Laser Synthesis and Microfabrication of Micro/Nanostructured Materials Toward Energy Conversion and Storage
2021-03-08LiliZhaoZhenLiuDuoChenFanLiuZhiyuanYangXiaoLiHaohaiYuHongLiuWeijiaZhou
Lili Zhao, Zhen Liu, Duo Chen, Fan Liu, Zhiyuan Yang, Xiao Li, Haohai Yu, Hong Liu, , Weijia Zhou
ABSTRACT Nanomaterials are known to exhibit a number of interesting physical and chemical properties for various applications, including energy conversion and storage, nanoscale electronics, sensors and actuators, pho‑tonics devices and even for biomedical purposes. In the past decade, laser as a synthetic technique and laser as a microfabrication technique facili‑tated nanomaterial preparation and nanostructure construction, including the laser processing‑induced carbon and non‑carbon nanomaterials, hier‑archical structure construction, patterning, heteroatom doping, sputtering etching, and so on. The laser‑induced nanomaterials and nanostructures have extended broad applications in electronic devices, such as light-ther‑mal conversion, batteries, supercapacitors, sensor devices, actuators and electrocatalytic electrodes. Here, the recent developments in the laser syn‑thesis of carbon‑based and non‑carbon‑based nanomaterials are compre‑hensively summarized. An extensive overview on laser‑enabled electronic devices for various applications is depicted. With the rapid progress made in the research on nanomaterial preparation through laser synthesis and laser microfabrication technologies, laser synthesis and microfabrication toward energy conversion and storage will undergo fast development.
KEYWORDS Laser synthesis; Laser microfabrication; Micro/nanostructured materials; Energy conversion and storage
1 Introduction
Nanomaterials have presented a number of interesting physi‑cal and chemical properties for various applications, includ‑ing energy storage and conversion [1], nanoscale electronics [2], sensors and actuators [3], photonics devices [4], and even for biomedical purposes [5]. The researches on nano‑material synthesis have a long history, and a large number of different synthesis approaches have been performed up until now, including the wet chemical method in solution conducting environment and the thermal treatment process in gas conducting environment. However, uniform and large‑scale production of nanomaterials remains a challenge, and the nanomaterials synthesized through these conventional approaches are non‑site specific in general. Thus, new technologies for large‑scale production and position con‑trollability are necessary.
The laser as a synthetic technique and laser as a microfab‑rication technique provide the alternative choice, which has the advantages of fast, scalable, environment friendly, cost‑effective and permitting in situ processing [6, 7]. Compared with the traditional synthesis and microfabrication tech‑niques, laser‑assisted processing techniques enable direct synthesis of nanomaterials in both gas environment and liquid environment with environment friendliness and less energy loss; especially, the suitability for processing of ther‑mally sensitive substrates has more advantages. On the other hand, recent advances in laser microfabrication incorporate mask unemployment for more advanced patterns of nanoma‑terials. Specially, as the synthetic technique, the traditional wet chemical methods are capable of producing nanomateri‑als with unique morphologies, but toxic or environmentally unfriendly reagents were frequently used. On the contrary, laser synthesis methods in solution conducting environment commonly used the target as precursor, avoiding the use of toxic reagents, and nanomaterials with smaller particle sizes can be obtained through regulation of the laser power, laser wavelength, laser focal length, laser pulse width and laser frequency [8]. Thermal treatment or annealing process is also frequently used methods for nanomaterial synthesis, which is conducted in the furnace at a high temperature by depending on the material formation thermodynamics. But this procedure undergoes some issues of time consuming, high thermal power and energy loss with sample dimensions considerably smaller than the heated volume. In addition, such methods are not suitable for thermally sensitive sub‑strates such as ITO glass or polymers, where microstructural changes and thermal‑expansion mismatch will occur under high temperatures. Alternatively, the laser synthesis tech‑nique with a focused irradiation beam enables a site‑specific growth of nanomaterials based on the local photo‑thermo‑chemical reaction, which possesses the general advantages of spatially confined reaction, non‑contact, fast process‑ing speeds by direct writing and 3D compatibility [9]. The advantage of spatially confined reaction for laser synthesis technology means that the laser‑induced photothermal effect or photochemical effect through synthesis of the purpose materials at a localized position to construct the patterned nanostructures. This can be called the spatially confined reaction by laser, which is strongly dependent on the laser process parameters, especially the pulse width. In addition, additional selectivity and unique properties of nanomaterials for some special applications can be conveyed by regulating the applied laser parameters [10]. As the microfabrication technique, employing masks for the definition of patterns on substrates and lithographic techniques are essential for the conventional fabrication method, which are awkward and expensive for constructing various devices for applications. The laser microfabrication technique obviates the need for masks and lithography, thereby enhancing the production yield of devices and promoting flexibility in the design of device geometry [11]. In conclusion, the laser provides not only an effective alternative to the conventional synthesis and microfabrication processes, but also innovative selective synthesis and microfabrication strategies for efficient usage of nanomaterials with minimized environmental require‑ments and damage on the substrate.
Herein, this review focuses on the nanostructures and nanomaterials using laser as synthesis and microfabrication technique, which are applied in energy conversion and stor‑age. As well known, the absorption of laser by the precursor materials and then resulting in various effects, such as melt‑ing, plasma formation and vaporization, are the basis of laser processing of materials. As the consequence, the characteris‑tics of the laser (laser intensity, wavelength and pulse width) and the photo‑thermophysical properties of the precursors determine the extent of these effects. In this review, we ini‑tially discuss laser processing as the synthetic technique for nanomaterial synthesis, including carbon nanomaterials and non‑carbon nanomaterials. Subsequently, we provide a comprehensive overview on the laser as a microfabrication technique applied in light-thermal conversion, batteries and supercapacitors, sensors, or actuators and electrocatalytic electrodes (Scheme 1). Finally, recent progress and advances in laser synthesis and microfabrication processing of nano‑materials for applications in various devices are discussed.
2 Laser as the Synthetic Technique
Recent technological advances in the development of diverse lasers have opened new avenues in material synthesis, no matter in solid form or in solution environment. The laser technique has long been utilized for material synthesis, which is realized by the photothermal reaction, photochem‑ical reaction or photo‑thermal‑chemical reaction derived from an irradiated laser by generating confined electromag‑netic field at a desired position with high controllability.
Scheme 1 Laser as a synthetic technique and a microfabrication technique and their applications in various functional devices
2.1 Carbon Nanomaterials
Among all of the synthesis methods, annealing or thermal treatment of the precursors is usually necessary to obtain the carbon nanomaterials [12, 13]. Conventionally, the thermal treatment of polymer precursors is conducted in a furnace or an oven at a high temperature, which suffer from a quite slow cooling rate and high energy loss due to the smaller sample dimensions than the heated volume. In addition, the morphology and structure of the precursors were difficult to inherit or create new nanostructures. As an alternative to the conventional annealing, laser processing offers a poten‑tial solution to the above issues and enables construction of different nanostructures from the precursors to carbon materials, on account of the localized thermal effect without any interference with the surrounding materials during laser processing [14].
Various kinds of carbon nanomaterials can be derived through laser processing technologies, including graphene‑related material, diamond‑like carbon, glassy carbon, and heteroatom‑doped carbon. Specially, graphene‑based struc‑tures have become a rising star owing to its unique physical and chemical properties [15, 16], which can be obtained from different precursors by laser processing, such as gra‑phene oxide, polymers, CH4, SiC and so on. In this section, the recent progress in the laser processing of different carbon materials from various precursors is reviewed.
2.1.1 Laser Synthesis of Graphene
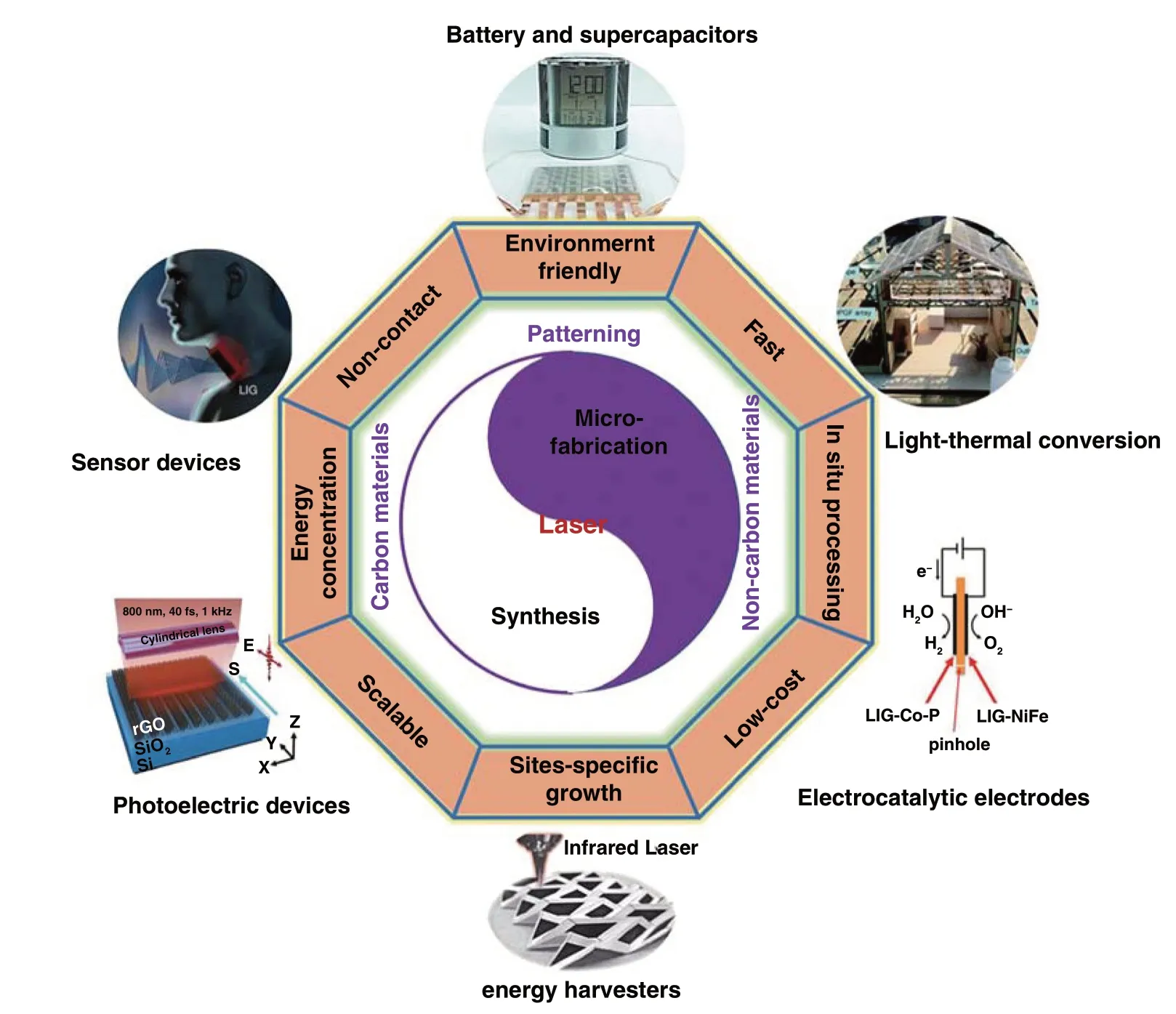
2.1.1.1 Laser Synthesis of Graphene from Graphene OxideGraphene oxide (GO) has been widely utilized as the precursors for synthesizing the reduced graphene oxide (rGO) to remove the oxygen‑containing groups by thermal reduction at a high temperature [17], chemical reduction by means of reductants [18], and photoreduction with light irradiation [19]. Among these, the laser synthesis of rGO has been demonstrated to be an efficient and facile approach for reduction of graphene oxide into graphene and provides an opportunity for localized reduction and controllable patterning [20]. Yannopoulos et al. compared the thermal reduction, chemical reduction, and laser‑assisted reduction approaches of GO to rGO. It turned out that high‑quality graphene‑like structures with efficient defect healing and ultralow sheet resistance were produced by the laser‑induced reduction of GO (Fig. 1a) [21], while the thermal reduction and chemical reduction methods cannot provide a high con‑ductivity for the as‑prepared rGO. This was because that the high temperature induced by laser (> 2500 °C) was able not only to remove oxygen‑containing species but also in favor of eliminating the structural defects in the formed rGO, which would result in a high conductivity for the rGO by laser‑assisted reduction method. Wong et al. [22] reduced the electro‑sprayed GO thin film into rGO and ablated the unwanted areas by using a 355 nm nanosecond laser. After tuning the output laser power, the patterned electrode arrays were completed. (Fig. 1b). In addition, Qu et al. fabricated an asymmetric Graphene/Graphene oxide fiber through the laser‑assisted partial reduction of GO fibers with graphene in the scanned region and GO in the unexposed region (Fig. 1c). The obtained asymmetric G/GO fiber was an ideal material as a moisture‑sensitive fiber actuator [23]. Thus, the laser synthesis of rGO possessed the advantage of con‑trollable reduction of partial GO to rGO. This group further reported a spontaneous reduction of GO aerogel strategy by laser triggering (Fig. 1d). Pure graphene, doped graphene, and multifunctional graphene composited metals or metal oxides with macroscopic bulk structure have been prepared by this fast solvent‑ and reagent‑free preparation method [24]. Only within 37.5 ms, a 5 cm3GO aerogel was com‑pletely reduced to graphene bulk. Unlike the dense struc‑ture of GO fiber in Ref. [23] or dense GO film in Ref. [22], where only the exposed region of GO was reduced to the rGO, the fewer stacked graphene sheets within the pore‑rich structure of GO aerogel and the available air stored in the pores played the crucial roles for the sustainable reduction of GO.
