Stability and optoelectronic property of low-dimensional organic tin bromide perovskites∗
2021-03-19Lei雷军辉Tang汤琼He何军andCai蔡孟秋
J H Lei(雷军辉), Q Tang(汤琼), J He(何军),†, and M Q Cai(蔡孟秋)
1School of Science,Hunan University of Technology,Zhuzhou 412007,China
2School of Physics and Electronics Science,Hunan University,Changsha 410082,China
Keywords: one/zero dimensional non-toxic organic tin bromide perovskites,first principle calculation,stability,Optoelectronic property
1. Introduction
Organic-inorganic hybrid perovskites have been investigated for their extensive applications in fields of photodetectors, solar cells and light-emitting diodes due to their superior properties such as the high absorption coefficient, charge carrier transport property,intense photoluminescence and low non-radiative charge recombination.[1-6]Hybrid perovskites can be classified into 3D, 2D, 1D, and 0D structures on the molecular level according to the distribution of inorganic octahedra (MH6, where M and H represent metal/halogen elements, respectively) in the organic cations.[7-9]When the inorganic octahedra are arranged by the corner-sharing with the small size cations fitting, the 3D structure with chemical formula AMH3(A=organic cation) is formed. If the inorganic octahedra are connected in layered sheet and sandwiched between large organic chains, the bulk material stacked by the alternate organic and inorganic layers is called the 2D perovskites. The bulk perovskites with 1D denote that the inorganic octahedra are connected in chain by sharing the corners,edges or faces while in the 0D perovskites the octahedra are divided completely by organic cations. The crystalline perovskites with the inorganic octahedra assembling in 2D, 1D,and 0D structures would exhibit particular properties due to the quantum confined effect. Great attentions are paid for 2D hybrid perovskites while the 1D/0D hybrid perovskites are little investigated. Furthermore,the stability and toxicity of traditional hybrid lead-halide perovskites are the major bottlenecks for practical applications. The toxic issues can be addressed by substituting toxic heavy lead with other friendly elements(such as Sn)and the ambient stability can be improved by tailored into a low-dimensional structure encapsulated by the organic chain due to its good hydrophobicity.[10-12]
Recently, 1D/0D hybrid perovskites have been synthesized successfully. Arad-Vosk et al.[13]have prepared organic metal halide perovskite(CH3NH3PbI3)nanorods with the 1D structure by porous silicon nanotube templates,and they found that transition from tetragonal structural to orthorhombic is inhibited due to the size effects. Fan et al.[14]fabricated the three-dimensional CH3NH3SnI3perovskite with the inorganic octahedra arrayed in 1D chain with nanoengineering templates, in which the nanowire integration and stability issues were both addressed. Especially, Zhou et al.[15]prepared the high-quality OTBP with the 1D/0D structures by controlling the reaction condition,and observed the structural transformation from 1D to 0D under the light. To provide insight into the optoelectronic properties of hybrid perovskites,the calculated results from the atomic and electronic structure are reliable and convincing.[16-18]The structure/property calculated from the density function theory (DFT) provides plenty intriguing results. For example, based on the calculated atomic/electronic structure,the intrinsic properties(such as structure,transport,excitation and optelectronic properties,which are closely related to the further development of hybrid perovskites)can be obtained to explain experimental facts and provide guidance in exploring new materials. As for the prepared 1D/0D OTBP recently,seldom theoretical researches have been launched. In this paper,the properties of atoms,the mobility and coupling effect of carriers, the light absorption coefficient in 1D/0D OTBP are obtained from first-principle approach.It is expected that the calculated results will provide guides to exploring new non-toxic opto-electronic materials.
2. Computational method
The work is implemented by Vienna ab initio simulation package (VASP), which employs the density functional theory plane wave method to perform quantum mechanics calculation.[19-21]The interaction between the valence electrons and ions is described by projected augmented wave formalism. Taken the weak interaction between the organic cations and inorganic octahedra into account, the nonlocal density functional (van der Waals density functional)is included in the generalized gradient approximation of the Perdew-Burke-Ernzerh functional (GGA-PBE).[22]The Monkhorst-Pack k-point mesh in Brillouin zone in both of 1D/0D OTBPs is sampled by 5×3×3 grid, and the wave function of a single electron is cut about 400 eV.For the structure optimization,the force acting on each atom and total energy convergence criterion are below 0.01 eV/˚A and 10−4eV,respectively.



