Interactive effects of elevated CO2 and nitrogen fertilization levels on photosynthesized carbon allocation in a temperate spring wheat and soil system
2021-03-17YuZHAOChaoLIANGShuaiSHAOJieLIHongtuXIEWeiZHANGFushengCHENHongboHEandXudongZHANG
Yu ZHAOChao LIANGShuai SHAOJie LIHongtu XIEWei ZHANGFusheng CHENHongbo HE∗and Xudong ZHANG∗
1Institute of Applied Ecology,Chinese Academy of Sciences,Shenyang 110016(China)
2University of Chinese Academy of Sciences,Beijing 100049(China)
3Jiangxi Provincial Key Laboratory of Silviculture,Jiangxi Agricultural University,Nanchang 330045(China)
(Received January 22,2019;revised June 24,2019)
ABSTRACT Increasing atmospheric CO2 concentration impacts the terrestrial carbon(C)cycle by affecting plant photosynthesis,the flow of photosynthetically fixed C belowground,and soil C pool turnover.For managed agroecosystems,how and to what extent the interactions between elevated CO2 and N fertilization levels influence the accumulation of photosynthesized C in crops and the incorporation of photosynthesized C into arable soil are in urgent need of exploration.We conducted an experiment simulating elevated CO2 with spring wheat(Triticum aestivum L.)planted in growth chambers.13C-enriched CO2 with an identical 13C abundance was continuously supplied at ambient and elevated CO2 concentrations(350 and 600µmol mol−1,respectively)until wheat harvest.Three levels of N fertilizer application(equivalent to 80,120,and 180 kg N ha−1 soil)were supplied for wheat growth at both CO2 concentrations.During the continuous 62-d 13CO2 labeling period,elevated CO2 and increased N fertilizer application increased photosynthesized C accumulation in wheat by 14%-24% and 11%-20%,respectively,as indicated by increased biomass production,whereas the C/N ratio in the roots increased under elevated CO2 but declined with increasing N fertilizer application levels.Wheat root deposition induced 1%-2.5% renewal of soil C after 62 d of 13CO2 labeling.Compared to ambient CO2,elevated CO2 increased the amount of photosynthesized C incorporated into soil by 20%-44%.However,higher application rates of N fertilizer reduced the net input of root-derived C in soil by approximately 8% under elevated CO2.For the wheat-soil system,elevated CO2 and increased N fertilizer application levels synergistically increased the amount of photosynthesized C.The pivotal role of plants in photosynthesized C accumulation under elevated CO2 was thereby enhanced in the short term by the increased N application.Therefore,robust N management could mediate C cycling and sequestration by influencing the interactions between plants and soil in agroecosystems under elevated CO2.
Key Words:C cycling,C sequestration,continuous 13C-enriched CO2 labeling,growing season,isotope composition,N management,wheat tissue biomass
INTRODUCTION
Increasing atmospheric CO2concentration has been one of the most pronounced global changes during the past 100 years.Elevated CO2has intimate effects on plant growth and the subsequent transfer of photosynthetically fixed carbon(C)(i.e.,plant-derived C)to the soil(Xuet al.,2013)and,thus,impacts the terrestrial C cycle.However,any response of plant or soil processes to elevated CO2can be constrained by soil nutrient availability,especially by nitrogen(N)(Billingset al.,2002;Liuet al.,2018).This phenomenon highlights the need to explore how CO2and N availability interactively affect C allocation and sequestration in plant-soil systems(van Kesselet al.,2000;Albarracínet al.,2013).Unfortunately,there remains large uncertainties in such effects in different ecosystems,owing to the highly variable physiological responses of plants(Butterlyet al.,2015)and the vastly different interactions between plants and soil(Jastrowet al.,2005;Hazraet al.,2019).
Despite increasing N deposition worldwide(Galloway,2005),the response of C fixation and allocation in plantsoil systems to increasing CO2concentrations in natural ecosystems is usually alleviated or even counteracted when N is still an intrinsic limiting factor(van Kesselet al.,2000;Reichet al.,2006;Bardgett,2011;Butterlyet al.,2015),especially in C3plant species,because they may respond sensitively to elevated CO2(Marhanet al.,2010).In managed agroecosystems,N fertilizer application is a common practice for increasing crop yields(Robertson and Vitousek,2009;Geet al.,2015;Zanget al.,2017,2019),and causally affects root growth and microbial activity as well as soil C turnover(Lee and Jose,2003;Geisseler and Scow,2014;Xiaoet al.,2019).Coupled with N fertilizer applications,there are usually positive responses of crop biomass to CO2enrichment(e.g.,Jensen and Christensen,2004;Kouet al.,2007;Hazraet al.,2019).In addition,elevated CO2has been observed to decrease N content in plant tissue and increase the C/N ratio(Stiling and Cornelissen,2007;Butterlyet al.,2015).Thus,the altered decomposability of plant tissues and the humification of plant-derived C in soil are highly dependent on the supply of N fertilizer.However,little is known about how N availability coupled with CO2enrichment influences photosynthesized C allocation and,consequently,C sequestration in agroecosystems,despite a previous investigation of C and N partitioning conducted in a wheat-soil system under water-limited conditions(Butterlyet al.,2015).Clarifying the interactive effects of CO2and N fertilizer is pivotal for improving our understanding of the role of N management in mediating C cycling and sequestration in agroecosystems under elevated CO2.For temperate agroecosystems with crops planted and harvested annually,determining the seasonal response of crops and soil is essential as first-hand information to predict their long-term consequences.
Compared with the visible responses of C fixation in plants to elevated CO2,small changes in soil organic C(SOC)are difficult to detect because of the large size of the soil C pool(Cardonet al.,2001;Jastrowet al.,2005;Carneyet al.,2007;Zhuet al.,2017;Pausch and Kuzyakov,2018).Thus,the influence of elevated CO2on the incorporation of photosynthesized C into soil remained elusive until isotopic techniques were applied to effectively differentiate the new portion(plant-derived C)from the old portion(native soil C)(Cardonet al.,2001;Liuet al.,2019;Zanget al.,2019).Pulse labeling of plants in14CO2or13CO2atmosphere can be used to trace the fate of recently assimilated C(Kuzyakov,2006);however,this approach results in an uneven distribution of labeled C between plant organs and C fluxes(Kuzyakov,2006;van Groenigenet al.,2017).A continuous supply of13C-labeled CO2in which13C is either depleted or enriched presents an advantage over13C pulse labeling for estimating the total amount of C transferred by plants into soil and belowground C pools during the labeling period(Kuzyakov,2006).However,in most experiments simulating rising CO2,13CO2has been supplied only at an elevated concentration,whereas CO2with natural13C abundance has been supplied at an ambient concentration(e.g.,Hagedornet al.,2013).In this case,the influence of elevated CO2on photosynthesized C incorporation into soil cannot be evaluated,due to the lack of quantification of new soil C under ambient CO2levels.Instead,for such an evaluation,13CO2should be supplied at both concentrations when plants are grown under ambient and elevated CO2.For shortterm plant growth,continuous13C-enriched CO2labeling is more powerful for tracing the flow of photosynthesized C belowground at each CO2concentration.