As for the solid GO film typically suffers from the restacking as a result of the strong van der Waals interac‑tions, which caused a low specific capacitance. The Light‑Scribe laser of DVDs can simultaneously cause reduction and exfoliation of GO and then produce an open network of laser‑scribed graphene (LSG). For instance, Kaner [25] used a Light Scribe DVD optical drive to perform on graphite oxide films, the graphene sheets were achieved and restack‑ing was avoided. It was realized by a drop‑cast GO thin film on a flexible substrate and then irradiating with an infra‑red laser inside a Light Scribe DVD optical drive. During the laser LightScribe process, the GO was reduced into the well‑exfoliated LSG sheets, which can be indicated by the change in film color and the SEM images. Kaner et al. [26] also used a LightScribe DVD burner to fabricate graphene micro‑supercapacitors over large areas by direct laser writ‑ing on graphite oxide films. Thanks to the precision of a laser, the desired graphene circuits were produced under the driving of the computer‑designed pattern onto the GO film. Large scale and patterning were realized within a short time. Luo et al. [27] also used the scribing process for the reduction and patterning of graphene oxide film to realize the LSG sensors fabrication. This will be discussed in detail in Sects. 3.2 and 3.3.
As well known, laser reduction of GO conforms to the mechanisms of photochemical reduction under a short‑wave‑length laser (e.g., UV laser) and photothermal reduction under a relative long‑wavelength laser (e.g., Vis and NIR laser). Recently, Guo et al. [28] proposed that by utilizing the transverse electric (TE) surface plasmons triggered by the gradient reduction of the GO film from its surface to the interior, a diverse laser-rGO interaction occurred through producing an inhomogeneous slab with the maximum die‑lectric permittivity (DP) at the surface and a smaller DP at deeper thicknesses that allows excitation of transverse electric (TE) mode surface plasmons (TE‑SPs). This TE‑SPs eventually resulted in interference intensity fringes and spatially periodic interactions (Fig. 1e). It was noted that the laser‑processed grating structure strictly parallel to the TE polarization state of the incident light. This implies that complex topography preparation is feasible by changing only the polarization (Fig. 1f).
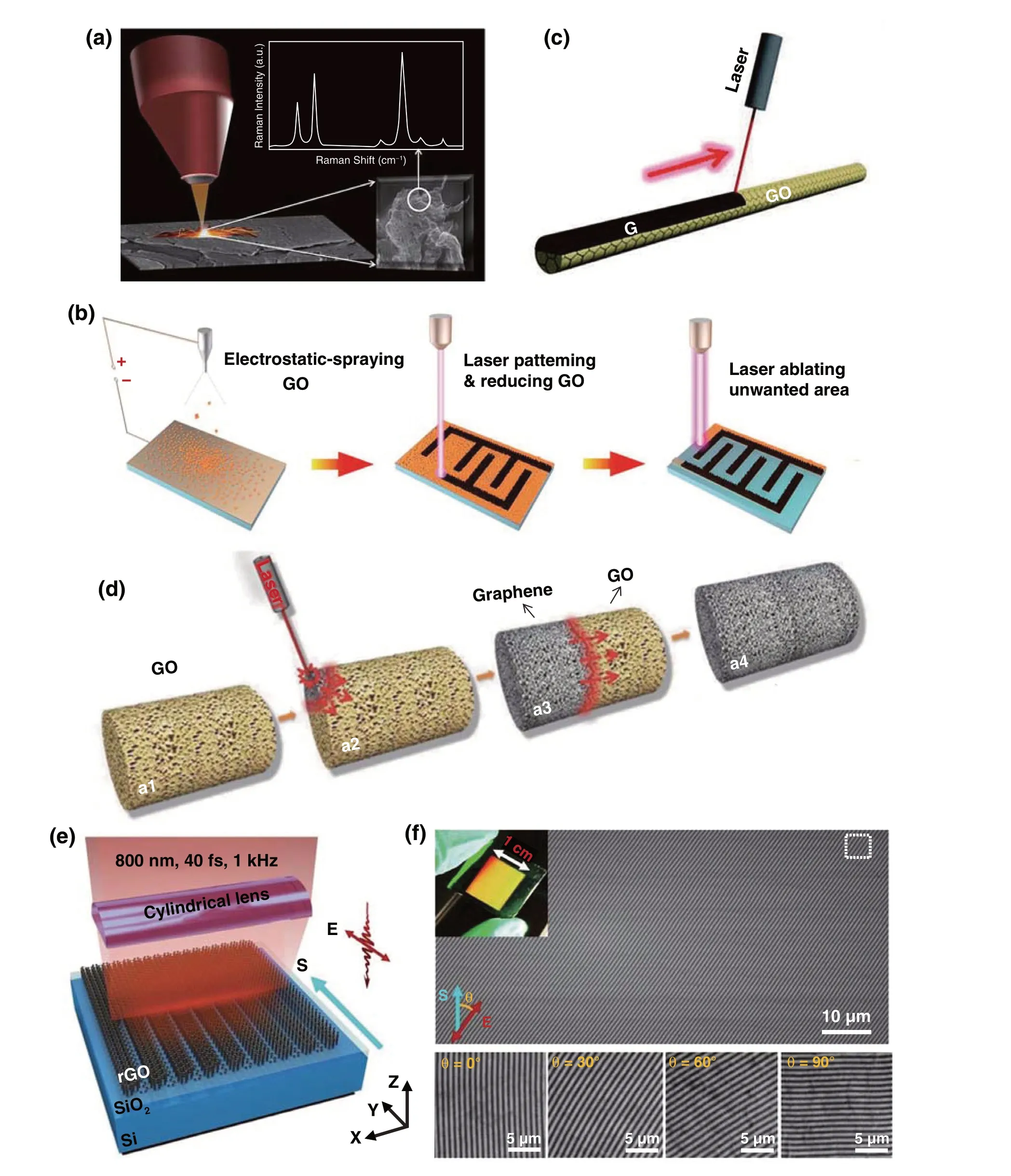
Fig. 1 a Reduction of GO by laser irradiation utilized a Nd‑YAG laser (pulse width in the range of ms) with an excitation wavelength of 1064 nm [21]. b Sche‑matic illustration of ultrathin laser‑processed graphene‑based micro‑planar supercapacitors. After the different powers of laser treatment, the reduction and ablation of GO film were completed [22]. c Representation of positioned laser reduction on one side of a GO fiber. The black region corresponds to the laser‑induced G region along the brown GO fiber [23]. d Schematic illustration of the preparation process of graphene bulks and functional counterparts induced by a laser shot within milliseconds [24]. e Schematic of grating processing of a GO film using cylindrical focusing of femtosecond laser pulses. f Photograph (insert) and SEM image of the large‑area (10 × 12 mm2) rGO. θ represents the angle between S and E [28]
2.1.1.2 Laser Synthesis of Graphene from PolymerBesides the GO, polymers were another frequently used carbon source for laser synthesis of graphene, especially for the polyimide (PI). Different from the 2D sheet‑like graphene derived from GO by laser, porous graphene with a larger specific surface area can be obtained by laser scribing of polymers. During the conversion process from polymers, thesp3‑carbon atom arrangement of polymers converted tosp2‑carbon atom arrangement under the pho‑tothermal effect induced by pulsed laser irradiation. Profes‑sor Tour’s group devoted a considerable amount of effort to laser‑induced graphene (LIG) conversion from poly‑imide or the other polymers [29-31]. In 2014 [32], they reported a one‑step laser scribing on commercial PI in air to form 3D‑graphene layers (CO2laser with a laser power of 3.6 W). The effect of laser power on the formation of LIG was investigated to obtain that the conversion from PI to LIG occurred at the threshold power of 2.4 W. The ther‑mal power dominated the quality of films when the laser power was < 4.2 W, and a higher degree of graphitization occurred with the increase of laser power. When the laser power increased to above 4.2 W, oxidation started to play an increasingly deleterious role in the film quality. In addi‑tion, the mechanism for laser scribing of polymers was also thoroughly investigated in this article. It was speculated that photothermal effects owing to the long wavelength (~ 10.6 μm) and relatively long pulse width (~ 14 μs) of the CO2laser caused lattice vibrations, which led to extremely high localized temperatures (>2500 °C). C-O, C=O and N-C bonds were broken under such high temperatures and atoms recombination occurred to release the correspond‑ing gases, which could be confirmed by the dramatically decreased oxygen and nitrogen contents in the polymer. The author also found out that the mechanism of laser graphitization in polymers was strongly correlated to the structural features of the repeat units, such as aromatic and imide repeat units. Through testing 15 different polymers by laser‑induced graphitization process, it demonstrated that only two polymers, PI and poly(etherimide), both of which contain aromatic and imide repeat units, can form LIG, while the other step‑growth polymers and the chain‑growth polymers cannot afford LIG. However, in 2018 [33], Their further research reported that multiple lasing method allows that any material that can be converted into amorphous carbon can be further treated by a CO2laser beam to obtain graphene, such as Kapton, Kevlar, polysul‑fones, poly(etherimide), polyphenylene sulfide, phenolic resin, and cross‑linked polystyrene. Finally, it can be con‑cluded that the wavelength of the laser irradiation as well as the number of exposures are important to the formation of LIG. Then by means of this laser graphitization from PI technique, Professor Tour’s group successively employed the laser‑induced graphene for flexible and embeddable gas sensors [34], and preparing porous B‑doped graphene with promising electrochemical performance by introduc‑ing H3BO3into PI [35]. Recently, Zhang et al. [36] used the laser writing of Kevlar textile in air to prepare a Janus graphene/Kevlar textile. With the motorized and computer‑controlled laser writing, LIG could be simply and rapidly written into various geometries from Kevlar textile. Similar to the conversion of PI, the C=O and N−C bonds in Kevlar being broken and the reorganization of remaining carbon atoms into graphene can be ascribed to photothermal effect induced by the laser irradiation.
Tour’s group [37] also developed a 3D LIG foam by combining the printing process on basis of laminated object manufacturing and the laser treatment process, which break through the limitation of only 2D products on the PI substrate being able to be synthesized by traditional laser‑induced graphene. They denoted this process as modified, automated 3D‑printing process based on laminated object manufacturing (LOM). During the preparation process, eth‑ylene glycol served as an adhesive through capillary forces between the layers, and stacked PI on top of one another was irradiated by a fiber laser, as shown in Fig. 2a. Except for laser‑induced graphene from PI, Professor Tour’s group also demonstrated a three‑dimensional (3D) printing graphene foam (GF) by using a mixture of Ni and sucrose as precur‑sor [38], which was also realized by in situ manually adding multiple layers of a mixture of Ni and sucrose to overcome the limitation of microsized scales due to the precursors contained in inkjet‑printable or UV‑curable inks for the tra‑ditional 3D printing technique. Graphene was formed under the template and catalyst effects of Ni and sucrose as car‑bon source. Besides, various naturally occurring substrates (Fig. 2b) such as paper, cloth, coconut shells, potato skins, cork [33] and polytetrafluoroethylene (Teflon, or PTFE) [39] have been tried to prepare LIG by this group. Some other groups also have done further researches on LIG based on the work of Tour’s group [40]. Luo et al. [41] prepared laser‑induced graphene paper (LIGP) with different shapes and structures. Similarly, they confirmed that the polymer precursor with considerable spaces was beneficial to a deep absorption of thermal energy resulted from laser and thus was conducive to the formation of LIGP. Alshareef et al. [42] used glucose‑derived amorphous carbon nanospheres (CNS) as carbon source, and a highly turbostratic graphitic carbon electrode was fabricated by laser scribing, which was composed of a 3D framework structure dominated by meso‑ and macro‑pores (Fig. 2c).
