非酒精性脂肪性肝病与阿尔茨海默病的相关性分析
2021-03-25张延霞郭宗君刘蕾张增红
张延霞 郭宗君 刘蕾 张增红




【摘要】 目的:通過分析阿尔茨海默病(AD)合并非酒精性脂肪性肝病(NAFLD)老年患者的临床特征,探讨NAFLD与AD的相关性及可能的机制。方法:选取2012年6月-2020年8月本院干部病房收治的AD患者66例,根据肝脏彩超分为AD+NAFLD组(观察组,n=32)和AD+肝脏正常组(对照组,n=34)。收集两组一般资料及疾病信息,并采用蒙特利尔认知评估量表(MoCA)和临床痴呆评定量表(CDR)评估其认知功能及日常行为能力。比较两组空腹血糖(FPG)、餐后2 h血糖(2 h PG)、糖化血红蛋白(HbA1c)、总胆固醇(TC)、甘油三酯(TG)、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)、尿酸(UA)、丙氨酸氨基转移酶(ALT)、天门冬氨酸氨基转移酶(AST)、胆红素(TB)、C反应蛋白(CRP)、血清补体C3、C4水平及MoCA、CDR评分,筛选AD的可能影响因素,分析各影响因素与MoCA评分和CDR评分的相关性。结果:两组血清ALT、AST、TB、2 h PG、UA、HbA1c、TG、HDL-C、LDL-C水平比较,差异均无统计学意义(P>0.05);观察组MoCA评分低于对照组,C3、C4、CRP、FPG、TC水平均高于对照组,差异均有统计学意义(P<0.05)。且观察组C3、C4、CRP、FPG、TC水平与MoCA评分均呈负相关(P<0.05),但与CDR评分均无相关性(P>0.05)。结论:NAFLD与AD患者认知障碍密切相关,可能是AD的危险因素,考虑NAFLD患者可通过升高TC水平损害脑细胞而导致神经细胞退行性疾病AD、升高FPG水平加重胰岛素抵抗导致AD发生发展、通过加重机体免疫炎症反应升高CRP、C3、C4水平而发生AD。
【关键词】 非酒精性脂肪性肝病 阿尔茨海默病 认知障碍
Analysis of Correlation between Nonalcoholic Fatty Liver Disease and Alzheimer’s Disease/ZHANG Yanxia, GUO Zongjun, LIU Lei, ZHANG Zenghong. //Medical Innovation of China, 2021, 18(28): 00-013
[Abstract] Objective: By analyzing the clinical features of Alzheimer’s disease (AD) patients with nonalcoholic fatty liver disease (NAFLD), to explore the correlation between NAFLD and AD. Method: From June 2012 to January 2019, 66 patients with AD admitted to cadre ward of our hospital were selected and divided into AD+NAFLD group (the observation group, n=32) and AD+liver normal group (the control group, n=34). The general data and disease information of two groups were collected, and the cognitive function and daily behavioral ability of two groups were evaluated by Montreal Cognitive Assessment scale (MoCA) and clinical dementia rating scale (CDR). Fasting blood glucose (FPG), 2 h PG, glycosylated hemoglobin (HbA1c), total cholesterol (TC), glycerol (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), uric acid, alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin (TB), C-reactive protein (CRP), complement C3 and C4 levels, and MoCA, CDR scores were compared between two groups. The possible influencing factors of AD were screened. The correlation between the influencing factors and MoCA score and CDR score was analyzed. Result: There were no significant differences in serum ALT, AST, TB, 2 h PG, UA, HbA1c, TG, HDL-C, LDL-C levels between two groups (P>0.05). The MoCA score of the observation group was lower than that of the control group (P<0.05), the levels of serum C3, C4, CRP, FPG, TC in the observation group were significantly higher than those in the control group (P<0.05). The levels of serum C3, C4, CRP, FPG, TC in the observation group were negatively correlated with the MoCA scores (P<0.05), but there were no correlation with CDR score (P>0.05). Conclusion: NAFLD is closely related to cognitive impairment in patients with AD and may be a risk factor for AD, considering that NAFLD patients can cause the neure cell degenerative disease AD by damaging brain cells with elevated TC levels, elevated FPG levels exacerbate insulin resistance leading to the development of AD, AD occurs by exacerbating elevated the levels of CRP, C3, C4 in the body’s immune inflammatory response.