The exciton binding energy (Eb) for the coupling electron/hole pairs can be obtained from the Wannier excitation model.[23,24]

The optical properties are estimated from the dielectric function depending on the incidence optical frequency:ε(ω) = ε1(ω)+iε2(ω). After obtaining the accurate electronic structure, the imaginary part ε2(ω) is calculated from the transitions between VB and CB directly. The ε1(ω)is obtained by the Kramers-Kronig transformation.[25,26]The optical absorption coefficient α is obtained according to the following formula:
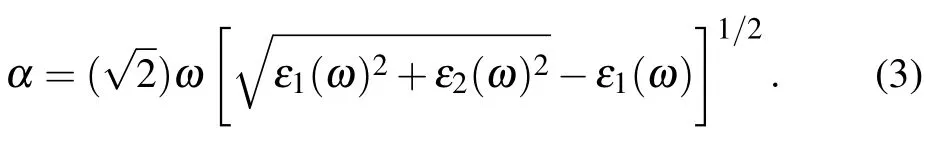
3. Results and discussion
3.1. Structure and stability
Figures 1(a) and 1(b) show the optimized structures of 1D/0DOTBP.As shown in Fig.1(a),the inorganic octahedra in 1D OTBP are arranged in chained by sharing the edge,which are surrounded by the organic molecules. The calculated average bond length of octahedra is about 2.98 ˚A,which is closed to the experimental values(3.02 ˚A).As shown in Fig.1(b),it is found that the inorganic octahedra are dispersed inorganic molecules and are completely isolated from each other. The calculated result shows that the average bond length of octahedra is about 3.02 ˚A. The experimental structure[15]is reproduced in our calculation, which ensures our methodology rationality.
The physical properties of hybrid perovskite are dominated by the inorganic octahedra distributed in the bulk material. To characterize the structural order degree, the bond lengths(between Sn and Br)and bond angles(among Br-Pb-Br)of inorganic octahedra in the 1D/0D OTBP are calculated and compiled in Table 1. In Table 1,the root-mean-square errors(RMSEs)of bond length and angle(relative to the normal octahedron with the average bond length)are also listed. It is found the RMSEs of the bond length and angle are changed from 0.422 ˚A,6.3◦into 0.416 ˚A,1.7◦,respectively,when the 1D OTBP is translated into 0D. That’s to say, the structure transition from the 1D to 0D is accompanied by structural relaxation,which is consistent with the experimental results.[15]

Fig.1. The optimized atomic arrangement of organic tin bromide perovskites: (a) 1-dimensional, (b) 0-dimensional. The calculated phonon dispersion relation of organic tin bromide perovskites: (c) 1-dimensional,(d)0-dimensional.
In the practical applications of the hybrid perovskite,the ambient stability, photo stability and heat stability should be take into account. In order to inspect the stability,the phonon spectrum of the 1D/0D OTBP is obtained from DFPT. The calculated phonon dispersion of 1D/0D OTBP is shown in Figs.1(c)and 1(d).As shown in Figs.1(c)and 1(d),it is found that the imaginary frequency is absent in both OTBPs through the whole Brillouin zone. This indicates that both OTBPs are dynamically stable and can exist themselves.[11]
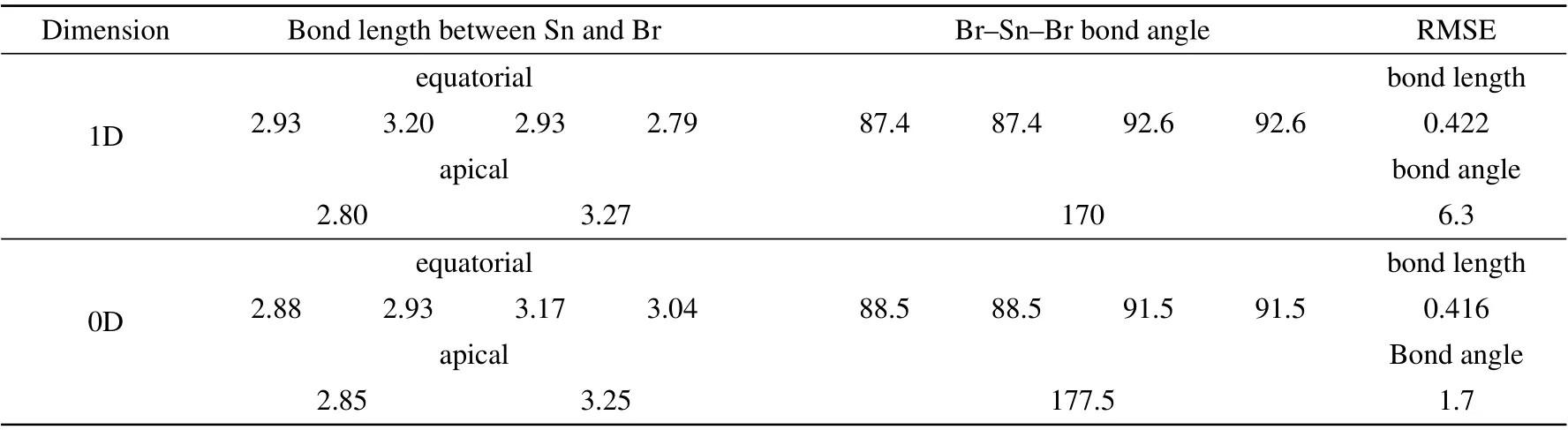
Table 1. The structural properties of inorganic octahedra [SnBr6] in the OTBP with the 1D/0D arrangement. The root-mean-square error(RMSE)of bond length(˚A)and angle(◦)is relative to the normal octahedron with the average bong length.