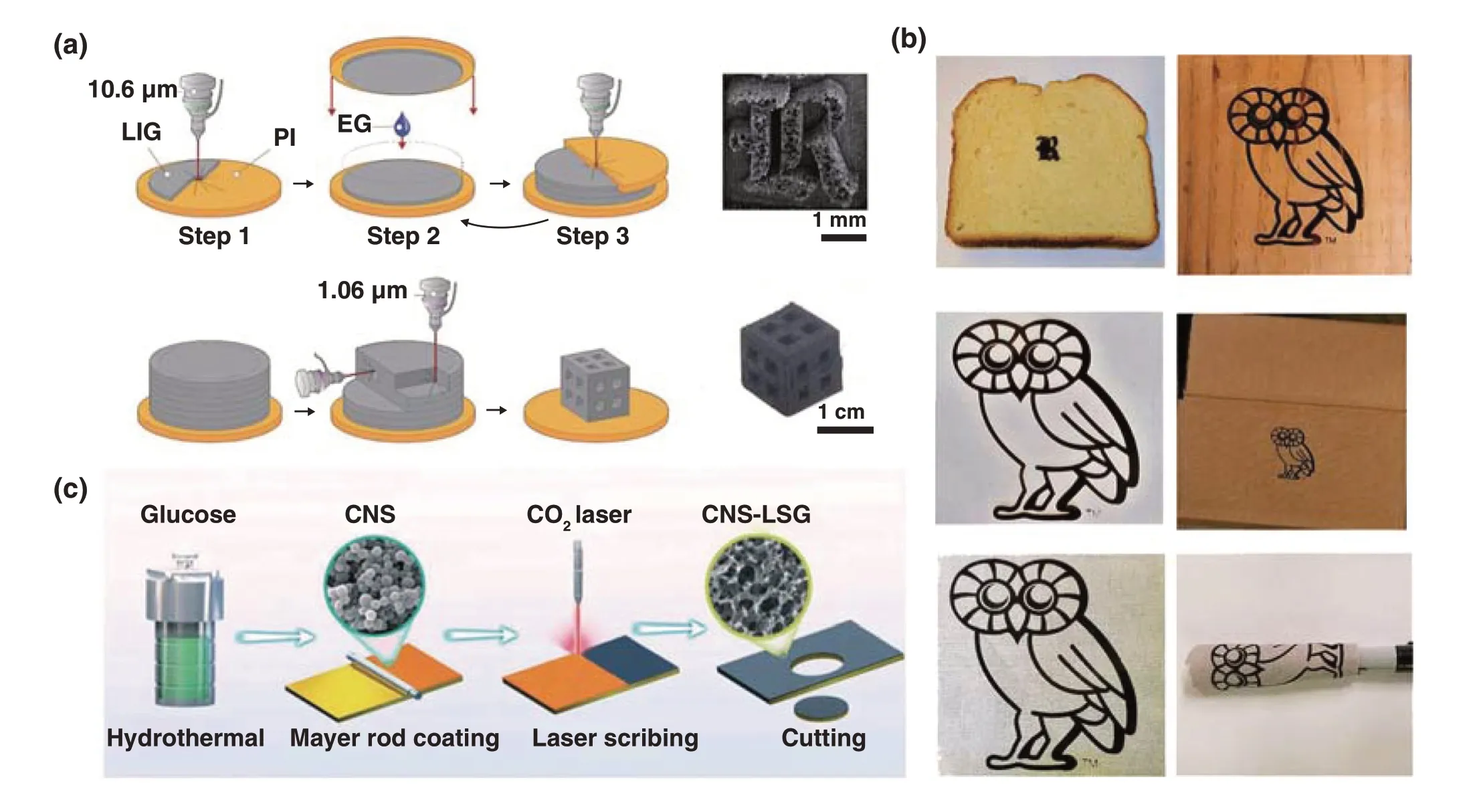
Fig. 2 a Manufacturing and processing of laser‑induced 3D GFs [37]. b LIG induced from bread, fire‑retardant treated pine wood, cotton paper, cardboard box, gray muslin cloth and muslin cloth wrapped around a marker [33]. c Schematic diagram of the laser scribing fabrication of CNS‑LSG electrode [42]
In conclusion, the polymer precursor for laser process‑ing is a more appropriate approach to prepare porous and 3D graphene, which is realized by photothermal effects induced by laser. Thus, the CO2laser with a long wave‑length (~ 10.6 μm) is preferable choice when using polymers as precursors. CO2lasers are unique compared to ordinary industrial lasers in that they usually have a wavelength of ~ 10.6 μm when output in the form of long‑wave infrared. Many organic materials, including paper, wood, plastics, rubber, textiles, and leather, are highly absorbent for such long wavelengths. The mechanism for laser scribing of poly‑mers was speculated that photothermal effects owing to the long wavelength of the CO2laser caused lattice vibrations, which led to the breaking of C-O, C=O, and N-C bonds and atoms recombination to form LIG. As reported [43], poly‑imide was ablated with a 308 nm XeCl excimer laser, and the carbon material was characterized after 200−800 pulses, but no graphene‑based 2D Raman peaks were detected. By contrast, a CO2laser could yield LIG from a wide variety of substrates for only 3−5 passes processing. Hence, the wave‑length of the laser irradiation is important to the formation of LIG. Furthermore, laser‑induced graphene from polymer precursors presents a novel strategy for simultaneous gra‑phene formation and patterning.
As mentioned above, the localized high temperature induced by CO2laser can be used to selectively convert some carbon precursors into graphene materials. In fact, the laser‑induced graphitization comprises various pho‑tothermal reactions, photochemical reactions, or thermal accumulation effect from high repetition rate laser. In terms of thermal accumulation effect, because the time interval between sequential laser pulses is significantly shorter than the time required for heat to diffuse out of the focal volume, hence the energy from successive laser pulses accumulates in and around the focal volume over time. Wang et al. [44] utilized the high‑repetition picosecond laser to processing the bakelite plate, which provides great potential to stimu‑late both research and industrial interest in the development of bakelite‑derived carbon materials. It demonstrated that the heat accumulation effect was critical for laser‑induced transient heating of bakelite materials, and the subsequent instantaneous high temperature field localized in the micro‑scale domain to generate the hierarchically macropores and mesopores. Young‑Jin Kim et al. [45] reported the direct laser writing of graphene oxide patterns using femtosecond laser pulses with different repetition rates. The results showed that the thermal accumulation effects dominantly affected the reduction degree and the linewidth of treated GO. Con‑sequently, it can be concluded that, photochemical reduction under a short‑wavelength laser and photothermal reduction under a relative long‑wavelength laser are commonly used for laser‑induced graphene. But the graphene produced by these two mechanisms is generally treated with nanosecond lasers, compared with ultra‑short ps and fs lasers, nanosecond laser caused the low level of achievable precision for patterns. Fortunately, thanks to the recent development of fs and ps laser sources operating at high repetition rates of hundreds kHz up to a few MHz, the development of thermal accumula‑tion effects is expected to be an alternative in LIG, which can achieve the high‑conductivity graphene under the premise of ensuring the precision of patterns.
2.1.1.3 Laser Synthesis of Graphene from CH4and SiCAs mentioned above, although the laser‑processed rGO had good electrical conductivity, abundant defects remain existed in thesp2carbon network. As a practical method, the chemical vapor deposition (CVD) and epitaxial growth (EG) of graphene were feasible methods to produce large‑scale graphene with high quality, while the fabrication of graphene patterns through CVD or EG was commonly time consuming and costly. Thus, developing a rapid approach for fabrication of graphene is essential. Lu et al. [46, 47] devel‑oped a fast growth method of graphene by laser direct writ‑ing (a focused continuous‑wave laser beam withλ= 532 nm, laser power = 5 W) on a thin Ni foil in a CH4and H2atmos‑phere, which can precisely control the position of graphene pattern and realize the improvement of growth rate than that of general CVD method. For instance, a graphene pattern with an area of 10 × 10 μm2can be rapidly grown in 0.2 s. A similar strategy was also reported by Zhong et al. [48]. Besides the CVD method, epitaxial growth of graphene was another choice. In order to solve the problem of high growth temperature for EG method, Salleo et al. [49] demonstrated a scalable epitaxial graphene synthesis technique based on laser‑induced surface decomposition of the Si‑rich face on silicon carbide (SiC) single‑crystal (pulsed KrF laser with 248 nm and 25 ns) (Fig. 3a). Furthermore, Choi and Lee et al. [50] reported epitaxial growth of solid‑phase N‑doped graphene on the doped silicon carbide (SiC) substrate driven by pulsed XeCl excimer laser (λ= 308 nm, pulse duration ~ 30 ns). Compared with the gas‑phase doped graphene method, this strategy can precisely control the doping con‑centration of graphene by tuning the dopant concentration of SiC substrate (Fig. 3b). The above reported laser epitax‑ial growth of graphene was all performed in a high‑vacuum condition. Yannopoulos and Siokou et al. reported that epitaxial growth of large‑area graphene on the surface of SiC(0001) using a continuous‑wave infrared CO2laser was realized (Fig. 3c) [51], which does not require high‑vacuum or strict sample‑chamber conditions. In addition, another advantage for the CO2laser was manifested that the very high heating rate derived from CO2laser efficiently avoided the different Si desorption rates from adjacent SiC steps to form homogeneous graphene. In conclusion, the laser syn‑thesis technique by means of EG presented many advan‑tages. Firstly, large‑scale production, time and cost saving as well as patterning without nanolithography were still exhib‑ited in laser processing. Secondly, this method operates at low temperatures on account of the laser‑induced local high temperature, which does not necessitate high vacuum and/or SiC pre‑treatment. Lastly, controllable doping concentra‑tion of graphene can be easily realized by tuning the dopant concentration of the SiC substrate.
