohol,Mindanao,Jolo,Basilan (Diesmoset al
.,2015;Frost,2020)Specimens:
EMD983,EMD 987,EMD988Boulenger,1897
Leyte Bubble-nest frog
Remarks:
Endemic.P.leitensis
(Figure 13) appears to have a very smooth dorsum since asperities and tubercles occur minimally.Philautus leitensis
is distinguished from its close congenerP.acutirostris
by the absence of a very pointed snout.This species can be distinguished from otherPhilautus
species in Mindanao through the presence of a very minimal tubercles or asperities on its back and the absence of vomerine teeth (Brown and Alcala,1994).Individuals of this species were found in arboreal microhabitats either perched to a leaf or leaf axil,adhering to a petiole,and positioned on dead branches as observed with some females.Other samples were found on ground microhabitats often concealing themselves between fern fronds or clustered in the moss on either ground or attached to the root or stem.Distribution:
Leyte,Bohol,Mindanao (Diesmoset al
.,2015;Frost,2020)Specimens:
EMD989,EMD1004,EMD1019,EMD1020cf.
Peters,1863
Luzon Bubble-Nest frog
Remarks:
Endemic.P.
cf.surdus
(Figure 14) has a very variable dorsal morphology but the presence of two heavily pigmented pair of tubercles on the shoulder level differentiates this species from otherPhilautus
species known from Mindanao (Brown and Alcala,1994).Samples were encountered in both ground(atop leaf litter,atop moss adhering to decaying log,lowlying vegetation less than 1 meter in height) and arboreal microhabitats (epiphytes beyond one meter from the ground,leaf axils,branches,and twigs one meter above the ground).This is the second most encountered species in terms of number of individuals for this survey.Although this species currently appears to show a combination of characters of the Luzon,Pollilo,and Mindanao specimens,Brown and Alcala (1994)noted deviation on extent of toe webbing of Mindanao samples.The variable dorsal morphology of samples from the current study supports prior observations of Sanguilaet al.
(2016) on the difficulty of identifying this species and that morphological data has to be backed up with molecular identification.Thorough inspection of more samples from other localities,coupled with molecular data,may clarify this gap.Distribution:
Bohol,Mindanao,Luzon Pollilo Islands (Diesmoset al
.,2015;Frost,2020)Specimens:
EMD992,EMD993,EMD996,EMD 997,EMD998,EMD999
Figure 12 Philautus acutirostris (Peters,1867) sampled in the montane and mossy forests.Photo by E.M.D.BARON.

Figure 13 Philautus leitensis (Boulenger,1897) sampled in the montane and mossy forests.Photo by E.M.D.BARON.
Brown and Alcala,1994
Remarks:
Endemic.P.surrufus
(Figure 15) can be identified using the following characters:absence of darkly pigmented pair of tubercles on it shoulder level,absence of knob-like appearance of the snout,and a reddish dorsal coloration(Brown and Alcala,1994).A single sample was encountered two meters above ground and perched on a leaf axil in the mossy forest of Mt.Kitanglad.This species is also among the four unaccounted species in the previous anuran surveys in the area.Distribution:
several localities in Mindanao (Diesmoset al
.,2015;Frost,2020)Specimen:
EMD1017Stejneger,1905
Mindanao Bubble-Nest Frog
Remarks:
Endemic.P.worcesteri
(Figure 16) is a stocky-bodiedPhilautus
primarily distinguished by the presence of a knoblike protrusion of its snout when viewed under the microscope(Brown and Alcala,1994).Two individuals of this species were encountered atop leaf axil,and atop frond ofAngiopteris palmiformis
in the mossy forest site.Distribution:
several localities on Mindanao (Diesmoset al
.,2015;Frost,2020)Specimen:
EMD1003,EMD10104.Discussion
Despite the short sampling period (approximately 2-3 days per site),13 species were accounted in the present survey.This relatively low count however increased the total known anurans to 26 species for Mt.Kitanglad.Although there is a reason to believe that there could be data from unpublished reports (ie unpublished theses),much of the publicly known knowledge of Mt.Kitanglad anurans depend heavily on limited published articles (Amoroso,2000;Beukema,2011;Heaney and Peterson,1992;Mohaganet al
.,2018).Thus,the basis of comparing previous species account and richness is solely based on these accessible literatures.Anuran species richness varied significantly among the reports including the current study.Several factors may contribute to this including weather condition at the time of sampling,sampling site selection,faunal focus,and sampling effort.Based on coordinates and site municipalities mentioned in previous published accounts cited here,there is a reason to believe that current site was not previously explored,or if explored,data was not published.Heaney and Peterson (1992)sampled extensively in Impalutao,Sumilao,Chinchona and Malaybalay covering lowland to higher elevation forests with special interest on mammals although other vertebrate groups like anurans were included.Amoroso (2000) may have sampled between the municipalities of Impasug-ong and Sumilao,but is assumed to have focused primarily on plant survey with possible opportunistic collections of anurans.Brownet al
.(2009) mentioned of the municipalities of Sumilao,and Libona as sources ofLeptobrachium lumadorum
in Mt.Kitanglad.Beukema in 2011 published survey results done on the disturbed lowland forest fragments within the Municipality of Sumilao to document anurans,and reptiles.The most recent paper of Mohaganet al
.(2018) covered an upper montane forest with an entirely different site coordinates.Moreover,all of the sites previously surveyed denoted presence of at least one freshwater body,a great contrast to the sites surveyed for the present study.
Figure 14 Philautuscf. surdus (Peters,1863) sampled in the montane and mossy forests.Photo by E.M.D.BARON.