Therefore,we conducted an experiment to simulate elevated CO2with spring wheat planted in growth chambers.13C-enriched CO2with an identical13C abundance was supplied at two CO2concentrations(350 and 600µmol mol−1)until wheat harvest.Three levels of N fertilizer application(equivalent to 80,120,and 180 kg N ha−1soil)were supplied for wheat growth at both CO2concentrations.We analyzed above-and belowground wheat biomass and soil for total C content and isotope composition(δ13C)to investigate how elevated CO2and N fertilizer interactively affect the allocation of photosynthesized C between crops and soil.Two main hypotheses were tested:i)elevated CO2favors the accumulation of photosynthesized C in wheat and increased N fertilizer application enhances this effect but alters the stoichiometric ratio of C and N and ii)the enhanced flow and incorporation of photosynthesized C into soil under elevated CO2may be strengthened by increased N fertilizer application,thereby favoring the accumulation of photosynthesized C in a wheat-soil system.By identifying how elevated CO2and N fertilizer interactively and differentially affected photosynthesized C allocation and the resultant soil C pool turnover,we aimed to improve our understanding of the influence of N management on the interactions between plants and soil in anthropogenic agroecosystems under the global climate change scenario.
MATERIALS AND METHODS
Site description
This study was conducted at the National Field Observation and Research Station of Shenyang Agroecosystems(41°31′N,123°24′E)in northeastern China.The site is located within a temperate,humid,continental monsoon climate region.The mean annual temperature is 7-8°C,and the mean annual precipitation is approximately 700 mm.Spring wheat(Triticum aestivumL.)is a representative annually seeded and harvested local crop.The soil type at the site is an Alfisol(Typic Hapludoll),and the pH is 6.4 using a soil:water(weight:volumn)ratio of 1:2.5.The SOC content at the site is 12.9 g C kg−1soil.The averageδ13C value of the soil is−23.7‰.The nutrient contents in soil are as follows:total N,1.04 g kg−1soil;total phosphorus(P),0.44 g kg−1soil;and total potassium(K),17.43 g kg−1soil.Before13CO2labeling,we periodically detected the atmospheric CO2concentration during the daytime at the experimental site with a CO2gas analyzer(LI-820,LI-COR Biosciences,USA).The CO2concentration ranged from approximately 350 to 380µmol mol−1in April 2015.
Continuous 13CO2 labeling experiment
Soil was collected at a depth of 0-20 cm from a wheat field that received an annual N application of approximately 120 kg N ha−1.After plant residues and stones in soil were removed by hand,the soil was sieved through a 5-mm sieve and then thoroughly homogenized.The soil was then air dried and weighed,and 4.0 kg soil samples(on an oven-dried weight basis)were added to each pot,which had an internal diameter of 20 cm and a height of 20 cm.The depth of the soil in the pot was approximately 17 cm.A KH2PO4solution was supplied as the basal fertilizer(equivalent to 60 kg P ha−1soil and 75 kg K ha−1soil).Twenty-five spring wheat seeds were sown in each pot on April 9 in 2015.On April 22,the seedlings were thinned to 20 per pot to maintain the same density as under field conditions.On the eighth day after thinning(April 30),(NH4)2SO4solution was applied at a rate of 33(N1),50(N2),or 75(N3)mg N kg−1soil,equivalent to 80,120,or 180 kg N ha−1soil,respectively,as the mean bulk density of the soil was 1.20 g cm−3.Then,the pots were transferred to sealed growth chambers(1.6 m×1.1 m×1.2 m),and the continuous13CO2labeling process was initiated.Soil without planted wheat was treated with the same fertilizer levels,CO2concentrations,and management procedures as those in the wheat-planted treatments throughout the experimental period.The13CO2-labeling experiment consisted of a split-plot design with two CO2concentrations(main plot factor)×three N levels(subplot factor)×three replicates.Six growth chambers,three at an ambient CO2concentration(350µmol mol−1)and three at an elevated CO2concentration(600µmol mol−1),were arranged in a completely randomized design.Each chamber contained nine pots for wheat planting,three pots at each N level at the beginning of labeling,and the pots were organized randomly.One pot from each N level was destructively sampled at each of the three growth stages(i.e.,the jointing stage,heading stage,and maturity stage)in each chamber.Just before13CO2labeling and N fertilizer application,five replicate wheat-planted pots were destructively sampled to measure theδ13C values of the initial soil(−23.6‰ on average),leaves(including stems,−27.6‰ on average),and roots(−25.4‰ on average).
13C-labeled CO2was produced by the reaction of13Clabeled Na2CO3(6.0 atom%13C,i.e.,δ13C=4 670‰)and HCl(2 mol L−1)from a commercial gas generator(Shsen-QZD,China).The purified CO2was continuously supplied to the sealed growth chambers at the set concentration(350 or 600µmol mol−1inside the growth chamber)during the daytime(between 5:00 a.m.and 6:00 p.m.)until wheat was harvested on June 30(Fig.1).The CO2concentrations in the chambers were monitored with nondispersive infrared CO2sensors(B-530,ELT SENSOR Corp.,Korea)and controlled at a fluctuation ofca.30µmol mol−1by automatically controlled solenoid valves located between the gas generator and the pipeline(Fig.1).During the night(6:00 p.m.-5:00 a.m.the next day),the CO2supply was automatically stopped.Simultaneously,to remove excess CO2above the set concentration in the chamber resulting from respiration from both the plants and soil during the night,the vacuum pump inside the chamber was switched on to absorb CO2into an alkali lime column,and the remaining CO2-free air was then returned to the chamberviainternal cycling until the CO2concentration in the chamber was reduced to the set value(Fig.1).To ensure a uniform CO2concentration inside each growth chamber,the air in the chamber was circulated using an internal ventilation system.The temperature in the chambers was automatically controlled within a 2-degree differential from that outside the chambers.Throughout the wheat growth period,water was added weekly at night to maintain soil water at 25% in the pots based on weight loss of the pots,and algae were carefully removed from soil.
