3-甲基己二酸稀土配位聚合物的合成、结构和磁学性质
2021-04-01谷博涵汪泉秀张巨文
谷博涵,徐 爽,汪泉秀,张巨文
(渤海大学 化学与材料工程学院,辽宁 锦州 121013)
Gu B H,Xu S,Wang Q X,et al.Syntheses, structures and magnetic properties of lanthanide 3-methyl-adipate coordination polymers[J].Journal of Engineering of Heilongjiang University,2021,12(1):42-46.
The crystal engineering realm of metal-organic coordination polymers (CPs) with intriguing architectures and topologies has been flourishing[1-2], which opened up great potential application of CPs in catalysis[3-4], adsorption[5-6], ion exchange[7], chemical sensing[8], luminescence[9-10], magnetism[11-12]etc. In this realm, there has been an enormous amount of research for constructing novel CPs by choosing functional metal ions and versatile organic ligands. The lanthanide ions have been comprehensively employed as building blocks to construct CPs which exhibit fascinating architectures due to their high coordination numbers and lanthanide contraction[13]. More importantly, the lanthanide ions have interesting properties such as luminescence and magnetism[14-15], making them promising functional materials. Moreover, it is well-known that the characteristic of the organic ligand plays a key role in the structural assembly of CPs. Thus, the selection of a suitable ligand is crucial in the building of lanthanide-organic CPs. The aliphatic polycarboxylic acids are a family of important flexible organic ligands which possess a variety of coordination modes in CPs[16]. In particular, the flexible aliphatic polycarboxylic acids with the flexibility and conformational freedom may bend, rotate and adjust when they coordinate to the metal centers, and cause the structural diversity[17-18]. It is noteworthy that, using them to construct the abundant and intriguing CPs has a growing trend in recent years[19-21].
So far, much work was focused on using flexible aliphatic acids to prepare lanthanide-organic CPs, such as glutaric acid, adipic acid[22-27]. However, the substituent adipate-based lanthanide-organic CPs have been less developed[28]. Thus, in this contribution, we chose a flexible substituent aidpic acid, 3-methyl-adipic acid (H2L), to construct two lanthanide-organic CPs [Er2(L)3(H2O)4] (1) and [Yb2(L)3(H2O)4] (2). Considering the binuclear Ln2unit in1and2, we also investigated the magnetic properties of1and2.
1 Experimental
1.1 Materials and measurements
All commercially available chemicals and solvents are of reagent grade and were used without further purification. Elemental analyses for C and H were performed on a Perkin-Elmer 2400 CHN elemental analyzer. FT-IR data were collected on a Magna FT-IR 560 spectrometer using KBr plate. Powder X-ray diffraction analyses were carried out on a Bruker AXS D8-Advanced diffractometer with Cu Kα (λ=0.154 06 nm) radiation. Thermogravimetric and differential thermoanalyses were simultaneously performed on a Pyris-Diamond thermal analyzer in the temperature range of 30~900 ℃ at a heating rate of 10 ℃·min-1under a flowing N2atmosphere. Magnetism was tested on a Quantum Design MPMS XL-7 SQUID-VSM magnetometer.
1.2 Syntheses of 1 and 2
A mixture of Ln(NO3)3·nH2O (Ln=Er or Yb, 0.2 mmol), 3-methyl-adipic acid (0.101 g, 0.5 mmol), aqueous NaOH solution (1 mL, 0.1 mol·L-1), EtOH (1.5 mL) and distilled water (10 mL) was placed in a 25 mL Teflonlined stainless steel vessel. The mixture was sealed and heated at 140 ℃ for 4 days, and then the reaction system was cooled to room temperature. The white block-shaped crystals were obtained. Elem. anal. calcd. for1: C, 28.63; H, 4.35. Found: C, 28.28; H, 4.31. Calcd. for2: C, 28.26; H, 4.29. Found: C, 28.02; H, 4.33. FT-IR (KBr, cm-1) for 1: 3 357 (m), 3 255 (m), 2 953 (m), 2 874 (w), 2 380 (w), 1 660 (m), 1 557 (s), 1 459 (s), 1 412 (s), 1 326 (w), 1 295 (w), 1 270 (w), 1 210 (w), 1 179 (w), 1 089 (w), 945 (w), 846 (w), 782 (w), 750 (w), 666 (w). For2: 3 366 (m), 3 256 (m), 2 954 (m), 2 869 (w), 2 373 (w), 1 660 (m), 1 557 (s), 1 458 (s), 1 419 (s), 1 326 (w), 1 294 (w), 1 272 (w), 1 216 (w), 1 179 (w), 1 092 (w), 945 (w), 845 (w), 781 (w), 759 (w), 666 (w).