3.2. Mobility and coupling effect of electrons and holes
One key parameter for photoelectric transformation efficiency in the hybridperovskite is the mobility of carriers. In order to obtain the carrier’s transport properties, the bands of 1D/0D OTBPs are calculated. For the band calculation, the spin-orbit coupling(SOC)effect should be adopted to predict the gap correctly.It is noted that the band could be obtained by GGA without the SOC due to the fact that the errors of using GGA and non-SOC cancel each other.[27]Therefore,in order to save the computational resource, the electronic properties are calculated by the GGA-PBE. As shown in Fig.2(a), one can find that the CB and VB are nearly flat along the 〈010〉,〈001〉directions while dispersed along〈100〉path,which indicates that the carriers are high mobile along the chains,but low perpendicular to the chains. While for the 0D OTBP,as shown in Fig.2(b), the fluctuation amplitude of energy in CB/VB is very small through the whole Brillouin zone,which means that the low mobile becomes isotropic. Intriguingly the discrete energy level (no electronic band formation) in the 0D OTBP originating from the strict quantum confinement will lead to exceptional purity emitting light when it is excited, which is according to experiment.[27]It should be noted that the calculated gaps of 1D/0D OTBPs are 2.0 eV and 3.25 eV, respectively. The gap of 0D OTBP is consistent with the experimental optical gap (3.4 eV) while the 1D OTBP is different from the measured value(3.1 eV).[15]This deviation between the calculated and observed values in 1D OTBP may be that the experimental value is corresponding to the inter band absorption,which is shown in Fig.2(a). To characterize the carriers’ transport properties, the electron/hole effective mass in 1D/0D OTBP is calculated from Eq. (1) and compiled in Table 2. The calculated average effective mass of electron/hole changes from 0.66/1.52mein 1D to 0.99/10.8mein 0D,which indicates that the mobility of 1D/0D OTBP is low(compared to the 2D/3D hybrid lead-halide perovskites).[16]The results will be reasonable if the quantum confinement is taken into account.
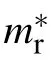

Fig.2. Band structure of organic tin bromide perovskites: (a) 1-dimensional and(b)0-dimensional with gap about 2.0 eV and 3.25 eV,respectively(blue arrow lines).The charges drifting along〈100〉,〈010〉,〈001〉directions in real space are denoted by along G-Y,Y-T,T-Z directions in the reciprocal space.
Table 2. Calculated effective masses of electrons in CBMand holes in VBM,reduced mass of excitations(in units of the free electron mass),dielectric constants(ε∞)and exciton binding energies(Eb (in meV))of 1D/0D OTBPs.

Table 2. Calculated effective masses of electrons in CBMand holes in VBM,reduced mass of excitations(in units of the free electron mass),dielectric constants(ε∞)and exciton binding energies(Eb (in meV))of 1D/0D OTBPs.