[Key words] Nonalcoholic fatty liver disease Alzheimer’s disease Cognitive impairment
First-author’s address: Cadre Ward of PLA 960th Hospital, Tai’an 271000, China
doi:10.3969/j.issn.1674-4985.2021.28.003
阿尔茨海默病(Alzheimer’s disease,AD)是以进行性认知障碍和记忆力损害为主的中枢神经系统退行性病变,近年来其发病率有上升趋势,对老年人群和其家人的生活质量造成严重威胁[1]。AD的特点是起病隐匿,自然病程长,往往不能做到早期确诊,多数患者直到发展到中重度具有明显临床症状时才被家人发现得以就诊、确诊[2]。AD的临床表现多样,主要是认知功能和日常行为能力降低及精神行为的异常[3-4]。AD相关的发病机制有30多种假说,而有关非酒精性脂肪性肝病(NAFLD)与AD的关系报道很少[5-6]。本研究拟通过分析AD合并NAFLD患者的临床特征,探讨NAFLD与AD的相关性,并进一步探讨其可能的机制。现报道如下。
1 资料与方法
1.1 一般资料 选取2012年6月-2020年8月本院干部病房收治的具有完整临床资料的AD患者66例。NAFLD诊断采用《2010年中华医学会非酒精性肝病诊疗指南》[7]中的诊断标准。AD诊断采用美国精神病学、语言障碍和卒中-老年性痴呆和相关疾病学会标准。纳入标准:符合AD诊断标准;临床资料完整。排除标准:血管性痴呆,自身免疫疾病,严重心、肺、肝、肾功能疾病,近期有感染史,可能影响认知功能的疾病,如严重的帕金森病、肿瘤。根据肝脏彩超将其分为AD合并NAFLD组(观察组,n=32)和AD+肝脏正常组(对照组,n=34)。本研究已经医院伦理学委员会批准,患者及家属均知情同意并签署知情同意书。
1.2 方法 收集两组一般资料及疾病信息,并采用蒙特利尔认知评估量表(MoCA)和临床痴呆评定量表(CDR)评估其认知功能及日常行为能力。
1.3 观察指标与评定标准 比较两组空腹血糖(FPG)、餐后2 h血糖(2 h PG)、糖化血红蛋白(HbA1c)、总胆固醇(TC)、甘油三酯(TG)、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)、尿酸(UA)、丙氨酸氨基转移酶(ALT)、天门冬氨酸氨基转移酶(AST)、胆红素(TB)、C反应蛋白(CRP)、血清补体C3、C4水平及MoCA、CDR评分,筛选AD的可能影响因素,各影响因素与MoCA评分和CDR评分的相关性采用Pearson相关性分析。MoCA评分标准:总分30分,<18分为重度认知功能障碍,18~23分为中度认知功能障碍,24~27分为轻度认知功能障碍,>27分提示无认知功能障碍;CDR评分标准:0分为日常行为能力正常,0.5分为可疑受损,1分为轻度受损,2分为中度受损,3分为重度受损。
1.4 统计学处理 采用SPSS 20.0软件对所得数据进行统计分析,计量资料用(x±s)表示,组间比较采用独立样本t检验;计数资料以率(%)表示,比较采用字2检验;各生化指标与MoCA、CDR的相关性采用Pearson相关性分析。以P<0.05为差异有统计学意义。
2 结果