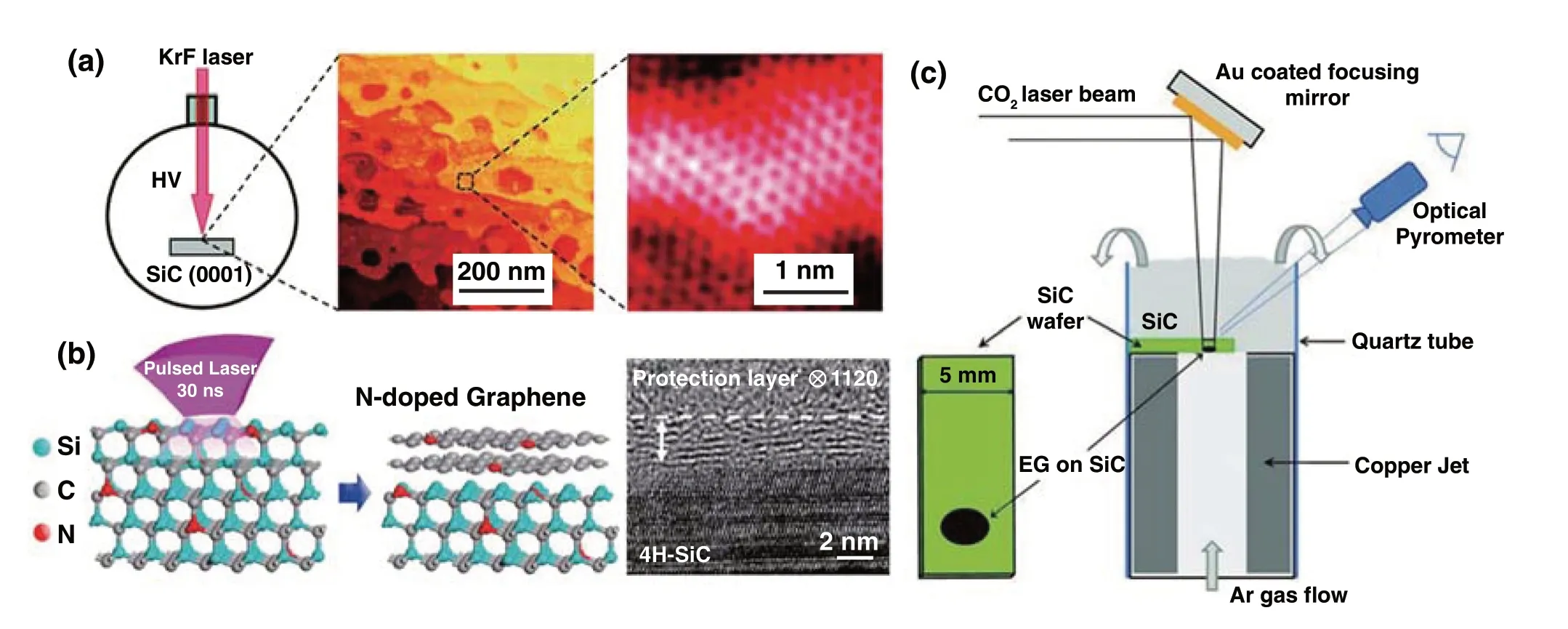
Fig. 3 a Illustration of laser‑induced epitaxial graphene synthesis [49]. b Schematic illustration of the synthesis method for laser‑induced N‑doped graphene on N‑doped SiC substrate and cross‑sectional HRTEM image of multilayer N‑doped graphene on 4H‑SiC (0001) [50]. c Schematic diagram of the CO2‑laser‑induced epitaxial growth of graphene on SiC wafers [51]
2.1.2 Laser Synthesis of Diamond‑like Carbon
Graphene has good electrical conductivity and transpar‑ency to some extent. Diamond‑like carbon (DLC) as another amorphous form of carbon exhibited the electrical insulator property due to kinship with diamond and visible and infra‑red range transparency. Therefore, combining the properties of DLC and graphene could innovatively obtain a transpar‑ent conductive material coating on an insulated transparent substrate. Laser synthesis of diamond‑like carbon and gra‑phene provided an accurate, convenient, clipping method to realize this innovative solution. Stock et al. [52] explored the preparation of DLC thin films from a high‑purity graphite target by pulsed laser deposition (PLD). It is worth noting that the high‑vacuum condition with residual pressure less than 10−8mbar was essential. Then by using the obtained DLC as substrate, UV laser surface annealing was per‑formed to modify the first atomic layers of DLC thin‑film structure by graphene‑like layers with high conductivity. It demonstrated that the obtained graphene layer on DLC had comparable conductivity and transparency performances to those of ITO. In addition, Fan et al. [53] used hydrocarbon species including a mixture of acetylene (C2H2), ethylene (C2H4) and oxygen (O2) as the precursor, a combustion torch was used to produce the flames and an ultraviolet (UV) laser was used to excite the combustion species in the direction of perpendicular to the combustion flames and parallel to the substrate (Fig. 4a, b). By analyzing the nucleation process of diamond growth, they proposed the two critical surface reactions during diamond growth: (1) addition of reactive hydrocarbon radicals to the active surface sites and (2) H‑abstraction from hydrocarbon radicals to create more reactive sites to accept hydrocarbons and stablesp3‑hybridized carbon bonds. Therefore, the H‑abstraction from hydrocarbons by UV laser‑induced photolysis to produce abundant reactive species play a dominant role in promoting diamond growth, which can shorten the diamond nucleation time and suppressed the nondiamond carbon accumulation.
2.1.3 Laser Synthesis of Heteroatom‑Doped Carbon
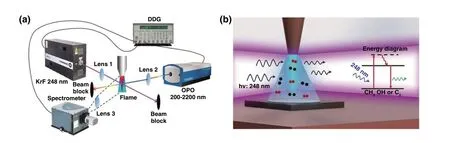
Fig. 4 a Schematic diagram of the optical emission spectroscopy (OES) and laser‑induced fluorescence (LIF) setup to characterize the species in the combustion flame with UV laser irradiation. b Schematic illustration of the UV‑laser‑assisted diamond combustion CVD setup [53]
During the laser processing, the high localized temperature caused by the photothermal effects of laser played a key role in material preparation, meaning that laser was used as a heat source. So ordinary materials prepared by heat‑ing synthesis can be similarly achieved by laser. Therefore, heteroatom‑doped carbon can also be rapidly synthesized by laser and realize patterning in a large scale. In terms of laser‑induced‑doped graphene, Professor Tour’s group prepared porous B‑doped graphene with promising electrochemical performance by introducing H3BO3into PI, as mentioned in Sect. 2.1.1.2 [35]. Alshareef et al. [54] prepared nitro‑gen‑atom‑doped graphene using urea‑containing polyimide as the precursor on Cu foil. The binder‑free, additive‑free, and conductive anodes for Na‑ion battery was fabricated by single‑step laser‑based transformation. Li et al. [55] prepared the S‑ and N‑doped graphene patterns on glass and polyethylene terephthalate (PET) substrates by using organic polybenzimidazole (PBI) ink as the precursor. Dur‑ing the fabrication process, a UV laser beam with 355 nm wavelength was used to break the C-S bond (2.8 eV) from dimethyl sulfoxide (DMSO) and the C-N bond (3.14 eV) from polybenzimidazole. In addition, Professor Yuge pre‑pared B‑ and N‑codoped single‑walled carbon nanohorns by CO2laser processing of a boron‑containing carbon tar‑get under nitrogen atmosphere [56]. Besides of nitrogen, other heteroatom‑doped graphene was also synthesized by laser processing. Ruoff et al. [57] reported a Raman laser (λlaser= 488 nm) irradiation to prepare the F‑doped graphene with fluoropolymer‑covered graphene as the precursors. The laser‑induced decomposition of the fluoropolymer produced active fluorine radicals and then reacts with thesp2‑hybrized graphene to form C-F bonds. Therefore, similar to laser syn‑thesis of graphene and other carbon materials, introduction of heteroatom precursors into the raw materials was essential for laser synthesis of heteroatom‑doped carbon. By consider‑ing the light‑absorption characteristics of the carbonization precursor and the precision of the laser, the appropriate type of laser can be selected, and then, heteroatom‑doped carbon materials were prepared through regulating parameters such as laser power, frequency, and pulse width of laser.
As mentioned above, laser synthesis of carbon materials from polymers is mainly based on the absorption of laser by the precursor, which results in the heating effects. As the consequence, the CO2laser is the preferable choice because that the infrared laser with a long wavelength is conducive to the photothermal reaction to carbonization. For the laser‑induced rGO from graphene oxides, although the photothermal effect and photochemical effect simultaneously happened, photochemical effect through rebinding of atoms within GO induced by laser was dominated. Thus, laser wavelength covering UV, Vis and NIR wavelength range is all adaptable. The effects of parameters of laser on the composition nature of obtained carbon materials are sum‑marized in Table 1.
2.2 Non‑carbon Nanomaterials
2.2.1 Laser Synthesis of Non‑carbon Nanomaterials in Non‑aqueous Environment
Except for the synthesis of different carbon nanomaterials, laser processing was also commonly developed to fabri‑cate some other non‑carbon nanomaterials, such as metal oxides, metal carbides and metal disulfides, especially as a large‑scale and fast fabrication method. Laser synthesis of non‑carbon nanomaterials is primarily based on the photo‑thermal effects and/or the photochemical effects induced by the irradiated laser, where the absorption of laser by the pre‑cursors results in some specific effects. Specially, ablation or heating dominate the nanomaterials formation during the laser synthesis of non‑carbon nanomaterials in non‑aqueous environment.
2.2.1.1 Metal and Metal OxidesMetal nanomaterials and metal oxide nanomaterials have been widely used in various applications in energy storage [58], energy conversion [59] and environmental treatment [60]. In recent years, many synthetic methods, including chemical vapor deposition (CVD) [61], liquid chemical method [62-65], hydrothermal method [66] and electrochemical method [67], have been developed. However, large‑scale manufacturing of these nanocrystals with high performance remained very difficult. Therefore, the laser synthesis method attracted increasing attention for its fast, facile and large‑scale fabrication pro‑cess, as well as operation at low temperatures [68].
As mentioned above, ablation or heating dominated the nanomaterials formation during the laser synthesis of non‑carbon nanomaterials in non‑aqueous environment. With regards to the laser ablation synthesis of nanomaterials, a target surface was irradiated by a beam of high‑energy pulsed laser. The reflection and absorption simultaneously occurred, and once the absorbed laser energy exceeded the evaporation temperature of the target, the target would melt and evaporate a large number of atoms, electrons and ions, thus forming a plasma on the surface of the target. After shifting the pulsed laser, the plasma expanded and then cooled and crystallized to prepare nanomaterials. For instance, Li et al. [69] reported a general strategy to manu‑facture a series of metal oxides (MOx, M = Ti, Mn, Fe, Co, Ni, Cu, Mo, Ag, Sn, W, and NiFe) with hierarchical nano‑structure on corresponding metal substrates by laser abla‑tion. When using as the electrocatalysts, additional binders were avoided to assist the adhesion of electrocatalyst nano‑particles on substrates. The universality for various metal oxides synthesis, large‑scale manufacturing and fast syn‑thesis process demonstrated the advantages of laser ablation synthesis (Fig. 5a, b). Zheng et al. [70] grew preferentially [001]‑oriented BiVO4on FTO substrates through the laser ablation of the BiVO4target. Compared with the BiVO4with randomly oriented grains produced by sol-gel drop‑casting method, the laser ablation method has a lot of advantages in sample preparation. This system is capable of controlling
the variables during the synthesis such as temperature, type of reactive gas and the pressure; thus, epitaxial films as well as various morphologies, compositions and phases of prod‑ucts can be fabricated. As demonstrated, this preferentially [001]‑oriented BiVO4exhibited higher performance as the photoanode materials for photoelectrochemical water split‑ting. Most of the laser ablation synthesis strategies of metal and metal oxides were utilized in the liquid environment, which was discussed in Sect. 2.2.2.