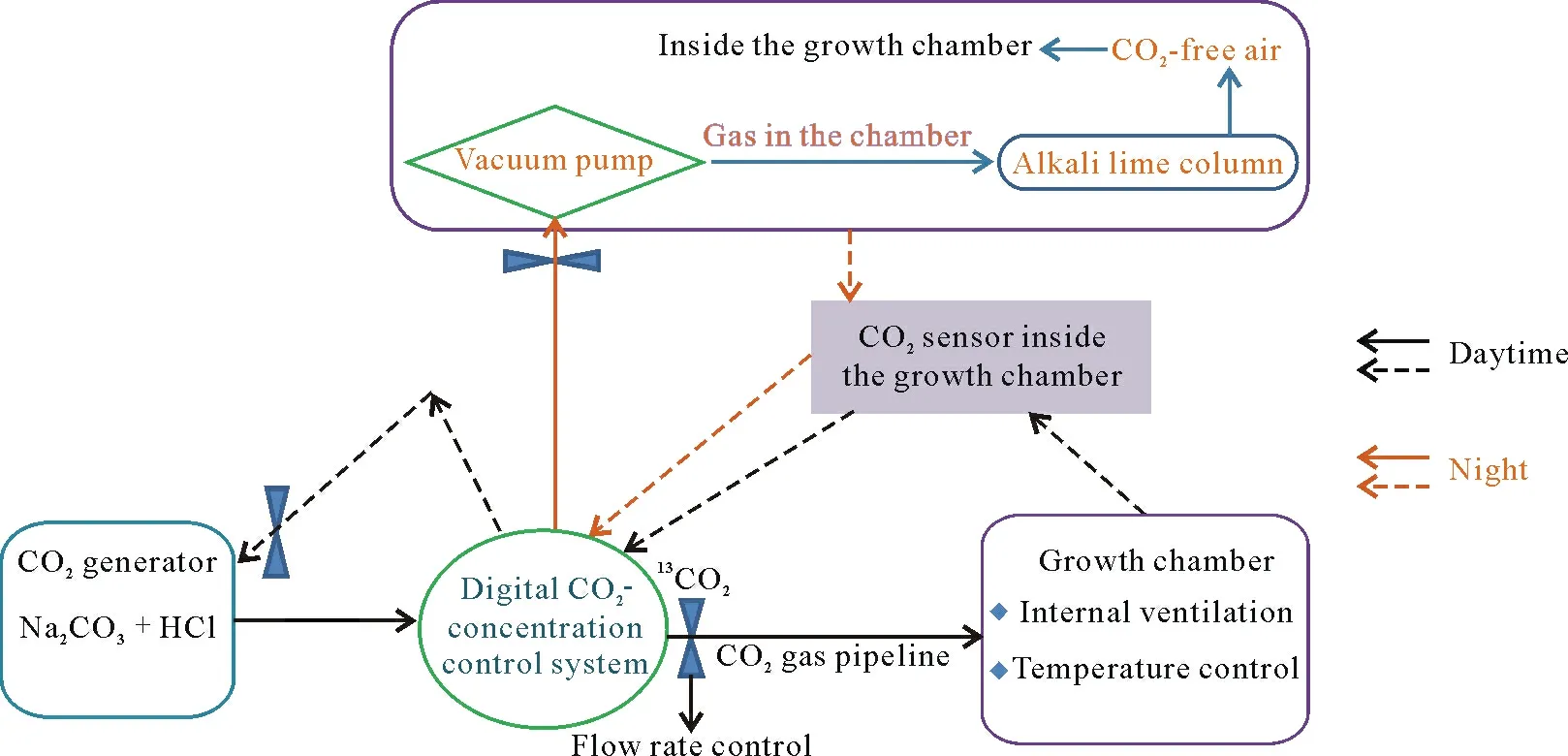
Fig.1 Schematic illustration of CO2-concentration control system inside sealed chamber during continuous 13CO2 labeling.
Sampling and measurements
Both wheat and soil were destructively sampled at the jointing(May 23),heading(June 3),and maturity stages(June 30).The aboveground vegetation was collected,and the roots were separated from soil by hand.The wheat tissues were divided into leaves(including the stems),roots,wheat ears,and grain.The soil samples were air dried after the residual roots were removed by hand,and the roots were washed with deionized water.The collected wheat tissues were oven dried at 85°C for 3 h and then dried at 65°C until a constant weight for mass measurements.Both the dried wheat tissues and dried soil samples were ground to pass through a 0.149-mm sieve for subsequent analyses.The C and N contents in the samples were measured with an elemental analyzer(Model CN,Vario Macro Elemental Analyzer System,GmbH,Germany),and theδ13C values of the soil and plant tissues were measured with an isotope ratio mass spectrometer(Thermo Finnigan MAT 253,Bremen,Germany)combined with an elemental analyzer(Flash EA-1112,Carlo Erba,Fisons,Italy).The results of the C isotope analyses are expressed inδunits as follows:
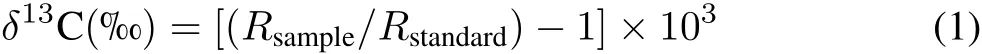
whereRis the ratio of13C/12C for both samples(Rsample)and standards(Rstandard).Theδ13C values are reported in relation to Pee Dee Belemnite.Notably,both theδ13C values and the total C content in all the unplanted soil did not change significantly during the13C-labeling period,indicating that the possible impact of either elevated CO2or N fertilizer application on the incorporation of new soil C was not derived from the fixation of CO2by soil autotrophic microorganisms.Therefore,the data from the unplanted soil were not included in our results and discussion.
Calculations
Photosynthesized C in wheat.During wheat growth in the sealed chambers,photosynthesized C accumulation was represented by biomass C accumulation.The photosynthesized C accumulation in wheat during13CO2labeling(MC,wheat,g pot−1)was calculated as the sum of the amount of photosynthesized C in each tissue:
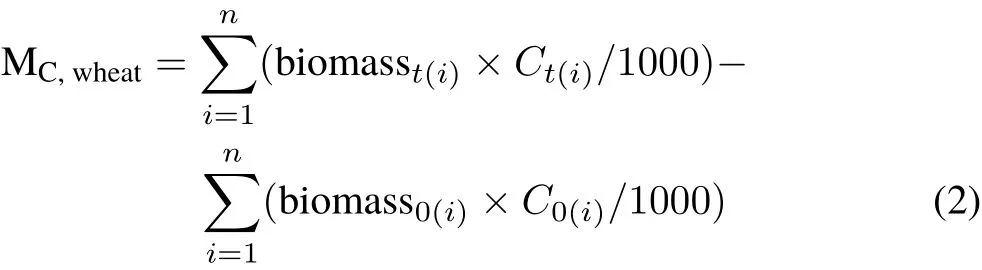
whereirepresents each wheat tissue,including the leaves,roots,wheat ears,and grains;biomass0(i)and biomasst(i)(g pot−1)represent the biomass of each wheat tissueiat the beginning and end of13C labeling,respectively;andC0(i)andCt(i)(g kg−1)represent the corresponding C contents in each wheat tissueiat the beginning and end of13C labeling,respectively.