2.1 两组一般资料比较 观察组,男25例,女7例;年龄75~94岁,平均(86.01±1.77)岁;平均吸烟时间(29.47±1.03)年;平均上学时间(6.25±0.78)年;平均体质指数(29.31±0.67)kg/m2;平均饮酒时间(46.09±1.68)年。对照组,男26例,女8例;年龄76~93岁,平均(85.81±1.72)岁;平均吸烟时间(28.95±1.01)年;平均上学时间(6.14±0.69)年;平均体质指数(29.07±0.65)kg/m2;平均饮酒时间(45.83±1.61)年。两组年龄、吸烟时间、文化程度、体质指数、饮酒时间比较,差异均无统计学意义(P>0.05),具有可比性。
2.2 两组各指标比较 两组血清AST、ALT、TB、2 h PG、UA、HbA1c、TG、HDL-C、LDL-C、CDR评分比较,差异均无统计学意义(P>0.05);观察组MoCA评分低于对照组,C3、C4、CRP、FPG、TC均高于对照组,差异均有统计学意义(P<0.05)。见表1。
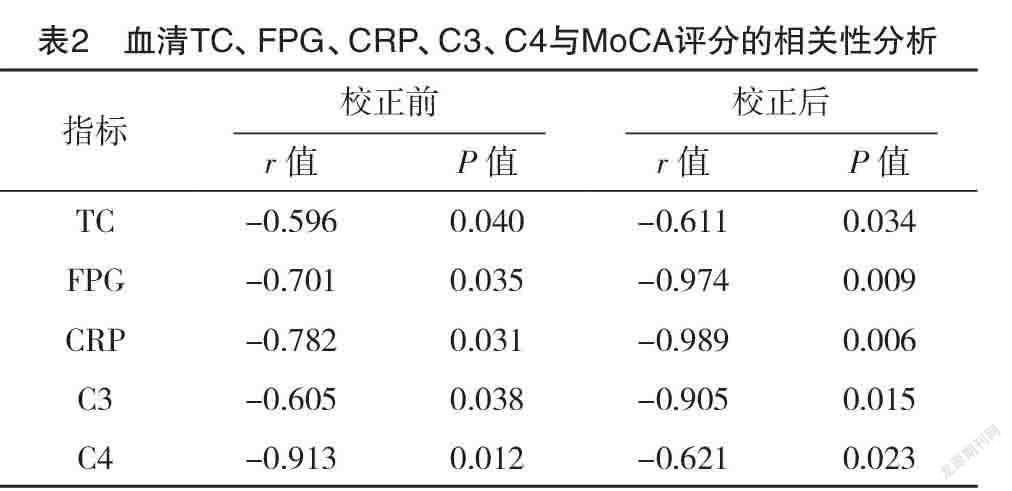
2.3 血清TC、FPG、CRP、C3、C4与MoCA、CDR评分的相关性分析 Pearson相关性分析显示,血清TC、FPG、CRP、C3、C4水平与MoCA评分均呈负相关(P<0.05),与CDR评分均无相关性(P>0.05),见表2、3。
3 讨论
阿尔茨海默病(Alzhemer’s disease,AD)是老年人最常见的痴呆类型,占老年人痴呆性疾病的50%~70%[8]。脑组织中老年斑(senile plaques,SP)形成、神经纤维缠结(neurofibrillary tangles,NFT)和神经元缺失为其主要病理表现[9]。非酒精性脂肪性肝病(nonalcoholic fatty liver disease,NAFLD)普通人群发病率为(28.01~52.34)/1 000人年[10-11],已成为全球最常见的肝病。NAFLD相关危险因素主要有肥胖、2型糖尿病、高血压、血脂异常等[12],发病机制与胰岛素抵抗、代谢综合征、糖尿病、氧化应激和脂毒性等多方面因素有关[13]。
已有研究发现,正常的TC水平对大脑结构具有重要的作用,大多数学者认为高TC水平能够增加AD的发病风险,机制為TC与神经突触的形成和其可塑性密切相关[14]。文献[15-16]研究发现痴呆患者在认知能力下降的早期,其机体内的TC水平高于无AD者,机体TC的代谢障碍能够促进AD的病理改变。本研究结果表明AD合并NAFLD患者MoCA评分显著低于无肝脏疾病AD患者,其TC水平与MoCA评分呈负相关(P<0.05),与上述结果一致。其机制为当TC水平升高时,大脑及血清中对神经细胞有明显毒性作用的24S-羟基TC水平也会随之升高,其可以造成神经元的退行性损害[17];也可能与其激活氧化应激途径,导致自由基生成过多有关[18];同时变性坏死的神经细胞可加速脑内TC的转化使24S-羟基TC含量明显升高[19],两者相互影响加剧了脑细胞的损害。NAFLD近年来被认为是一种代谢综合征在肝脏的表现形式,胰岛素抵抗是代谢综合征与NAFLD共同的病理基础[20],NAFLD可导致机体血脂代谢障碍使TC升高,本研究与上述报道相符,AD合并NAFLD患者TC水平显著高于无肝病患者(P<0.05)。因此,NAFLD可通过升高TC水平而导致神经细胞退行性疾病AD。