2 Results and discussion
2.1 Structural description
In previous work, we reported a series of lanthanide 3-methyl-adipate CPs [Ln2(L)3(H2O)4] (Ln=Nd, Sm, Eu, Gd, Tb and Dy, H2L=3-methyl-adipic acid). Although all of their single-crystal X-ray diffraction data were collected, only the single-crystal structures of [Ln2(L)3(H2O)4] (Ln=Nd, Eu, Gd and Tb] were successfully solved after a lot of efforts. However, the powder X-ray diffraction analyses reveal the isostructural nature of these six CPs[28]. Similarly, in this work, the single-crystal structures of1and2could not be solved due to the disorder problems of the 3-methyl-adipate anions. Thus, the powder X-ray diffraction analyses were used to characterize the molecular structures of1and2. The PXRD patterns of the as-synthesized crystalline samples for1and2are in good agreement with the simulated PXRD pattern based on the single-crystal data of [Tb2(L)3(H2O)4] (Fig.1). These results can show the isostructural nature of1,2and [Tb2(L)3(H2O)4].
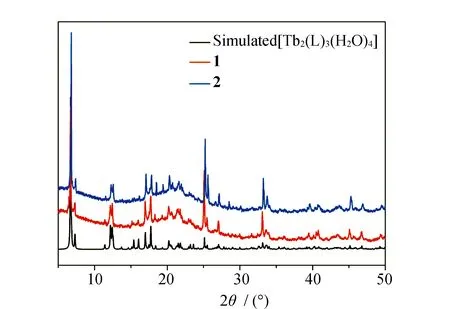
Fig.1 Simulated PXRD pattern of [Tb2(L)3(H2O)4] and as-synthesized PXRD patterns of 1 and 2
In order to understand the molecular structures of1and2, herein, we used the single-crystal X-ray diffraction data of [Tb2(L)3(H2O)4] to describe the single-crystal structure of1. As shown in Fig.2(a),CP1consists of two crystallographically independent ErIIIions, three 3-methyl-adipate anions and four coordinated water molecules. The Er1 ion is coordinated by nine oxygen atoms from four 3-methyl-adipate anions and two coordinated water molecules. The coordination polyhedron of Er1 is a muffin-shaped geometry (Fig.3(a))[29]. The coordination geometry of Er2 is similar to that of Er1. The 3-methyl-adipate anion possesses two types of coordination modes I (Fig.3(b)) and II (Fig.3(c)) in CP 1. The carboxyl groups of 3-methyl-adipate (I) bridge two Er1 ions and two Er2 ions to generate two types of Er2units, respectively. These two types of Er2units are linked by 3-methyl-adipate (I) into two types of 1D chains (Fig.2(b) and 2(c)), respectively. Additionally, these two types of 1D chains are alternately connected by 3-methyl-adipate (II) to construct a 2D layer (Fig.2(d)).
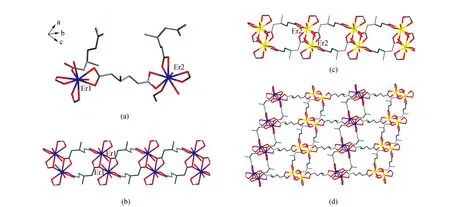
Fig.2 Crystal structure of 1. All hydrogen atoms are omitted for clarity. (a) Coordination environments of two ErIII ions. (b) 1D chain formed by the 3-methyl-adipate (I) anions bridging the Er12 units. (c) 1D chain constructed from the 3-methyl-adipate (I) anions bridging the Er22 units. (d) 2D layer built from the 3-methyl-adipate (II) anions bridging two types of 1D chains.