Incorporation of photosynthesized C into soil.Based on the mass balance of13C(Boutton and Yamasaki,1996;Bernouxet al.,1998),in each wheat-planted treatment,the soilδ13C value at the end of the13CO2labeling(δt)was expressed as follows:
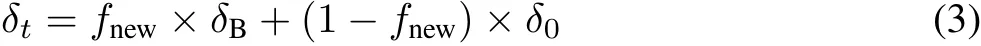
whereδ0represents the initial soilδ13C at the beginning of13CO2labeling(i.e.,t=0)andδBrepresents the isotopic composition of soil C derived from the wheat grown under13C-enriched CO2and is assumed to be equivalent to theδ13C of the wheat roots in the corresponding treatment(i.e.,δroot)in this root C input-dependent pot cultivation experiment.During the labeling period,the proportion of photosynthesized C(new C)in soil under13CO2(i.e.,fnew)in the corresponding treatment(following Talhelmet al.,2009)was calculated as follows:

Since

Thus
Accordingly,the incorporation(i.e.,net input)of photosynthesized C into soil(MCsoil)was calculated as follows(Hofmockelet al.,2011):
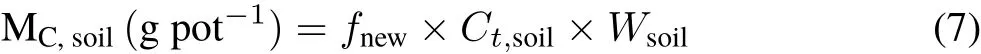
whereCt,soil(g kg−1)andWsoil(4 kg pot−1)represent the soil C content and soil weight at the end of the experiment corresponding tofnew,respectively.
Amount and distribution of photosynthesized C in the wheat-soil system.The amount of photosynthesized C in the wheat-soil system(MCwheat-soil)after the wheat growing season was calculated by summing the amounts of photosynthesized C in the wheat and soil(following Luet al.,2002):
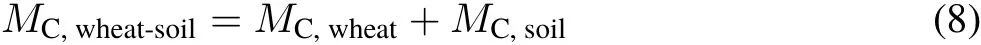
The percent distribution of photosynthesized C in the wheat-soil system was calculated as follows(following Luet al.,2002):
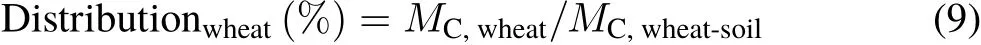
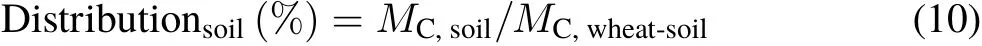
Statistical analysis
A split-plot statistical design was applied in which the CO2concentration was the main plot factor(n=2)and the N level(n=3)was the subplot factor.Means and standard deviations were based on three replicates.Effects of CO2concentration and N fertilization level and their interactions were analyzed by repeated measures analysis of variance.For each sampling time,two-way analysis of variance was used to test the significance of the treatment effects(CO2concentration and N fertilization level)and their interactions.The data were tested for normality using the Shapiro-Wilk test and for homogeneity of variance using the Levene test.The data were natural log transformed as needed prior to statistical analyses to meet the assumptions of normality and homoscedasticity;however,the untransformed means and standard deviations were reported in the figures and tables.All statistical analyses were carried out using the SPSS Version 16.0(SPSS Inc.,USA)software package.Differences at theP<0.05 level were considered statistically significant,and differences at the 0.1≥P>0.05 level were considered trends.
RESULTS
Wheat biomass,C and N contents,and photosynthesized C accumulation in wheat tissues
Under ambient CO2,the wheat biomass increased from the jointing(approximately 10.0 g pot−1)to maturity(approximately 28.0 g pot−1)stages(Fig.2).Until the maturity stage,the biomass tended to increase with an increasing N application level.Compared to ambient CO2,elevated CO2increased the biomass of all wheat tissues at all three N levels during the 62-d labeling period,and the percent increase was the greatest at the N3level.For both leaves and roots,the increases in biomass under elevated CO2were greater than those under increased N application levels.At the maturity stage,the increase in root biomass under elevated CO2ranged from approximately 17.7% in the N1treatment to 32.7% in the N3treatment.A similar increase was observed in the leaves,and the increases in both the root and leaf biomass were greater than in the grain.

F ig.2 Amounts of wheat tissue biomass at the jointing,heading,and maturity stages under ambient(350µmol mol−1)and elevated(600µmol mol−1)CO2 concentrations(350CO2 and 600CO2,respectively)in combination with three N fertilizer application levels.N1,N2,and N3 represent the addition of 33,50,and 75 mg N kg−1 soil,respectively.Vertical bars are the standard deviations of the means(n=3).For a given N application level,bars with the same uppercase letter are not significantly different between elevated and ambient CO2 at P<0.05;for a given CO2 concentration,bars with the same lowercase letter(s)are not significantly different among N application levels at P<0.05.
The C content varied slightly among wheat tissues(i.e.,approximately 426.4 g C kg−1in leaves,403.0 g C kg−1in wheat ears,389.6 g C kg−1in roots,and 428.5 g C kg−1in grain,on average);however,they were not affected by either the CO2concentration or N application level(Fig.3).Therefore,during our continuous 62-d labeling period,the difference in the amount of photosynthesized C between treatments was positively related to that in wheat biomass production between treatments.The accumulated photosynthesized C in wheat(calculated by Eq.2)increased significantly with an increasing CO2concentration(P<0.05),and the increases were approximately 14.1%,18.8%,and 23.8% in the N1,N2,and N3treatments,respectively(Fig.3).The effect of N application level on the amount of photosynthesized C in wheat tissues differed between the two CO2concentrations.For instance,the amount of photosynthesized C in roots was 5.3% higher at N3than at N1under ambient CO2;however,it was 41.4% higher at N3than at N1under elevated CO2(Eq.2)(Fig.2).