Figure 15 Philautus surrufus (Brown and Alcala,1994) sampled from the mossy forest.Photo by E.M.D.BARON.
Despite these variations,the increasing number of species from Beukema’s (2011) record of 10 species (this being the highest species richness prior to this study) to the account of 13 species of the present study,is positively suggesting an increasing species count and thereby implies that given more survey efforts,especially intensive surveys in more localities,anuran richness and diversity in Mt.Kitanglad is expected to increase.Such effort however needs to be coupled with actions ensuring that data are made accessible so that future researchers will have reference data when comparing anuran diversity in this mountain range.

Figure 16 Philautus worcesteri (Stejneger,1905) sampled from the mossy forest.Photo by E.M.D.BARON.
Eight species were accounted from the mossy forest site,although only four of these were exclusively recorded there:Ansonia muelleri
,Pelophryne brevipes
,Philautus surrufus
,andP.worcesteri
.There were five species accounted from the agroecosystem site (Rhinella marina
,Limnonectes magnus
,Leptobrachium lumadorum
,Pulchrana grandocula
,andSanguirana mearnsi
),butL
.magnus
andL.lumadorum
were also encountered in the montane forest site.The bigger proportion of the species accounted shows greater affinity towards a forested site,montane and/or mossy,than non-forested ecosystem like the agro-ecosystem(Figure 1).This data seems to agree with previous accounts that more anuran species were documented in forested sites than entirely open ecosystems (Aureo and Bande,2017;Cruz and Afuang,2018;Delimaet al
.,2007;Diesmoset al
.,2004).Closed canopy forests provide an ambient condition such as a moist environment,and a relatively cooler understory (Hillerset al
.,2008;).This is significant since anurans are sensitive to temperature fluctuations (Bickfordet al
.,2010;Piedrahitaet al
.,2017;Schefferset al
.,2013).Forested habitats with canopy cover also allow leaf litter to accumulate and eventually contribute to a thicker humus deposition on the forest floor.These conditions allow diversification of microhabitats that are beneficial to anuran assemblage (Alcalaet al
.,2004).The observation ofR
.marina
in high elevation habitats such as the agro-ecosystem site in the current study parallels reports of Diesmos (1998) on the presence of this species in the summits of Mount Makiling (1090 masl) and Mount Arayat (1026 masl).This however magnifies evident anthropogenic activities in this side of Mt.Kitanglad Range.Rhinella marina
was previously accounted in sites where anthropogenic activities are prominent such as areas near human habitations,and in artificial habitats such as forest edges,and agricultural and cultivated sites (Alcala and Brown,1998;Diesmoset al
.,2015;Gaulke,2011).Although this invasive species was not documented inside the forested sites,the proximity of the agro-ecosystem site to the forested area may also entail how progressive anthropogenic activities are in the area.Since 12 out of the 13 species accounted are endemic species and appears to have dependence on forested habitats,the increasing magnitude of anthropogenic activities might eventually affect these endemic species.Studies to confirm this appear timely.Additional anuran inventories are highly encouraged due to the presence of anthropogenic pressures in the western slope of Mt.Kitanglad Range.Acknowledgments
The researchers are thankful to the generosity of time and talent of the local parabiologists who assisted them during the sampling.The help of the local officials of Barangay Lirongan and municipality of Talakag for ease of site access by the researchers is also commended.The regional office of DENR X issued the Gratuitous Permit for voucher collection.Arvin C.Diesmos helped in the confirmation of identification of some samples.Researchers are also thankful to Guiler Opiso for the help in preparing the map.杂志排行
Asian Herpetological Research的其它文章
- An Integrative Taxonomy of Amphibians of Nepal:An Updated Status and Distribution
- A New Species of the Gekko japonicus Group (Squamata:Gekkonidae) from Southwest China
- Genetic Diversity and Population Structure of the Oriental Garden Lizard,Calotes versicolor Daudin,1802 (Squamata:Agamidae) along the Mekong River in Thailand and Lao PDR
- Species Diversity,Distribution,and Microhabitats of Anurans on Mt.Kalo-Kalo of the Mt.Kalatungan Range Natural Park,Bukidnon,Philippines
- Behavioral and Neurogenomic Responses to Acoustic and Visual Sexual Cues are Correlated in Female Torrent Frogs
- The Influence of Environmental Factors on the Behavior and Mortality Risk of Polypedates megacephalus Tadpoles