既往研究表明,糖尿病患者的认知功能障碍发生率较高,也更容易发展成为AD。国内外近些年研究认为AD和T2DM发病的共同病理生理联系,可能与胰岛素细胞信号转导异常即胰岛素抵抗、炎症反应、氧化应激、线粒体功能障碍和遗传等因素有关,PFG升高亦可产生胰岛素细胞信号转导异常现象,从而与AD认知障碍的发生发展相关联[21]。可能的机制有以下几种:首先胰岛素可通过选择性地分布胰岛素受体蛋白进入中枢神经系统,在AD患者脑组织中大脑胰岛素受体的敏感性改变而产生胰岛素抵抗,影响脑组织的代谢降解以及β淀粉样蛋白和tau蛋白的表达[22]。其次,研究表明Aβ寡聚体能使树突胰岛素受体(IRs)从胞膜上移除下来使受体数目减少而导致AD。另外,AD患者胰岛素细胞信号转导异常,使胰岛素或IGF-1受体阳性神经元的联合定位减少,乙酰胆碱的生成也相应减少促进AD的发生发展[23]。再者,胰岛素降解酶(IDE)可有效降解聚集的Aβ[24],当处于胰岛素抵抗的环境时,胰岛素可能竞争性抑制胰岛素降解酶,从而阻碍Aβ蛋白的降解,增加其神经毒性[25]。此外,胰岛素抵抗也能通过活化糖原合成酶激酶-3β而增加tau蛋白的磷酸化[26]。本研究结果显示,AD合并NAFLD患者FPG水平与MoCA评分呈负相关,与上述研究结论相符。近年来研究提示,胰岛素抵抗包括空腹血糖受损在内的代谢综合征与NAFLD共同的病理基础[20],NAFLD与高水平的FPG两者间相互影响,NAFLD促使胰岛素抵抗进一步加重而导致FPG水平升高,FPG升高也通过胰岛素抵抗进一步加重,使NAFLD继续进展。因此,NAFLD可能通过升高FPG水平加重胰岛素抵抗导致AD发生发展。
Serpente等[27]研究发现,在AD发病机制中炎症免疫反应具有重要作用。超强的免疫反应可引起误导攻击神经组织,造成神经元细胞的损伤和凋亡、死亡[28]。因为有血脑屏障的存在,既往认为中枢神经系统是不受免疫反应攻击的免疫豁免器官,但是,最近多个研究揭示,通过血脑屏障进入脑内的炎症因子和淋巴细胞,两者可引起AD的病理炎症反应[29-31]。本研究结果显示,AD合并NAFLD患者CRP、C3、C4与MoCA评分均呈负相关(P<0.05),与以上研究结果一致。肝脏是生成炎症因子的重要器官,研究发现,NAFLD患者CRP、C3、C4水平升高,是因为肝细胞对游离脂肪酸的输入和运出失去平衡,导致肝细胞出现脂质沉积过多,当游离脂肪酸增加超过肝细胞的氧化能力时,细胞内有毒代谢产物清除障碍发生堆积,即可激活炎性反应通路[32]。其次,红细胞补体受体1(CR1)可抑制补体激活,而NAFLD肝损伤患者存在着CR1活性及数量表达缺陷,补体激活水平明显提高,使机体免疫功能紊乱程度加重[33]。另外,何涛君等[34]发现,血清补体C3、C4水平和肝损伤的病变程度关系密切。本研究結果显示,AD合并NAFLD患者CRP、C3、C4水平较对照组均显著升高(P<0.05),与以上结果相符。因此,NAFLD可能通过加重机体免疫炎症反应导致AD发生发展。
综上所述,在AD合并NAFLD患者中,血清TC、FPG、CRP、C3、C4水平较高,NAFLD可能通过升高上述因子水平导致AD,并促进认知功能障碍的发生发展,在临床工作中通过上述指标的检测有助于早期发现AD,以进行早期临床干预,提高患者的生活能力和生活质量。本研究尚存在一定局限性,在以后的工作中通过扩大样本量和增加试验方法给予进一步验证。
参考文献
[1] Pongan E,Tillmann B,Leveque Y,et al.Can musical or painting interventions improve chronic pain,mood,quality of life,and cognition in patients with mild Alzheimer’s disease?Evidence from a randomized controlledtria[J].J Alzheimers Dis,2017,60(2):663-677.