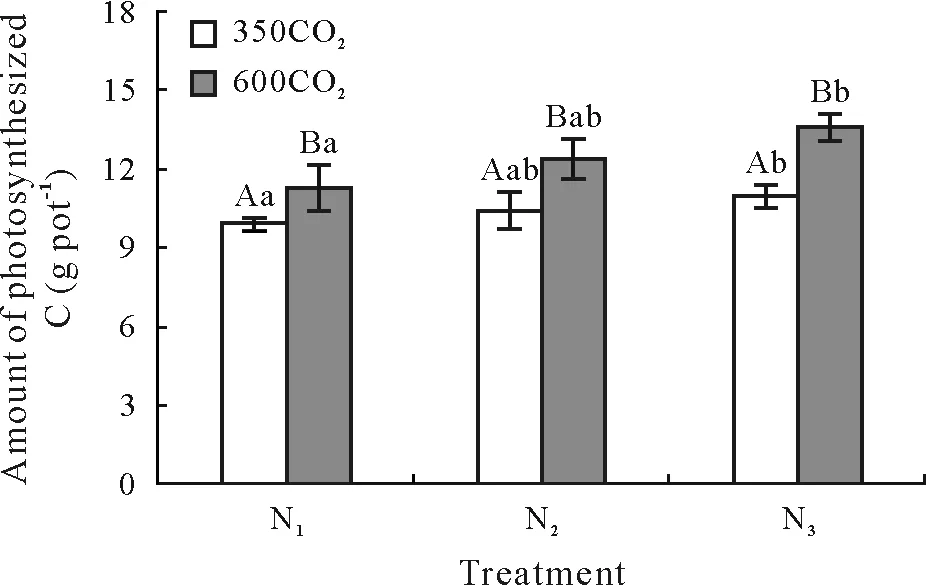
Fig.3 Amounts of photosynthesized C in whole wheat plants over the growing season under ambient(350µmol mol−1)and elevated(600µmol mol−1)CO2 concentrations(350CO2 and 600CO2,respectively)in combination with three N fertilizer application levels.N1,N2,and N3 represent the addition of 33,50,and 75 mg N kg−1 soil,respectively.Vertical bars are the standard deviations of the means(n=3).For a given N application level,bars with the same uppercase letter are not significantly different between elevated and ambient CO2 at P<0.05;for a given CO2 concentration,bars with the same lowercase letter(s)are not significantly different among N application levels at P<0.05.
The N content in wheat tissues decreased with the growth of wheat under both CO2concentrations,and the lowest N content was observed in the N1treatment(Fig.4).Compared with ambient CO2,elevated CO2reduced the N content in all wheat tissues and accordingly increased the C/N ratio,especially in the leaves at the jointing stage.At both CO2concentrations,the higher N application level increased the N content in roots.At the maturity stage,the N content in roots in the N3treatment was approximately 14.6% higher than that in the N1treatment under ambient CO2,and it was approximately 13.0% higher than that in the N1treatment under elevated CO2.The C/N ratio in roots decreased as the N fertilization level increased,and the extent of these changes was similar between the two CO2treatments(Table I).
13C enrichment in wheat tissues during the growing season
Theδ13C values of wheat tissues increased significantly from the jointing stage to the maturity stage at both CO2concentrations(Fig.5).Compared with ambient CO2,elevated CO2significantly increased theδ13C values of wheat tissues;however,the relative increase caused by elevated CO2(expressed in percentage)varied among tissues.For leaves,the relative increase tended to increase with wheat growth,and this increase remained higher in the N2and N3treatments(approximately 15.2%-18.0%)than in the N1treatment(approximately 14.2%-16.2%).In contrast,the increase inδ13C in roots caused by elevated CO2tended to decrease as the N application level increased at the maturity stage.
Soil C content,13C enrichment,and plant-derived C incorporation into soil
During the growth of wheat,soil C content remained equivalent to the initial value of 12.8 g C kg−1soil,regardless of CO2concentration and N fertilizer application level.However,theδ13C values of the cultivated soil increased with wheat growth under both ambient and elevated CO2(Fig.6).From the heading to maturity stages,theδ13C of the soil under elevated CO2remained higher than that under ambient CO2at all three N levels.Compared with the ambient CO2treatment,the soilδ13C values at the maturity stage in the elevated CO2treatment were 9.36‰,8.46‰,and 6.01‰higher in the N1,N2,and N3treatments,respectively.
When average rootδ13C of the three growth stages(the jointing,heading,and maturity stages)was used as a parameter in Eq.6,the calculatedfnewvalues were similar to the theoretical values obtained based on the root and shoot residue input according to the method described by Lobeet al.(2005)(Table II).Under ambient CO2,the greatestfnewvalue was observed at the N3level(1.90%),whereas no significant difference infnewwas observed between the N1(1.70%)and N2(1.68%)levels.Under elevated CO2,fnewat the N1level was 2.45%,whereasfnewunder both the N2and N3levels wasca.2.28%.Elevated CO2increasedfnewby 44.1%,36.8%,and 19.5% at the N1,N2,and N3levels,respectively.When rootδ13C after the vegetation stage was used as a parameter in the mixing model in Eq.6,the estimatedfnewvalues under both CO2treatments were lower than those estimated using the average rootδ13C of the three growth periods.Thefnewunder ambient CO2ranged from 1.32% to 1.48% and followed the order N3>N1≈N2.Under elevated CO2,the greatestfnewwas observed at the N1level(1.92%),whereasfnewunder both the N2and N3levels wasca.1.77%.Elevated CO2increasedfnewby 41.0%,34.0%,and 20.3% at the N1,N2,and N3levels,respectively,exhibiting no significant difference from those estimated using the average rootδ13C of the three growth periods.
Accumulation and allocation of photosynthesized C in the wheat-soil system
The total amount of plant-derived C(photosynthesized C)incorporated in soil ranged from 0.86 to 1.28 g pot−1(Table III).The photosynthesized C in plants ranged from 9.92 to 13.59 g pot−1(Fig.3).The amount of photosynthesized C in the wheat-soil system,which ranged from 10.8 to 14.8 g pot−1,increased significantly with increasing CO2concentration(P<0.05)and increasing N fertilizer application level(P<0.05)(Table III).The largest increase caused by elevated CO2occurred when fertilizer was applied at the N3level(23.1%).During the 62-d labeling period,more than 7% of the photosynthesized C was distributed to the soil,and accordingly,less than 93% of this C was distributed to wheat.The allocation of photosynthesized C between wheat and soil was dependent on both CO2concentration and N application level(P<0.05),and a significant(P<0.05)CO2×N interaction was observed.Under ambient CO2,the proportion of photosynthesized C allocated to soil did not vary significantly among the three N levels,whereas under elevated CO2,increased N application significantly decreased the proportion of photosynthesized C allocated to soil.

Fig.4 Content of N in wheat tissues at the jointing(a),heading(b),and maturity(c)stages under ambient(350µmol mol−1)and elevated(600µmol mol−1)CO2 concentrations(350CO2 and 600CO2,respectively)in combination with three N fertilizer application levels.N1,N2,and N3 represent the addition of 33,50,and 75 mg N kg−1 soil,respectively.Vertical bars are the standard deviations of the means(n=3).For a given N application level,bars with the same uppercase letter are not significantly different between elevated and ambient CO2 at P<0.05;for a given CO2 concentration,bars with the same lowercase letter(s)are not significantly different among N application levels at P<0.05.