Note: the reduced mass in 1D is calculated based on the in conduction band and valence band due to the obvious anisotropy.[16]
Dimension Conduction band Valence band m∗xx m∗yy m∗zz m∗xx m∗yy m∗zz〈m∗e〉 〈m∗h〉 〈m∗r〉 ε∞ Eb 1D 0.23 11.1 11.1 0.55 13.7 13.7 0.66 1.52 0.16 3.29 201 0D 0.95 0.95 1.1 11.5 10.9 10.1 0.99 10.8 0.91 3.20 1201
3.3. Absorption coefficient
Due to the nonequilibrium carriers injected by light in the hybrid perovskites are of great importance on exploiting solar energy, the absorption coefficient of 1D/0D OTBPs is calculated from Eq. (3). The calculated absorption coefficients of 1D/0D OTBPs with the incidence optical energy ranging from 1.5 eV to 4 eV are depicted in Fig.3(a). According to the gap of 1D/0D OTBPs as shown in Fig.2,the onset of intrinsic absorption should locate at the incidence light with 2.0 eV and 3.25 eV, respectively. However, the obvious below-band gap light absorption of 0D OTBP is observed, which may originate from the gap state absorption as shown in Fig.3(b).The gap state is resulted from energy level splitting from the photon perturbation. As shown in Fig.2(b), the degeneracy of occupy orbits in the 0D OTBP is higher than that in 1D,which leads to the intensive energy level splitting. As a result,the 0D OTBP with wider gap has similar absorption spectrum with the 1D OTBP.

Fig.3. (a)The calculated absorption coefficients of the organic tin bromide perovskites with 1/0-dimensional structures from PBE functional corrected by vdW-DF. (b) The sketch map of intrinsic absorption and gap state absorption,which are shown in red or purple arrows,respectively.(c)The projected density of states(PDOS)of organic tin bromide perovskites with the 1 dimensional structure.
In addition, as depicted in Fig.3(a), it can be found that both 1D and 0D OTBPs exhibit distinguishing absorption efficiency with the coefficient approaching to 105cm−1, which is similar to the traditional perovskites.[24]In order to trace the origination of high absorption ability, the projected density of states(PDOS)of 1D OTBP is calculated. As shown in Fig.3(c), the VBM/CBM are dominated by p orbits of Br/Sn respectively. Based on the PDOS, it can be referred that the direct transitions from the occupied to unoccupied p-orbitals lead to the high absorption ability.[16,29,30]
3.4. Application prospect
From the above discussion,the stable non-toxic 0D OTBP is a potential friendly photoluminescence material. Firstly,the plenty excitation energy level provides the platform for the carriers generation/recombination. Secondly, the discrete energy level will lead to the excellent purity emitting light under illumination. At the same time the low transport properties resulted from the spatial confinement of the carriers promote the effective radiative relaxation.[31-37]Furthermore, the excellent absorption within the whole visible light will lead to high quantum efficiency(QE).
4. Conclusion
In summary,the atomic and electronic properties of nontoxic 1D/0D OTBP are calculated based on the first-principles calculation. The results indicate both the structures are stable and the inorganic octahedra in 0D have higher order. In contrast to the traditional 2D/3D perovskites,the average electron/hole effective masses in the 1D/0D ODBPs(0.66/1.52me,0.99/10.8merespectively) are much larger. The low mobility along with the large excitation binding energy(1201 meV)restricts the photovoltaic application. However, for the stable 0D OTBP, the whole visible light can be absorbed due to the gap states absorption. At the same time the high absorption ability(approaching to 105cm−1)indicates that 0D OTPB is excellent solar energy absorber.In a word,the non-toxic stable 0D OTBP with low transport property, large excitation binding energy and high absorption ability is expected to provide potential photoluminescence materials.
Acknowledgement
The authors thank the Changsha Supercomputer Center for computation.
杂志排行
Chinese Physics B的其它文章
- Transport property of inhomogeneous strained graphene∗
- Beam steering characteristics in high-power quantum-cascade lasers emitting at ~4.6µm∗
- Multi-scale molecular dynamics simulations and applications on mechanosensitive proteins of integrins∗
- Enhanced spin-orbit torque efficiency in Pt100−xNix alloy based magnetic bilayer∗
- Soliton interactions and asymptotic state analysis in a discrete nonlocal nonlinear self-dual network equation of reverse-space type∗
- Discontinuous event-trigger scheme for global stabilization of state-dependent switching neural networks with communication delay∗