[2] Zhang X,Schmitt F A,Caban-Holt A M,et al.Diabetes mitigates the role of memory complaint in predicting dementia risk:results from the preventionof Alzheimer’s disease with vitamin E and selenium study[J].J Prev Alzheimers Dis,2017,4(3):143-148.
[3] Stites S D,Karlawish J,Harkins K,et al.Awareness of mild cognitive impairment and mild Alzheimer’s disease dementia diagnoses associated with lower self-ratings of quality of life in older adults[J].J Gerontol B Psychol Sci Soc Sci,2017,72(6):974-85.
[4] Xing S,Shen D,Chen C,et al.Effect of the herbal formulation Shen-Zhi-Ling on an APP/PS1 mouse model of Alzheimer’s disease by modulating the biliverdin reductase/heme oxygenase 1 system[J].Exp Ther Med,2017,14(3):1961-1966.
[5] Huang Z,Muniz-Terrera G,Tom B D M.Power analysis to detect treatment effects in longitudinal clinical trials for Alzheimer’s disease[J].Alzheimers Dement(N Y),2017,3(3):360-366.
[6] Wu Z,Nakanishi H.Old and new inflammation and infection hypotheses of Alzheimer’s disease:focus on microglia-aging for chronic neuroinflammation[J].Nihon Yakurigaku Zasshi,2017,150(3):141-147.
[7]范建高.非酒精性脂肪性肝病診疗指南[J].胃肠病学,2016,15(11):676-680.
[8]唐丽娜,许小明,李艳红.炎症因子与阿尔茨海默病的相关性研究进展[J].中国老年学杂志,2016,36(17):4378-4379.
[9] Reitz C.Alzheimer’s disease and the amyloid cascade hypothesis:a critical review[J].Int J Alzheimers Dis,2012,66(1):1155-1160.
[10] Zlber-Sagi S,loton R,Shlomai A,et al.Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective followup[J].J Hepatol,2015,56(5):1145-1151.
[11] Younossi Z M,Koenig A B,Abdalatif D,et al.Global epidemiology of nonalcoholic fatty liver disease meta-analytic assessment of prevalence,induce,and outcomes[J].Hepatoligy,2016,64(1):73-84.
[12] Byme C D,Targher G.NAFLD:a multisysystem disease[J].
J Hepatol,2015,56(2):62-71.
[13] Chalasani N,Younossi Z,Lavine J E,et al.The diagnosis and management of non-alcoholic fatty liver disease:practice guideline by the American Association for the Study of Liver Diseases,American College of Gastroenterology,and the Amercan Gastroenterological Association[J].Hepatology,2015,55(1):2005-2023.
[14]刘梦姣,曾慧,王晓松,等.老年人躯体功能与认知功能的关系研究进展[J].中国全科医学,2014,17(3):242-245.
[15] Whitmer R A,Sidney S,Selby J,et al.Midlife cardiovas cularrisk factors and risk of dementia in late life[J].Neurology,2005,64(6):277-281.