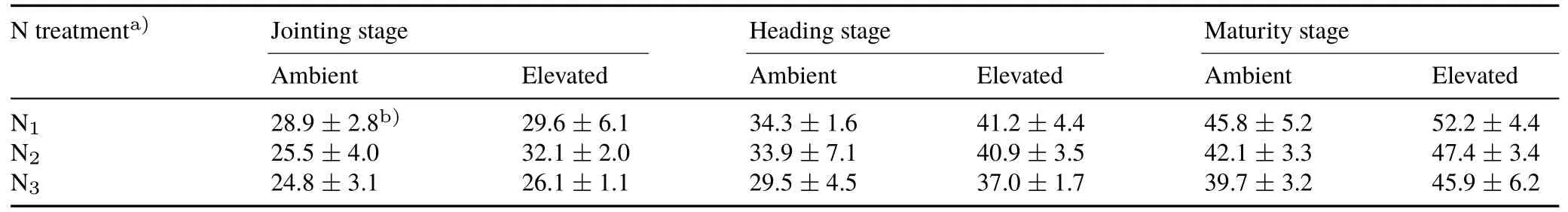
TABLE IRatio of C/N in wheat roots at the jointing,heading,and maturity stages under ambient(350µmol mol−1)and elevated(600µmol mol−1)CO2 concentrations in combination with three N fertilizer application levels
DISCUSSION
Interactive effects of elevated CO2 and N fertilizer on photosynthesized C accumulation in wheat
During the 62 days of13CO2labeling,the accumulation of photosynthesized C in wheat could be explicitly indicated by biomass production,due to the constant C content in the tissues.The increase in wheat biomass caused by elevated CO2observed in the current study was within the range(12%-48%)reported in a previous meta-analysis(Wanget al.,2013).Although a short-term response of wheat growth to elevated CO2was possible at a relatively low rate of N application,such as the N1treatment,a further increase in wheat biomass under elevated CO2with increased N application(Fig.2)indicated that improved soil nutrient availability can induce a stronger positive response of plant growth and resultant photosynthesized C accumulation to elevated CO2(Fig.3).

Fig.5 Isotope composition(δ13C)of wheat tissues at the jointing(a),heading(b),and maturity(c)stages under ambient(350µmol mol−1)and elevated(600µmol mol−1)CO2 concentrations(350CO2 and 600CO2,respectively)in combination with three N fertilizer application levels.N1,N2,and N3 represent the addition of 33,50,and 75 mg N kg−1 soil,respectively.Vertical bars are the standard deviations of the means(n=3).For a given N application level,bars with the same uppercase letter are not significantly different between elevated and ambient CO2 at P<0.05;for a given CO2 concentration,bars with the same lowercase letter are not significantly different among N application levels at P<0.05.
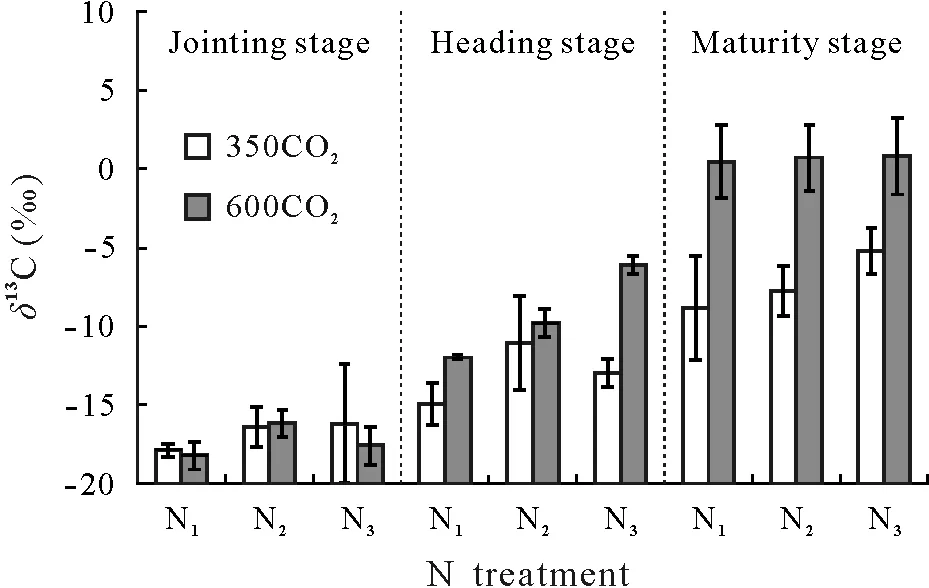
Fig.6 Isotope composition(δ13C)of wheat-planted soil at the jointing,heading,and maturity stages under ambient(350µmol mol−1)and elevated(600µmol mol−1)CO2 concentrations(350CO2 and 600CO2,respectively)in combination with three N fertilizer application levels.N1,N2,and N3 represent the addition of 33,50,and 75 mg N kg−1 soil,respectively.Vertical bars are the standard deviations of the means(n=3).
Despite the application of N fertilizer,elevated CO2decreased the N content in wheat tissues(Fig.4),thereby increasing the C/N ratio(Table I)and possibly reducing the decomposability of roots(Butterlyet al.,2015).Decreases in tissue N content have been commonly observed under elevated CO2(Stiling and Cornelissen,2007;Bloomet al.,2010;Butterlyet al.,2015),especially during active photosynthesis,as recorded at the vegetative stage of wheat in this study(Fig.4).Considering the asynchronous changes in C and N contents caused by elevated CO2,the decrease in N content could not be derived from the dilution effect and should instead be largely attributed to the physiological responses of plants.As reported previously,elevated CO2can reduce the activities of some key enzymes related to the conversion of inorganic N to organic forms(e.g.,protein)in C3plants(Bloomet al.,2010).Additionally,the increased water use efficiency under elevated CO2can reduce the flow of nutrients to the root surface,causing a shortage of N in the root environment(Lamberset al.,1995;van Vuurenet al.,1997).As a compensatory mechanism,the increasedN application level in this study favored N retention in the leaves under elevated CO2(Fig.4),possiblyviaenhanced N uptake by plants and the conversion of inorganic N into proteins(Panget al.,2006).However,the N content in wheat under elevated CO2concentrations remained lower than that under ambient concentrations,even when the N fertilizer application level was high enough for wheat growth(Fig.4).This finding indicated that the physiologically reduced N uptake per unit plant under elevated CO2could not be completely counteracted by improving N availability.Therefore,compared with increasing N supply,increasing CO2concentration has the potential to more profoundly influence both the quality and quantity of fixed CO2in wheat,especially in roots(Eq.2)(Fig.2,Table I).