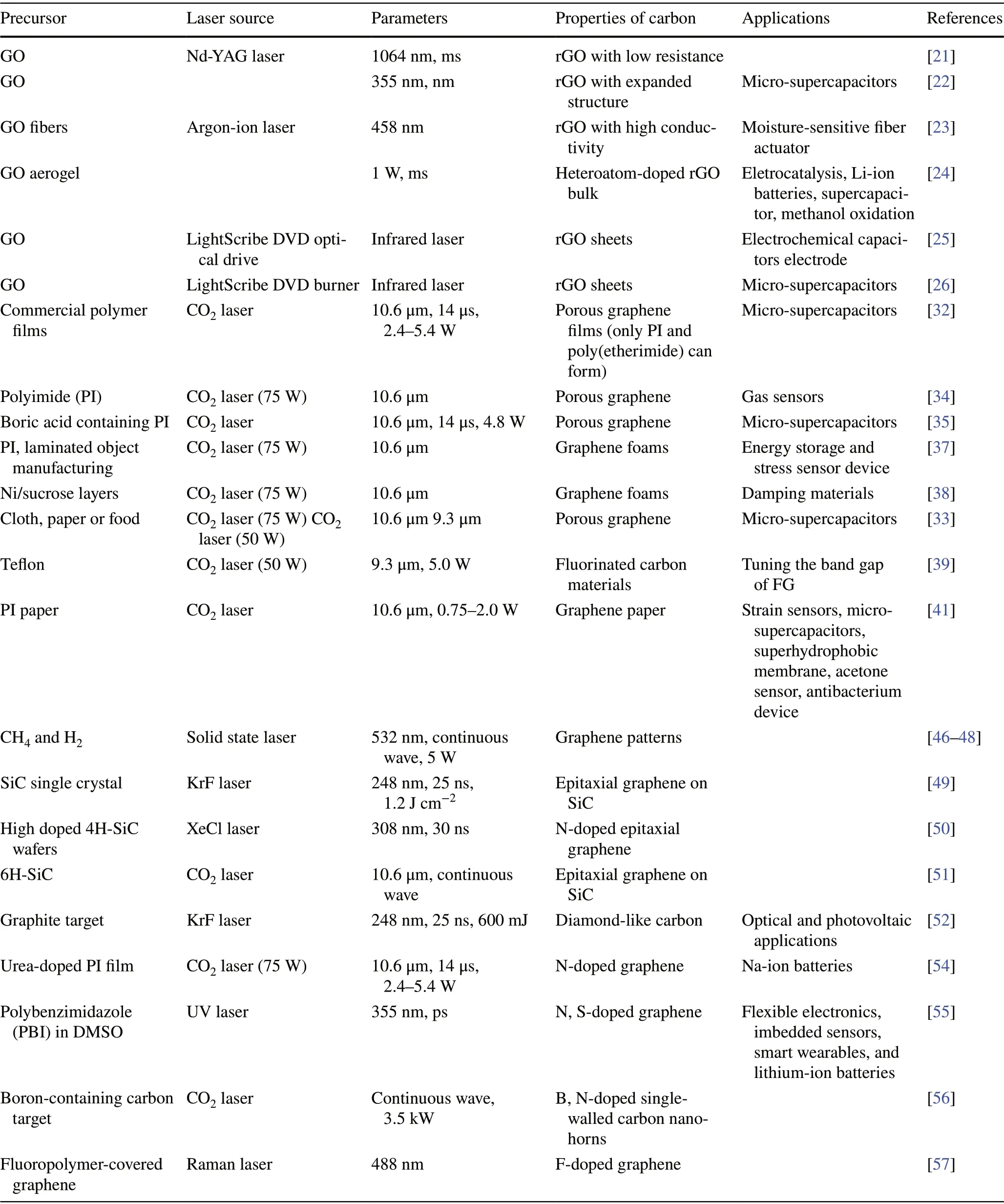
Table 1 Effects of parameters of laser on the composition nature of obtained carbon nanomaterials
With regards to the laser heating synthesis of nanomateri‑als, the significantly localized and rapid photothermal effect induced by a focused laser was employed to replace the con‑ventional thermal treatment for the nucleation and growth of nanomaterials. For instance, the laser‑assisted pyrolysis method was a traditional synthesis strategy for metal oxide nanomaterials [71-73]. Xu et al. [74] fabricated N‑doped SnO2nanoparticles through this laser‑assisted pyrolysis process, which using Sn(OH)4suspension as the precur‑sor with NH3for doping and a gaseous sensitizer (C2H4) for absorbing laser photons (Fig. 5i). The as‑synthesized N‑doped SnO2nanoparticles exhibit excellent cycling sta‑bility and rate capability as anode materials in lithium‑ion batteries (LIBs), which was attributed to the formed unreac‑tive Sn-N bonding in the structure during charge/discharge and the small particle sizes as a result of laser pyrolysis. However, the heat transfer of the laser‑assisted pyroly‑sis process was usually limited by the gaseous precursor and generally obtained the product with poor phase purity. Metal-organic frameworks (MOFs) provided ideal contact between carbonaceous and metallic precursors on an atomic scale and preserved efficient photothermal conversion under laser irradiation [75]. Ogale et al. [76] selected MOFs of zeolitic imidazolate frameworks (ZIF‑67) as precursors to achieve the metal and heteroatom‑doped, porous graphene hybrid electrodes by laser direct writing, which set a prec‑edent for laser carbonization of MOF under ambient for micro‑supercapacitor application. Deng et al. [77] selected different porous MOFs to be processed by pulsed laser, a series of metal nanoparticles including Fe, Ni, Co, Cu, Cd, Zn, In, Pb, and Bi with uniform sizes and gaps were pro‑duced (Fig. 5c). The self‑reduction process of metal ions atomically dispersed in MOFs and then assembled across the pores under laser irradiation, which contributed to the formation of metal nanoparticles (MNPs). In addition, the formed thin layers of graphene on the surface can efficiently protect metal nanoparticles from oxidation and aggregation. By controlling the processing position of laser, the conver‑sion sites from MOFs to MNPs were controlled and the desirable patterns were finally generated (Fig. 5d-h). Thus, this method can be called nanoscale laser metallurgy and patterning (nano‑LaMP). The mechanism of the MNPs pro‑duction through laser irradiation on MOFs was proposed and then confirmed in this article (Fig. 5f). Firstly, the coordina‑tion between metal ions and organic ligands in MOFs leads to high light absorptivity. Secondly, the high photothermal conversion efficiency leads to the rapidly increased tempera‑ture of the MOFs, and the increased temperature was high enough to decompose the organic ligands in the MOF. Then, a local reductive atmosphere composed of H2, CH4, CO, and C2H2was created to reduce the metal cations in MOF and subsequently assembled to form MNPs with protective gra‑phene layers under the synergy effect of the reductive atmos‑phere and extremely high temperature at the local position resulted from laser movement. This work provided a new perspective for laser synthesis of nanopowders free of pre‑fabrication of films. By using the same nano‑LaMP method, Deng et al. [78] recently found out that metal species in the MOF resulted in different final products after nano‑LaMP. For instance, the metal featuring with unsaturated d orbit‑als [Hf(5d2), Zr(4d2), Ti(3d2), Cr(3d5), V(3d3), Fe(3d6) and Mo(4d5)] (Fig. 5j) in MOF was inclined to convert to transi‑tion metal carbide nanoparticles (TMCs), because that these metals could form hybrid orbitals with carbon to form metal carbides rather than metal nanoparticles. In addition, these TMCs needed an extremely high temperature to form sta‑ble metal carbides compared with the formation of metals (Fig. 5j); therefore, the extremely high temperature resulted from pulsed laser made it possible for the reduction of metal ions to TMCs. In conclusion, the characteristic of metal spe‑cies in MOFs as well as the laser synthesis method along with high temperature, reductive atmosphere (CO and H2) and instant heating and cooling facilitated the formation of a large variety of TMCs (Fig. 5k).
Hybrid materials composed of graphene and metal oxides were also developed to be fabricated by laser processing, as laser‑induced graphene from polymers has been widely studied and significant advances have been made. Tour et al. [79] scribed a metal‑complex containing polyimide film by the CO2laser, different metal oxide nanoparticles includ‑ing Co3O4, MoO2, and Fe3O4embedded in porous graphene were formed. This work provided an advanced method for synthesis of metal oxide nanoparticles/graphene hybrid
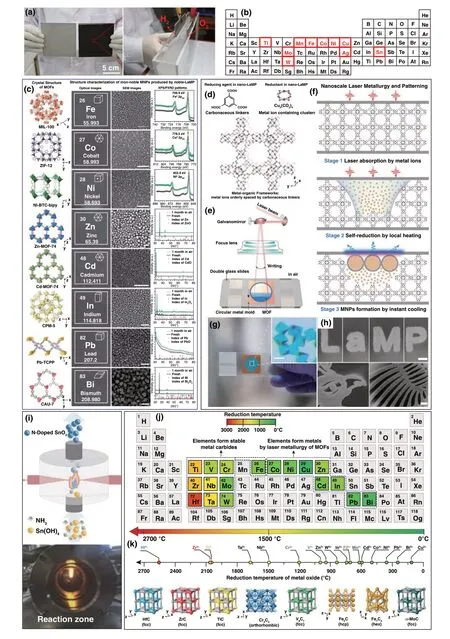
Fig. 5 a Digital photograph of Ni plates (10 × 10 cm2) before (left) and after (right) laser ablation and the overall water splitting device by Ni plate. b The applied metals and alloys highlighted within a periodic table [69]. c Various non‑noble MNPs produced by the nano‑LaMP method in air displayed along with their correspond‑ing MOF crystals. From left to right, the crystal structure of MOFs, optical images, SEM images, PXRD patterns, and XPS patterns of MNPs. HKSUT‑1 precursor d and illustrations of the experimental setup e for the nano‑LaMP method. f Mechanism for the production of MNPs by laser irradiation on MOFs in air. g Optical image of the MOF crystals prepared for nano‑LaMP. The scale bars are inset in g 20 μm and h 200 μm [77]. i Illustration of the laser pyrolysis process of N‑doped SnO2 [74]. j Periodic table shows the reduction tempera‑tures of various metallic elements according to Ellingham diagram. k TMCs synthesized in this study using MOF as precursor and laser as energy source [78]
materials by laser processing. Meanwhile, through two‑step laser scribing, Co3O4/LIG, MnOx/LIG and NiFeOx/LIG were also prepared by this group [80], where the first laser‑induced graphene and then soaking the LIG by metal ions solution for second‑time laser processing were contained. In addition, they verified that the nanoparticles could convert from metal oxides to metal dichalcogenides, but the lateral sulfating treatment was essential.
2.2.1.2 Metal CarbidesAs laser scribing is a relatively common method for synthesis of carbon materials, the metal carbide‑graphene composites synthesized by laser were also developed. Lin et al. [81] developed a laser direct‑write patterning process to fabricate molybdenum carbidegraphene (MCG) conductive composites directly on paper substrates, which was realized by soaking the fibrous paper in the Mo‑gelatin ink and then direct laser conversion of MCG by a CO2laser with wavelength of 10.6 μm (Fig. 6a, b, e). After the conversion, the coexistence of graphene and Mo3C2was verified by Raman and XRD results (Fig. 6c, d). It was demonstrated that gelatin‑based ink could produce higher conductivity than other hydrogel polymers, which was attributed to the formation of lamellar morphology in layer‑by‑layer form and the absorption enhancement of CO2laser by strongly binding with Mo5+ions under the effect of porcine skin peptides with rich amino acid functional groups. This work potentially promoted laser synthesis of metal carbide‑graphene composites for practical applica‑tions in recyclable and disposable paper‑based electronics. Based on this work, this group further proposed the ultrathin carbides on versatile substrates through CO2laser process‑ing of a lamellar hydrogel/metal‑ion matrix [82]. Different metal ions were investigated, and it can be observed that Co2+and W6+gelatin produced the composites of carbides and metal hybrid phases. The Ti4+and Zr4+gelatin hydro‑gels were converted to carbide and oxide composites, while Fe3+and Ni2+gelatin dominantly produced metal oxides with small portions of carbides. No crystalline phase was produced by Zn2+gelatin (Table in Fig. 6f). Therefore, the finally formed products are determined by two critical factors, including the efficiency of laser absorption by the metal‑gelatin and the activation energy of carbonization, which determine whether the carbides can be formed.
2.2.1.3 Metal DisulfidesIn the past decades, numerous attempts to fabricate the transition metal dichalcogenides (TMDs) with monolayer by the chemical vapor deposi‑tion (CVD) process had been performed [83]. However, on account of being time‑consuming, low thermal efficiency and required transfer of the CVD process, developing alter‑native synthesis methods were required. Laser synthesis of the transfer‑free TMDs provided a promising strategy. In terms of laser synthesis of TMDs nanomaterials, laser thin‑ning and laser annealing were two commonly used strate‑gies. The laser thinning method relies on the sublimation of the outermost layer and heat is introduced by the absorption of laser. Because that the van der Waals force between TMDs layers is very weak, the produced heat is difficult to transfer through the substrate; thus, the sublimation of the outermost layer occurs with the bottom layer of TMDs which remain tightly attaching on the substrate. For example, Steele et al. [84] employed a focused laser beam to fabricate monolayer MoS2from multilayered MoS2flakes by the laser‑thinning method (Fig. 7a). A MoS2monolayer domain surrounded by multilayer film through the growth of MoS2films and sub‑sequent laser thinning process was also produced by Sow et al. [85]. With this approach, functional junctions with dif‑ferent band gaps were readily fabricated by laser thinning MoS2domains with different thicknesses, which exhibited superior photoresponse characteristics (Fig. 7b). In addi‑tion, laser ablation‑induced defects on TMDs also depends on the sublimation of part of the TMDs through the pho‑tothermal effects by laser. In this case, a series of van der Waals heterostructure arrays, including VSe2/WSe2, NiTe2/WSe2, CoTe2/WSe2, NbTe2/WSe2, VS2/WSe2, VSe2/MoS2, and VSe2/WS2, were prepared by laser‑patterning periodic arrays of nucleation sites on monolayer or bilayer semicon‑ducting TMDs to selectively grow metallic TMDs. The laser ablation for patterning played an important role in providing nucleation sites for growth of TMDs [86].