[16] Solomon A,Kareholt I,Ngandu T,et al.Serum cholesterol changes after midlife and late life cognition:twenty-one-yearfollow-up study[J].Neurology,2007,68(10):751-756.
[17]林岩,李焰生.他汀类药物与痴呆[J].国外医学:脑血管疾病分册,2005,13(9):689-693.
[18]何小明,张振馨.胆固醇24S-羟化酶与阿尔茨海默病[J].中国老年学杂志,2006,25(8):634-636.
[19] Wada-Isoe K,Wakutani Y,Urakami K,et al.Elevated interleukin-6 levels in cerebrospinal fluid of vascular dementia patients[J].Acta Neurol Scand,2004,110(2):124-127.
[20] Birkenfeld A L,Shuman G I.Nonalcoholic fatty liver disease,hepatic insulin resistance,and type 2 diabetes[J].Hepatology,2014,59(2):713-723.
[21] de La Monte.Alzeimer’s disease is type 3 diabetes-evidence reviewed[J].Journal of Diabetes Scidence and Technology,2008,2(6):1101-1113.
[22] de Felice F G,Lourenco M V,Ferreira S T.How does brain insulin resistence develop in Alzeimer’s disease Alzeimer’s disease?[J].Alzeimer’s Dement,2014,10(1):826-832.
[23] Cai Z Y,Xiao M,Chang L Y,et al.Role of insulin resistance in Alzeimer’s disease[J].Metab Brain Dis,2015,30(4):839-851.
[24] Moloney A M,Griffin R J,Timmos S A,et al.Defects in IGF-1 receptor,insulin receptorand IRS-1/2 in Alzeimer’s disease indicate possible resiling[J].Neurobiol Aging,2010,31(2):224-243.
[25] Plum L,Schuber T M,Bruing J C.The role of insulin receptor signaling in the brain[J].Trends Endocrinol Metab,2005,16(2):59-65.
[26] Kremer A,Louis J V,Jaworski T,et al.GSK3 and Alzeimer’s Disease:Facts and Fiction[J].Front Mol Neurosci,2011,4(1):17-25.
[27] Serpente M,Bonsi R.Innate immune system and inflammation in Alzheimer’s disease:from pathogenesis to treatment[J].Neuroimmunomodulation,2014,21(23):79-87.
[28] Ceccom J,Loukh N.Reduced sphingosine kinase-1 and enhanced sphingosine 1-phosphate lyase expression demonstrate deregulated sphingosine 1-phosphate signaling in Alzheimer’s disease[J].ActaNeuropathol Commun,2014,27(2):2-12.
[29] Minogue A M,Jones R S,Kelly R J,et al.Age-associated dysregulation of microglial activation is coupled with enhanced blood-brain barrier permeability and pathology in APP/PS1 mice[J].Neurobiol Aging,2014,35(6):1442-1452.
[30] Cudaback E,Jorstad N L,Yang Y,et al.Therapeutic implications of the prostaglandin pathway in Alzheimer’s disease[J].Biochem Pharmacol,2014,88(4):565-572.
[31] Solberg N O,Chamberlin R,Vigil J R,et al.Optical and SPION-enhanced MR imaging shows that trans-stilbene inhibitors of NF-κB concomitantly lower Alzheimer’s disease plaque formation and microglial activation in AβPP/PS-1 transgenic mouse brain[J].J Alzheimers Dis,2014,40(1):191-212.
[32]王亮.血清補体C3、C4的表达变化与肝癌患者不同child-pugh分级的关系[J].中国误诊学杂志,2012,12(5):1076-1077.
[33]宋清玲.拉米夫定和干扰素α序贯治疗慢性乙型肝炎肝损伤患者48周前后血清补体C3、C4变化[J].医学综述,2015,21(10):1914-1916.
[34]何涛君,吴正林,钟小强,等.乙肝患者HBV载量与IgA,IgG、IgM及C3、C4相关性研究[J].现代检验医学杂志,2015,30(4):67-70.
(收稿日期:2020-11-24) (本文编辑:程旭然)