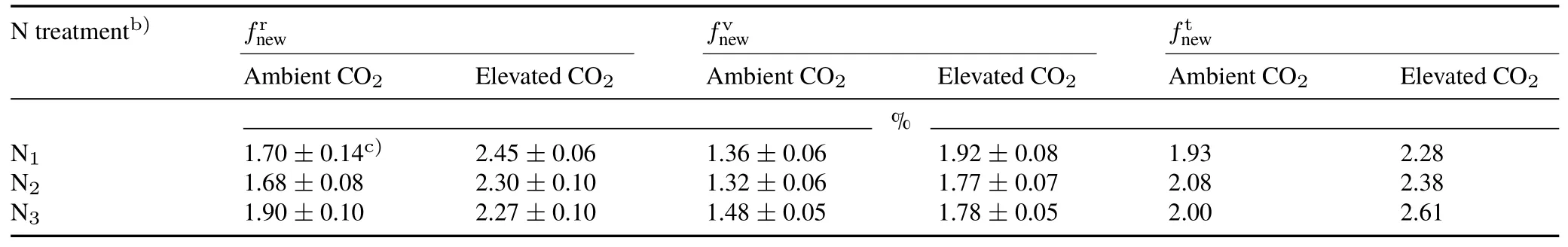
TABLE IIProportions of photosynthesized C(new C)in soil(fnew)a)under ambient(350µmol mol−1)and elevated(600µmol mol−1)CO2 concentrations in combination with three N application levels after wheat harvest
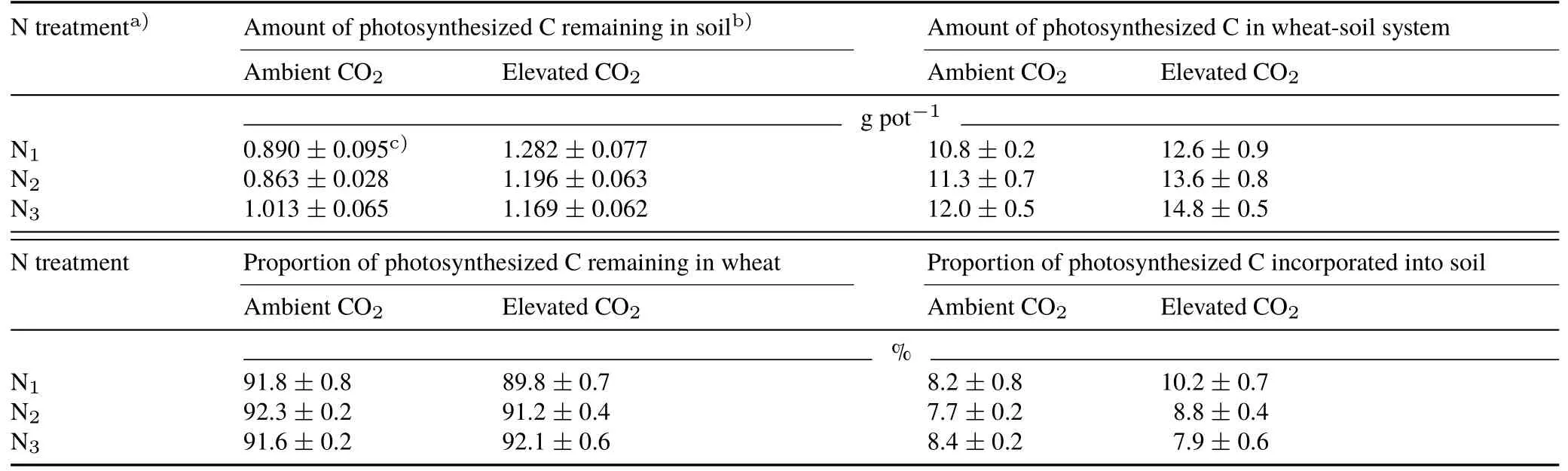
TABLE IIIAmounts of photosynthesized C remaining in soil and the amount and percent distribution of photosynthesized C in the wheat-soil system after one wheat growing season under ambient(350µmol mol−1)and elevated(600µmol mol−1)CO2 concentrations in combination with three N fertilizer application levels
Although CO2was supplied with identical13C abundance at both concentrations,theδ13C of photosynthetic products in wheat leaves remained higher under elevated CO2than that under ambient CO2,and the difference became larger at higher N levels(Fig.5).This phenomenon may have been attributed to the altered ratio of the partial pressure of CO2concentrations within and outside the leaves,controlled by the narrowing of the stomatal pores of leaves induced by elevated CO2and an increased level of N fertilizer application(Freyet al.,1996;Huanget al.,1999;Ainsworth and Rogers,2007;Griepentroget al.,2015).With the transfer of photosynthetic products from leaves to heterotrophic organs,the rootδ13C values were also dependent on the CO2concentration and N fertilizer application level(Fig.5).Therefore,during our13CO2-labeling experiment,only biomass C accumulation(and not the13C amounts in wheat)was valid for comparing the difference in photosynthesized C accumulation in plants and the resultant photosynthesized C flow to the soil among treatments.
Interactive effects of elevated CO2 and N fertilizer on the incorporation of plant-derived C in the soil
Under both ambient and elevated13CO2,the increasing soil13C signature with wheat growth indicated the incorporation of plant-derived C into soil,which could be effectively distinguished from old soil C,even in the short term,viacontinuous13C-enriched CO2labeling(Fig.6).However,the intrinsically different13C signatures in wheat roots at different CO2concentrations and N application levels(Fig.5)implied that the13C amounts in soil could not be used directly to compare the differences in photosynthesized C incorporated into soil among treatments.Essentially,the influence of elevated CO2on the incorporation of photosynthesized C into soil can only be estimatedviaa mixing model(Boutton and Yamasaki,1996;Bernouxet al.,1998).In the applicable formula of the mixing model,the initial soil should be taken as a reference,and the correspondingδ13C of the plants(e.g.,roots in our experiment)and soil at each CO2concentration should be used as parameters.Generally,in simulated elevated CO2experiments,theδ13C of plants is obtained at the end of the experiment(e.g.,Griepentroget al.,2015).However,unlike the homogeneity ofδ13C in C3and C4vegetation in natural isotope methods,the variation in plantδ13C with plant age,as being an intrinsic physiological response(Chevillatet al.,2005),was observed during13CO2labeling in our experiment(Fig.5).Thus,it is more appropriate to acquire theδ13C values of plants by averaging plantδ13C values over the labeling period(Talhelmet al.,2009).In our experiment,we evaluated the influence ofδ13C acquisition in plants on thefnewestimation and found thatfnewvalues calculated based on the average rootδ13C of the three growth stages were more similar to the theoretical values obtained according to the method described by Lobeet al.(2005)(Table II).