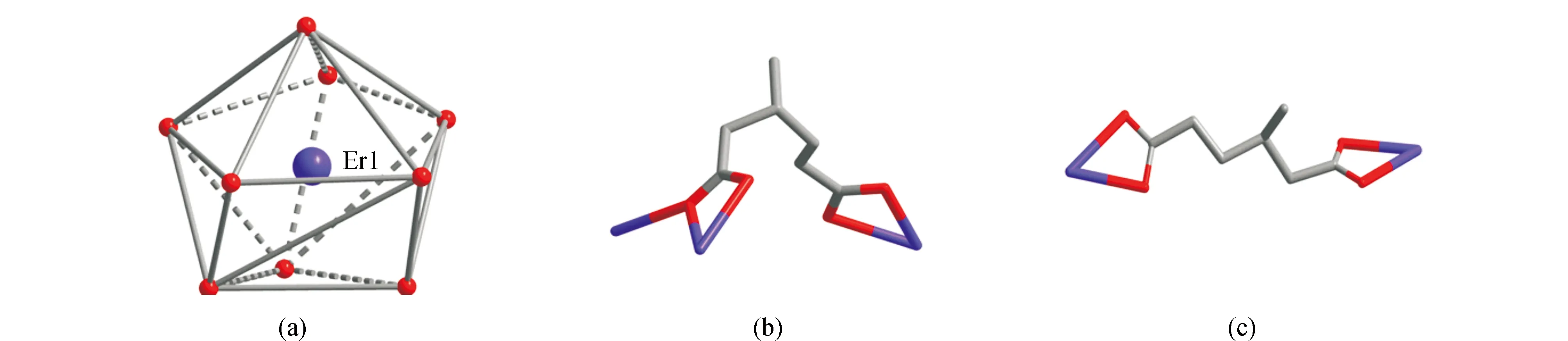
Fig.3 Coordination geometry of the ErIII ion in 1(a),Coordination modes I (b) and II (c) of the L ligand in 1
2.2 Thermal analyses
The TG curves of CPs1and2are shown in Fig.4. CPs1and2exhibit similar thermal stability. Thus, we chose CP1as a representative example to illustrate their thermal behaviors. CP1possesses two weight loss steps. The first weight loss of 9.63% from 90 to 160 ℃ is due to the loss of four coordinated water molecules (calcd. 8.17%). The second weight loss at 350 ℃ is attributed to the decomposition of the 3-methyl-adipate anions.

Fig.4 TG curves of 1 and 2
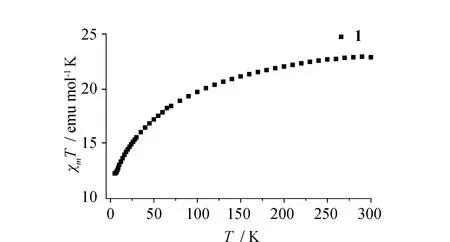
Fig.5 Temperature dependence of χmT for 1.
2.3 Magnetic properties
Owing to the presence of the binuclear Ln2units in1and2, the direct-current variable-temperature magnetic susceptibilities of1and2were investigated in the 2~300 K range in an applied magnetic field of 1 000 Oe. As shown in Fig.5 and Fig.6, at room temperature (300 K), theχmTvalues of1and2are 22.97 and 5.16 emu mol-1K, which are close to the theoretical values of 22.96 (ErIII) and 5.14 (YbIII) emu mol-1K for two isolated LnIIIions[30]. Upon cooling, theχmTvalues of1and2decrease gradually within the entire temperature range to 12.30 and 1.57 emu mol-1K at 2 K. Such behaviors may be attributed to either the presence of antiferromagnetic couplings between two LnIIIions or the thermal depopulation of Stark sublevels of LnIII[31].
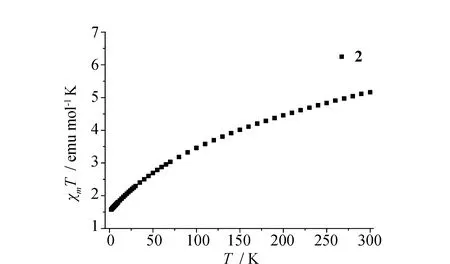
Fig.6 Temperature dependence of χmT for 2
3 Conclusions
Two lanthanide-organic CPs [Er2(L)3(H2O)4] (1) and [Yb2(L)3(H2O)4] (2) have been successfully prepared by the solvothermal method and structurally characterized. CPs1and2display a 2D layer structure built from the 3-methyl-adipate anions bridging two types of binuclear Ln2units. The weak antiferromagnetic interactions between two LnIIIions may exist in CPs1and2.