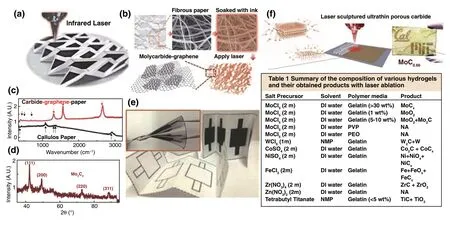
Fig. 6 a An optical photograph of a fabricated origami structure after the direct laser‑write MCG patterning process (black color areas) on a paper substrate (white color areas). b Schematic illustration of the simplified MCG process from fibrous paper, soaked with the gelatin‑mediated ink containing Mo5+ ions, laser conversion process, to the resulting MCG composites. c Raman spectroscopy and d XRD patterns of the MCG sample. e Two partially folded, four 2 × 2 cm2 electrodes on a paper substrate: (top) after the laser conversion process and (bottom) before the conversion process. (inset) A fully folded device with two electrodes on top and two electrodes at the bottom for a two‑capacitor in sandwich structure to be connected in series or parallel as supercapacitors [81]. f IR laser ablation generates highly porous structures and the summary of the composition of various hydrogels and their obtained products with laser ablation [82]
The laser annealing method is using laser as a heating source to promote the synthesis reaction between different precursor species. For instance, Tenne et al. [87] creatively used ultrashort‑pulse laser to process a mixture of bulk transition‑metal dichalcogenides and Pb/PbO to fabricate inorganic fullerene‑like and nanotube‑like TMDs nanostruc‑tures, the catalytic effects of metal assisted with the ultrafast laser‑induced thermal annealing contributed to the forma‑tion of different TMDs nanostructures. Lamberti et al. [88] reported rapid one‑pot synthesis of MoS2‑decorated laser‑induced graphene (MoS2‑LIG) by direct writing of polyim‑ide foils coating with a layer of MoS2dispersion. After laser writing, the MoS2can be in situ decorated into the surface of 3D arrangement of agglomerated and wrinkled graphene flakes. The metal sulfide (CdS or PdS) decorated graphene composites by laser‑induced synchronous carbonization and sulfidation of a metal‑complex‑containing polyethersulfone layer were also reported [89]. The prepared LI‑CdS/PdS‑G@ITO afforded both the porous structure and uniform distri‑bution of metal sulfides, which presented an efficient pho‑toanode for photoelectrochemical detection of Cu2+with significant selectivity and sensitivity. WO3and Se powders can also be used as the reaction precursors, Chueh et al. [90] utilized laser annealing as a heating source to synthesize few‑layer WSe2by the reaction between WO3and Se gas (Fig. 7c). The phase transformation from WO3to WSe2was confirmed by Raman spectrum, and the distinct interface between WO3/WSe2and SiO2can be clearly observed in the optical images of the deposited film before and after laser annealing. This laser irradiation‑assisted selenization (LIAS) process not only had the advantages of being ultra‑fast (< 20 min), low‑cost and low synthesis temperature, but also exempted from the additional transfer process of TMDs. Furthermore, the laser irradiation‑assisted synthesis pro‑cess can also be adapted to the other TMDs such as MoSe2, which demonstrated wide applicability. It is noted that most of the fabrication process of transition metal dichalcogenides by laser is based on two‑step treatment, including the first growth of TMDs film and then laser treatment; the LIAS method provides a new strategy to synthesize TMDs by the gas-solid reaction utilizing laser as heating source.
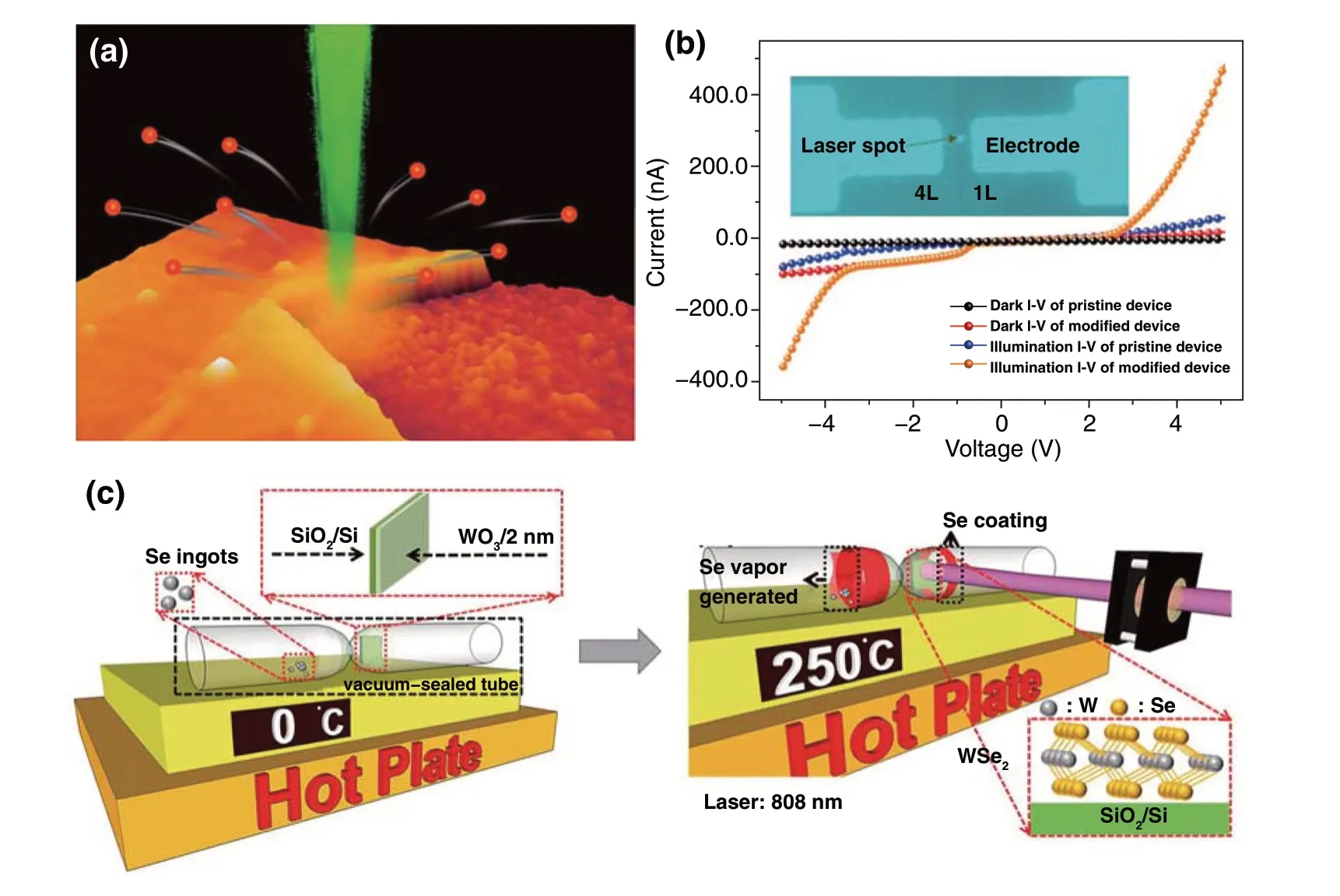
Fig. 7 a Schematic of controllably thinning multilayered MoS2 down to single layer by laser [84]. b Typical I-V characteristics of pristine and modified photodetection devices at dark and laser illumination condition [85]. c Schematic illustration of the rapid synthesis of the WSe2 by the LIAS process [90]
2.2.1.4 Heteroatom‑Doped Transition Metal Com‑poundsExcept for the laser synthesis of heteroatom‑doped carbon, the laser processing technique was also utilized for incorporation of heteroatoms into the transition metal com‑pounds. For instance, the laser‑induced chemical modifica‑tion of suspended TMD monolayer films via local exchange of the chalcogen atoms has been reported [83]. With the proposed method, total or partial locally replacing selenium by sulfur atoms was achieved, which exhibited an excellent performance in nanodevices composed of heterogeneous 2D materials. In addition, the synthesis of oxide thermoe‑lectric material of Bi2Sr2Co1.8Oxvia laser floating zone [91] and the synthesis of rare‑earth‑doped SrTiO3(Y, Dy, Sm, Pr, La‑doped SiTiO3) via laser melting [92] also have been reported, demonstrating the potential advantage of laser synthesis of heteroatom‑doped transition metal compounds. Liu et al. [93] used the laser‑drilled micropores strategy to prepare the hybrid electrocatalyst of Fe‑doped NiS2/MoS2on a CNT film with periodic micropores, the hybrid elec‑trocatalyst provided strengthened interfacial interactions and abundant active sites, improving both thermodynamic catalytic activity and active site density. Therefore, different laser synthesis strategies can be utilized for incorporation of heteroatoms into transition metal compounds, the optimiza‑tion of laser power, exposure time, and reactive atmosphere is crucial to control the heteroatoms dopant on the transition metal compounds.
2.2.1.5 Other Non‑carbon NanomaterialsOrganic-inor‑ganic halide perovskites have emerged as promising mate‑rials for optoelectronics, especially photovoltaics [94, 95]. As well known, the optimum annealing crystallization tem‑perature for MAPbI3perovskite film was 100 °C. However, it is limited by the energy and time consuming, and incom‑patibility with low temperature fabrication requirement of flexible polymers or plastic substrates for the traditional thermal‑annealing process, laser annealing‑induced crys‑tallization process provided a novel annealing approach for precisely controlling the crystallization position and achiev‑ing larger grains with lower density of trap states and higher carrier mobility, especially for reducing carrier recombina‑tion and enhancing photovoltaic performance of perovskite solar cells (PSCs). Yu et al. [96] introduced a continuous‑wave laser (λ= 450 nm) irradiation to rapidly crystallize and prepare CH3NH3PbI3films with dense and homogene‑ous grains. The obtained planar‑heterojunction solar cells showed the optimal efficiency of 17.8% with a remarkably high open‑circuit voltage of 1.146 V. In addition, Kim et al. [97] used the near‑infrared (NIR) laser (λ= 1064 nm) to introduce controllable crystallization of MAPbI3perovskite solar cells, the inverted‑type perovskite solar cells with effi‑ciency of 11.3 and 8.0% on typical glass and flexible poly‑mer substrates, respectively, were demonstrated. Recently, Yan et al. [98] also used the fast laser annealing to induce a higher temperature and selectively grew large perovskite grains. By tuning the laser processing parameters, including the scanning speed, laser wavelength and output power, the optimum perovskite films with high crystallinity and per‑ovskite solar cells with high energy conversion efficiency and durability were successfully fabricated. Besides the crystallization of perovskite films for PSCs, the fs laser pro‑cessing Bi2WO6with a strong enhancement of the structural organization and crystallinity were also reported [99].
Different lasers commonly induced different temperatures due to a different absorption capability of the precursor, thus produced different types of non‑carbon materials. In conclusion, the characteristics of precursor species and the laser radiation (laser intensity, wavelength, and interaction time, etc.) are of vital importance. The effects of precursors and parameters of laser on the composition nature of the obtained non‑carbon materials are summarized in Table 2.
As mentioned above, laser as a synthetic technique can construct various nanostructure during the laser processing. However, more nanostructure styles have not been realized compared with those constructed by wet chemical methods. Therefore, in situ transformation from the preconstructednanostructures through the thermal effect of the unfocused laser is also a potential proposal for expanding the appli‑cation of laser synthesis process. Furthermore, laser as a synthetic technique is not limited to the aforementioned applications. Laser synthesis have great potential for many other development directions. For instance, more compound species can be controllably synthesized by laser ablation under different atmospheres instead of only air or Ar atmos‑phere, such as the synthesis of nitrides under the N2or NH3atmosphere, synthesis of sulfides under the H2S atmosphere, synthesis of carbides under the CH4atmosphere and even synthesis under the CO2atmosphere for oxides or carbides with unique properties.