Over 62 days of continuous13CO2labeling,when the average rootδ13C of the three growth stages was used as a parameter in Eq.6,it was estimated that 1%-2%renewal of soil C was induced by wheat root deposition under ambient CO2(Table II).This rate was consistent with that obtained in a wheat field experiment using the13C natural abundance approach(approximately 1.29% year−1)(Qiaoet al.,2015).Comparatively,elevated CO2enhanced the flow of photosynthesized C to soil,as indicated by the largerfnewunder elevated CO2than under ambient CO2(Table II).Therefore,the reduced decomposability(indicated by the increased C/N ratio)of wheat roots under elevated CO2(Table I)could be counteracted by the increased input of root biomass and deposits(Fig.2),thus ultimately favoring photosynthesized C incorporation in soil(Schlesinger and Andrews,2000;Pendallet al.,2004).
In the current study,the incorporation of photosynthesized C into soil depended on the interactive effects of CO2and N fertilizer on its quantity and decomposability.Under ambient CO2,the improved root decomposability(indicated by a decreased C/N ratio)and increased input of root biomass recorded at higher N levels jointly contributed to the increased net input of plant-derived C in soil with increased N fertilizer application(Tables II and III).In contrast,the net input of plant-derived C in soil under elevated CO2was lower at the higher N levels than that at the lowest level(N2and N3vs.N1),thus partly opposing our hypothesis.It was noted that the influence of the N fertilization level on root decomposability was independent of the CO2concentration(Table I);however,the increase in root biomass production(indicating the amount of photosynthesized C)induced by a higher N fertilization level was significantly greater under elevated CO2than that under ambient CO2(41.4%vs.5.3%)(Eq.2)(Fig.2).The increased input of available substrates with increasing root biomass(Cheng and Johnson,1998;Cardonet al.,2001)could result in a shift in soil microorganisms from consuming old SOC to utilizing easily degraded rhizodeposits(Carneyet al.,2007),thus increasing the loss of plant-derived C associated with increased root and/or soil microbial respiration(Heathet al.,2005).Therefore,under elevated CO2,higher application rates of N fertilizer reduced the net input of root-derived C in soil in our experiment(Tables II and III).Nevertheless,van Groenigenet al.(2017)reported that the influence of elevated CO2,in combination with high N addition,on new soil C stocks varied significantly(from negative to positive),implying that the interactive effects of CO2and N on the inputs and losses of photosynthesized C depended at least partly on the ecosystem and management practices.
Allocation of photosynthesized C in the wheat-soil system
Photosynthesized C accumulation in the wheat-soil system increased synergistically with elevated CO2and increased N fertilizer application level(Table III),which could partially alleviate rising CO2levels.In contrast to the N levelindependent photosynthetic C allocation between wheat and soil observed under ambient CO2,the increasing N fertilizer application under elevated CO2favored the retention of photosynthesized C in plants but retarded the translocation of photosynthesized C into soil(Table III),thereby enhancing the pivotal role of plants in photosynthesized C accumulation in this plant-soil system under elevated CO2in the short term(Table III).It has been established that short-lived tissues of wheat are usually recycled as plant residues in agroecosystems.A portion of plant-fixed C can be transformed into refractory soil humic materials,while a certain portion is respired as CO2back into the atmosphere.Considering both the microbial assimilation and catabolism processes regulated by N application levels,further experimental work is required to understand the long-term responses of photosynthesized C allocation in the plant-soil system to elevated CO2coupled with N application management.
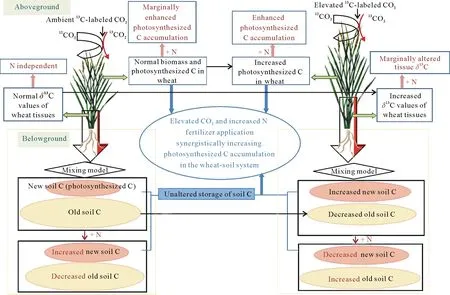
Fig.7 Schematic illustration of the interactive effects of elevated CO2 and N fertilization levels on photosynthesized C allocation in a wheat-soil system.
CONCLUSIONS
We applied continuous13CO2labeling in all CO2treatments to evaluate the interactive effects of elevated CO2and N fertilization levels on soil C turnover and the allocation of photosynthesized C between wheat and soil(Fig.7).Increased CO2concentration coupled with N fertilization may alter C fixation and allocation in agroecosystems by impacting both the quantity and decomposability of photosynthesized C in to plants and,accordingly,the incorporation of photosynthesized C into soil.During the 62 days of continuous13CO2labeling,elevated CO2and increased N fertilizer application levels synergistically increased the amount of photosynthesized C in wheat.Elevated CO2increased the amount of photosynthesized C incorporated into soil at all three N levels.In contrast to the N level-independent photosynthetic C allocation between wheat and soil observed under ambient CO2,the increased N fertilizer application level under elevated CO2increased photosynthesized C accumulation in wheat tissues but retarded the incorporation of photosynthesized C in soil in the short term.Thus,the pivotal role of plants in photosynthesized C accumulation under elevated CO2was enhanced by increased N fertilizer application in the short term.For the entire wheat-soil system,elevated CO2and increased N fertilizer application levels synergistically increased the amount of photosynthesized C,and such a process could,to a certain extent,alleviate rising atmospheric CO2levels.Notably,our findings suggest that robust N management can mediate C cycling and sequestration under elevated CO2viainfluencing the interactions between plants and soil in agroecosystems under climate change.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China(No.41630862),the National Key Research and Development Program(No.2017YFD0200100),and the“China Soil Microbiome Initiative:Function and Regulation of Soil-Microbial Systems”of the Chinese Academy of Sciences(No.XDB15040200).We highly acknowledge the members of the Interdisciplinary Innovation Team of the Chinese Academy of Sciences.
杂志排行
Pedosphere的其它文章
- Notes to Authors
- Agricultural and environmental challenges in agroecosystems
- In Commemoration of Professor Tianren YU’s 100th Anniversary
- Microbial diversity assembled from series-diluted suspensions of diseasesuppressive soil determines pathogen invasion resistance
- Global patterns of phosphorus transformation in relation to latitude,temperature and precipitation
- Chemical and spectroscopic characteristics of humic acid from a clay loam soil in Ontario after 52 years of consistent fertilization and crop rotation