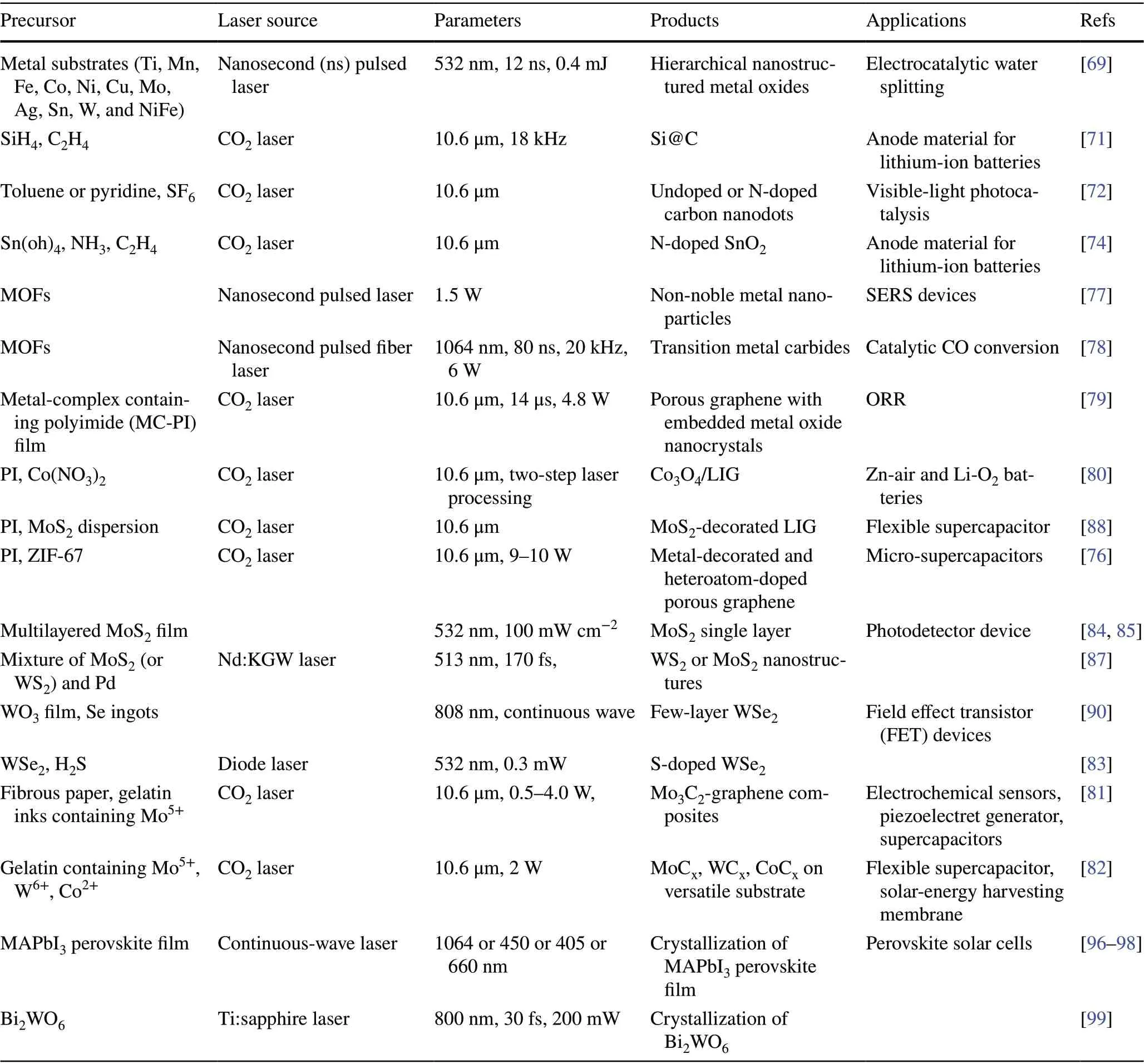
Table 2 Effects of precursors and parameters of laser on the composition nature of the obtained non‑carbon nanomaterials
2.2.2 Laser Synthesis of Nanomaterials in Liquid
The laser synthesis of nanomaterials can be performed in two distinct conditions, including the non‑aqueous environ‑ment and the liquid environment. Therein, laser synthesis of nanomaterials in liquid environment has been widely applied as a versatile technique to construct various colloidal nanostructures. According to the processing mode and the formation mechanism of nanoparticles, laser synthesis can be classified into three methodologies: laser ablation in liq‑uids (LAL), laser fragmentation in liquids (LFL), and laser melting in liquids (LML) [100]. As a synthesis technique of nanomaterials, LAL and LFL were the most commonly uti‑lized methods to prepare nanomaterials. Then in this section, we reviewed on the recently researched nanomaterials with innovative phases through LAL and LFL in liquid.
2.2.2.1 Laser Synthesis of Nanomaterials by Laser Abla‑tion in LiquidThe LAL technique has inspired extensive researches on nanomaterial synthesis and modification in liquid as well as the properties and applications of the LAL‑induced nanomaterials. Two LAL techniques, includ‑ing the “top‑down” synthesis of pulsed laser ablation of a solid target in liquids (PLAL) and the “bottom‑up” method of pulsed laser irradiation of colloidal nanoparticles in liq‑uids (PLICN) were promising liquid‑based laser protocols for complex nanomaterials. Most PLAL synthesis processes were realized by laser heating the solid target, and then, the initial products including plasma, vapor, and droplets with micro‑nanosizes could be produced, which further reacted with the liquid solvent to form the final colloidal nanoparti‑cles [101]. The PLICN technique was that pulsed laser irra‑diation of a precursor solution, where nucleation and growth of nanomaterials occurred as a result of the laser‑induced chemical reactions [102].
As mentioned above, the PLAL process went through the plasma and the gasification stage, and the subsequent sub‑stance diffusion and interaction with liquid solvents after bubble collapse. In general, lasers with a short pulse‑width and high‑energy density were beneficial to the generation of plasma and vapor products, while nanodroplet products were usually caused by lasers with low‑power density. The formation of nanostructures was attributed to their interac‑tion with the surrounding liquid solvents and the ultrafast quenching of laser. Owing to the variety of solid targets and liquid solvents, nanomaterials including alloys, metals, oxides, hydroxides, nitrides, carbides, sulfides and compos‑ites were achievable by PLAL [103].
In terms of the synthesis of metals and alloys by PLAL, the “bare” unprotected Au nanoparticles were frequently pre‑pared by laser ablation of Au target or Au particles in solu‑tion [104-106], which were applied for the catalytic applica‑tions. In order to enhance the catalytic activity of individual gold nanoparticles (AuNPs), using AuFe alloys plate [107] or Pt‑Au powder‑mixture compression [108] as the target for PLAL synthesis method, a homogeneous Au‑Fe nanoalloy with a Fe content up to 11 at% for OER and PtAu alloys for the 4‑NP reduction were achieved. Replacing the deionized water by some metal ions solution, the PLAL technique can also be utilized to synthesize alloys. Mukherjee et al. [109] proposed a laser ablation of Co target in K2PtCl4solution, the PtCo nanoalloy embedded in CoOxmatrices was syn‑thesized. Liang et al. [110] prepared Au‑ZnO nanospheres with Au NPs in different particle size encapsulated in ZnO nanospheres through laser irradiation of the Au target and Zn target in liquid, respectively. Otherwise, Liu and Du et al. reported the synthesis of RuAu single‑atom alloys (SAAs) [111] and RhO2clusters embedded in the surface of Rh nanoparticles [112] by PLAL technique, the strong quench‑ing effect played an important role in fabricating metastable nanostructures with novel properties (Fig. 8a). The ternary metal alloy AuAgPt was developed by laser‑irradiation of chemically synthesized Au@AgPt yolk‑shell nanocubes in liquid to induce alloying (Fig. 8b) [113]. In addition, laser driven the plasma resonance absorption performance for noble metal nanoparticles was applied to realize some plas‑mon‑driven chemical process. For instance, Pan et al. [114] reported that through two steps of the first synthesis of Au nanoparticles by PLAL of Au plate and then photochemical reaction between Au nanoparticles and CuCl2under 532 nm pulsed laser, the Cu nanoclusters were achieved due to the reduction of Cu2+ions by plasmon‑generated “hot electrons” from Au.
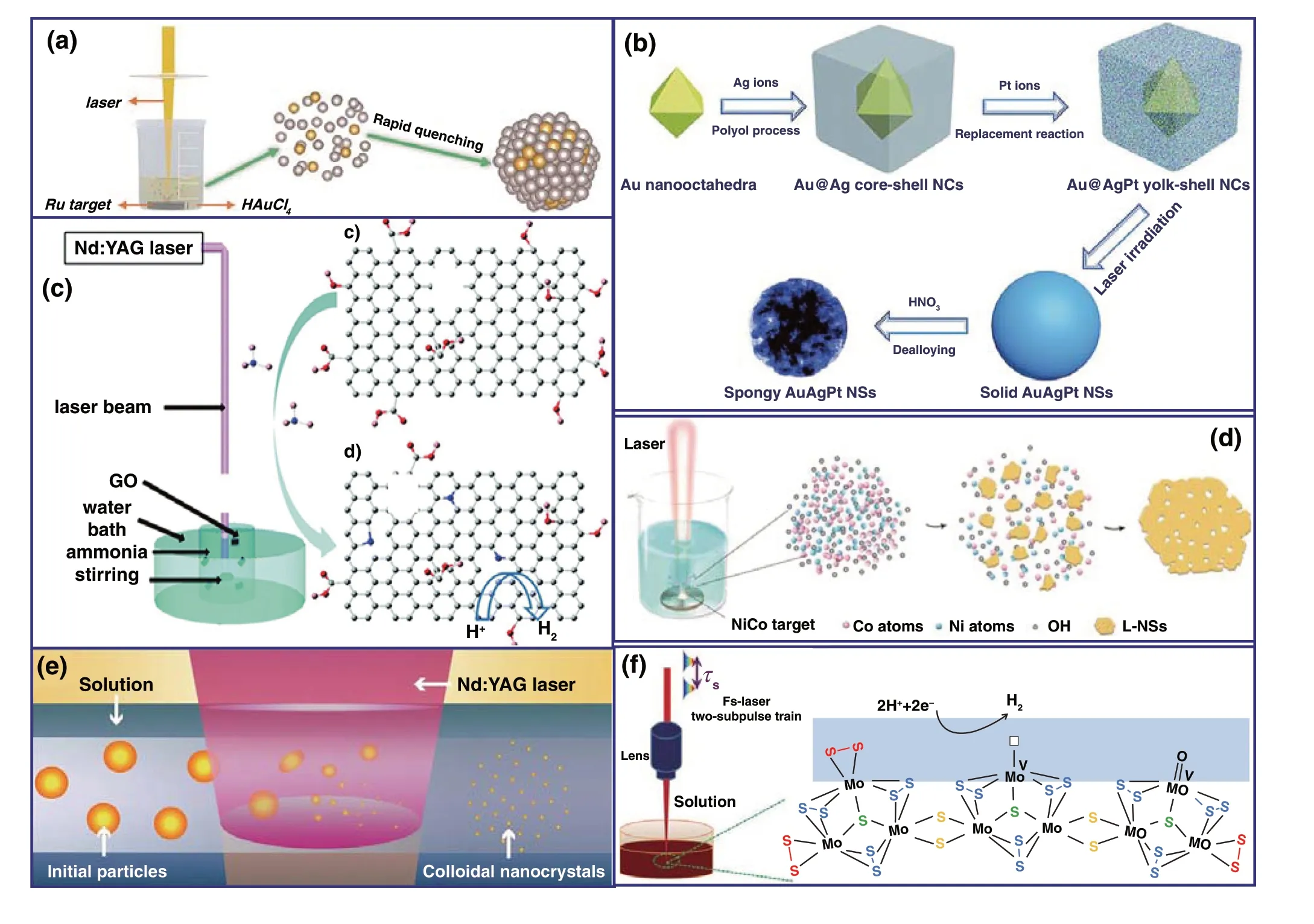
Fig. 8 a Schematic setup of PLAL and the formation process of RuAu SAA nanoparticles [111]. b Schematic diagram for the formation process of spongy AuAgPt [113]. c Illustration of the preparation of nitrogen‑doped graphene oxide by laser irradiating a solution containing graphene oxide and ammonia [115]. d Laser ablation of CoNi alloy target in 1 M KOH solution [119]. e Schematic illustration of the fabrication processes of laser synthesis and processing of colloids [127]. f Controllable synthesis of nanosized amorphous MoSx by fs laser [132]
In terms of the synthesis of metals oxides and hydrox‑ides by PLAL, Du et al. synthesized an N‑doped graphene oxide by PLAL of a solution composed of ammonia and graphene oxide, which achieved a high pyridinic N dopant (51%) (Fig. 8c). The PLAL process provided a simple and fast approach to prepare N‑doped graphene oxide compared with the conventional method, and the obtained N‑doped GO exhibited an improved electrochemical HER perfor‑mance [115]. Bao et al. [116] ablated CoO micropowders by femtosecond (fs) laser pulses in pure water, and the gen‑erated CoO nanoparticles suspended in water were collected directly for solar water‑splitting. Müller et al. [118] reported the crystalline Co3O4nanoparticles (< 5‑nm) [117] and [Ni − Fe]‑layered double hydroxides (LDHs) as highly active catalysts for oxygen evolution. Co0.75Ni0.25(OH)2nanosheets with high‑density pores via PLAL process were similarly realized (Fig. 8d) [119]. Kim et al. [120] reported thin NiFe LDH nanostructures on 1D‑CdS nanorods synthesized by using PLAL. The efficient carrier transport attributed to het‑erostructures greatly inhibits the recombination of carriers.
Ternary oxide nanocrystals (TONs) have received increas‑ing attention for their inherent optoelectronic properties, such as good electrical conductivity and multiple active sites; thus, they have been well exploited for energy and catalytic applications [121, 122]. However, adoption of sur‑factants or growth‑guiding agents for traditional synthetic methods will decrease the charge transport in products and hence degrade the device performance. Therefore, PLAL provides an attractive, imperative, and green approach to synthesize TONs. Zeng et al. reported a universal PLAL and subsequent hydrothermal growth for various TONs synthesis. A series of TONs, including Zn2SnO4, NiCo2O4, Zn2GeO4, ZnFe2O4, Fe2GeO4, and ZnMnO3were synthe‑sized, which confirmed the universality of this method. Dur‑ing the synthesis process, the PLAL‑generated highly reac‑tive species, such as metal ions, clusters, H+and OH−will facilitate the formation of various TONs in the subsequent hydrothermal process [123]. Based on this work, Zeng et al. prepared Zn2SnO4nanocrystals with an average size of 140 nm by PLAL and subsequent hydrothermal synthesis to construct high‑performance nanoscale photodetectors [124]. Yang et al. [125] reported a defective α‑Ag2WO4nanorods prepared by PLAL. The thermodynamic disequilibrium cre‑ated by laser ablation combined with the weak bond energy of Ag2WO4induced abundant [WO6] cluster distortions into the crystal lattice. Similarly, the hydrogen‑interstitial CuWO4nanomesh was prepared by PLAL for the applica‑tion of single‑component full spectrum‑active photocatalysts for hydrogen evolution [126]. Wang et al. [127] reported the laser synthesis of ligand‑free La:BaSnO3nanocrystals by PLAL, and by embedding this laser generated nanocrystals in BiVO4photoanode matrix, an enhanced charge transport for photoelectrode was achieved (Fig. 8e).
In terms of the synthesis of molybdenum sulfide by PLAL, spherical MoS2nanoparticles with onion‑like struc‑ture and internal shrinkage cavities can be prepared through laser ablation of MoS2target in deionized water [128]. Lee et al. [129] prepared a high concentration of few‑layer MoS2via laser exfoliation of bulk MoS2in liquid, the S vacancy and crystal phase transformation were controlled and a dra‑matically improved electrocatalytic activity toward HER was obtained. Furthermore, the author demonstrated that the presence of protons from solvents with lowpKavalues was conducive to creating S vacancies by extracting S atoms to form H2S, and simultaneously facilitated the 2H‑to‑1 T phase transition of MoS2by sliding S atoms. Amorphous molybdenum sulfide (a‑MoSx) with abundant defect‑rich active sites was synthesized by the femtosecond laser abla‑tion of ammonium tetrathiomolybdate aqueous solution with PLICN (Fig. 8f) [130], which was reviewed in the following as the “bottom‑up” method. Besides of MoS2, very recently, Du et al. [131] used C and O co‑doped BN nanospheres as the precursor for PLAL treatment, it demonstrated that the laser‑modified boron nitride exhibits unique electrical conductivity and high corrosion resistance under oxidizing conditions, which attributed to the interlayer B-B dipolar interaction.
In contrast to the “top‑down” synthesis of PLAL on bulk targets, the “bottom‑up” method of PLICN is also a prom‑ising liquid‑based laser strategy for nanomaterial design [133]. Liang et al. utilized the photo‑excited electrons gen‑erated by laser processing SnOxin a PtCl62+solution, and hybrid catalysts composed of Pt clusters anchored on the surface of SnO2were obtained, which presented high activ‑ity and long‑term durability for methanol oxidation [134]. Zheng et al. [102] proposed two distinct reaction pathways of a Co2+, Ni2+, and Mn2+ion‑containing aqueous solu‑tion irradiated by lasers with different wavelengths (532 or 1064 nm). The different laser wavelengths resulted in dif‑ferent product formation mechanisms. As demonstrated, the ionization of water molecules to produce reducing species such as solvated electrons (eaq−) and hydrogen radicals (H·) in the solution dominantly occurred when the 532 nm laser was utilized, and hollow (Ni0.18Mn0.45Co0.37Ox) metal oxide nanoparticles as the final product were formed after the reduction of metal ions by these reducing species and further laser heating. While the 1064 nm laser mainly induced vibra‑tion of water molecules, then the stretched OH groups of water molecules facilitated the hydrolysis reaction of metal ions and led to the formation of ([Ni0.15Mn0.15Co0.7(OH)2](NO3)0.2·H2O) hydroxide nanostructures. Recently, Liu et al. [135] reported a laser‑assisted, continuous, solution route for the simultaneous reduction and modification of graphene oxide with Pt, PtPd alloys, RuO2and MnOxnanoparticles, which demonstrated the versatility of PLICN in synthesizing functional nanoparticle‑modified graphene materials.
Laser‑based photohydrothermal synthesis of metal oxides was another kind of PLICN technique. Digital ZnO nanow‑ires arrays were achieved by this rapid and one‑step selective digital direct growth method, which was realized by local‑ized temperature increase after the thin metal layer absorbed the focused laser, and then induced rapid photohydrothermal reaction of Zn(NO3)2solutions near the focal spot region [136, 137]. However, the spatial size was generally limited by the thermal diffusion and the size of the focused laser spot. Ko et al. grew ZnO or TiO2nanowires on a selected area that was even smaller than the laser focus size by laser‑based photohydrothermal synthesis. The size of laser‑induced temperature field can be effectively reduced through reducing the physical dimension or decreasing thermal con‑ductivity of the laser absorption layer substrate. A smaller nanowire array on PI substrate instead of metal absorption layer can also be successfully synthesized, due to the visible‑light absorption capacity and low thermal conductivity of PI [138].
Besides the metal oxides and metal hydroxides, the PLICN technique was also used for other components. For example, Wang et al. [139] prepared Cl‑functionalized car‑bon dots (Cl‑CDs) in chlorobenzene via simple laser treat‑ment, which were embedded in perovskite films to regulate both the films morphology and the electronic structures. An improved performance for perovskite solar cells was achieved. Du et al. [122] firstly prepared c‑BN NPs with an average size of 3.5 nm at ambient temperature and pres‑sure by exploiting a laser‑induced photochemical effect on a dioxane solution of ammonia borane. Beyond that, Irannejad et al. [140] used a focused femtosecond laser to process the air/graphene oxide solution interface to produce rGO gels without any reductive additives, the reduction of the GO and gel formation occurred simultaneously by removing the hydroxyl, carboxyl functional groups and water in the same time. However, when the laser beam was focused inside the graphene oxide solution, only the reduction of GO solution occurred, no gel formed.
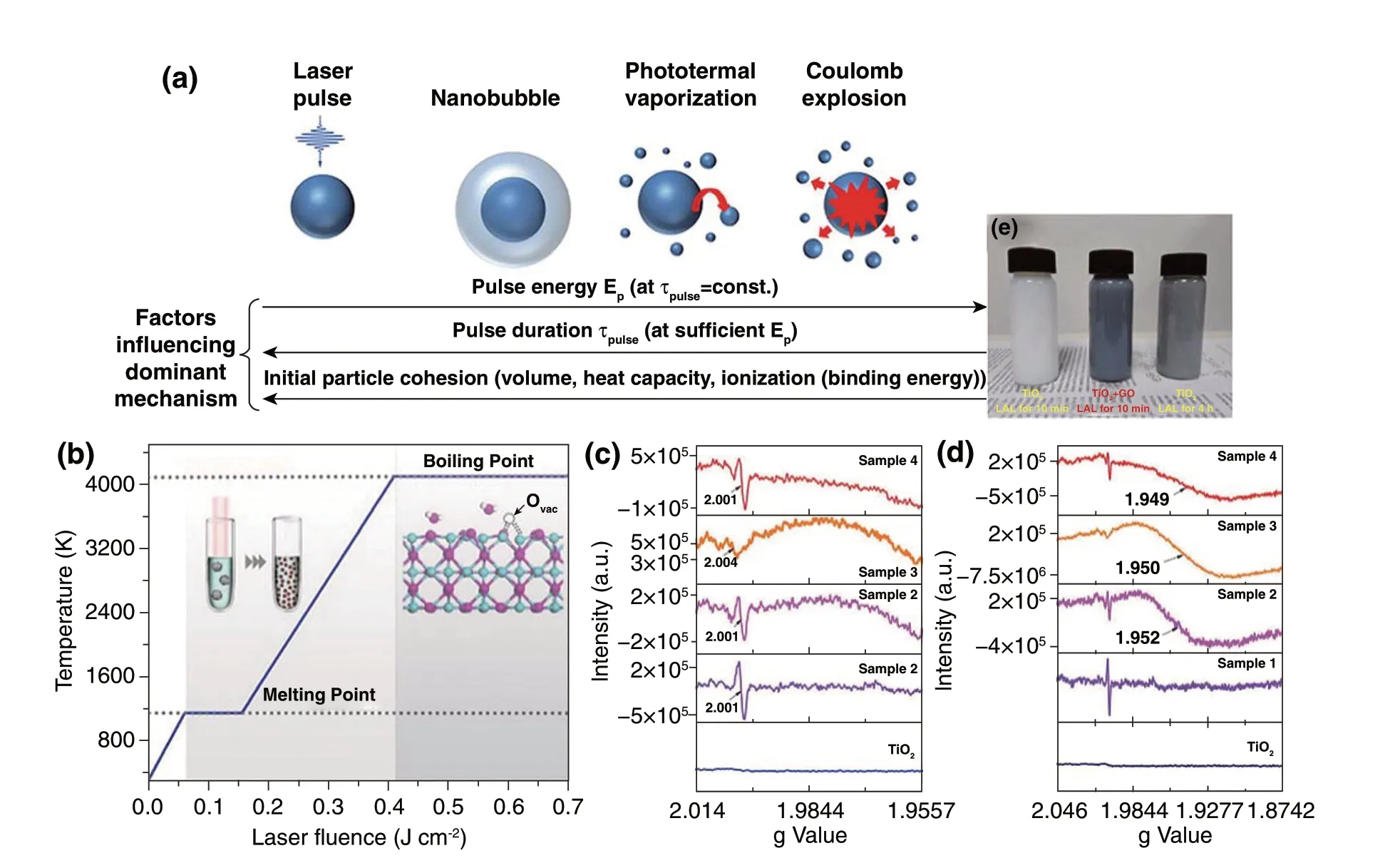
Fig. 9 a Schematic illustration of the size‑reduction and fragmentation mechanisms of plasmonic NPs in a colloidal solution [141]. b Prepara‑tion of a highly active Co3O4 catalyst via laser fragmentation [142]. c, d EPR spectra of different samples for confirmation of oxygen defects. e Photographs of TiO2‑10 min, TiO2 + GO‑10 min, TiO2‑4 h [143]
2.2.2.2 Laser Synthesis of Nanomaterials by Laser Frag‑mentation in LiquidLaser synthesis of nanomaterials by LFL is induced by the absorption of laser energy with microparticle powder suspensions or nanoparticle colloids. Photothermal vaporization and coulomb explosion are the mechanisms for LFL (Fig. 9a) [141]. To trigger an LFL pro‑cess in the liquid, commercial solid‑state lasers are often combined. Lasers with the pulse width of nanosecond level frequently trigger the photothermal vaporization, while cou‑lomb explosion is triggered by the ultrashort lasers with ps or fs level and is caused by charge repulsion of ionized NPs. LFL can combine both the advantages of size reduction and defect introduction into products, which regulate the elec‑tronic structure and lead to a significant improvement of the catalytic activities. Zhou et al. reported that Co3O4nanopar‑ticles with a high activity were prepared via the LFL pro‑cess (Fig. 9b). Ultrafine nanoparticles with abundant oxy‑gen vacancies induced by laser irradiation can significantly improve both electrical conductivity and energy adsorption [142]. Defect‑induced electronic reconstruction has also been realized by LFL of photocatalysts and electrocatalysts. G. Yang et al. performed LFL to obtain TiO2−x‑graphene oxide mixtures (Fig. 9c-e). After LFL, the conduction band (CB) moved below 0 eV in the range of − 0.01 to − 0.55 eV, which resulted in a 0.87 eV decrease of the band gap for the visible‑light absorption [143]. Zhao et al. employed LFL to liberate the N‑doped carbon nanotubes (N‑CNTs) confined Co single‑atom sites as an efficient selective‑hydrogenation material for quinoline. Furthermore, the catalytic conver‑sion of quinoline derivatives with methyl, hydroxyl, and halogen groups into corresponding 1,2,3,4‑tetrahydroquino‑lines was also realized by this liberated catalyst [130]. Mus‑selman et al. [144] utilized the nonresonant intense pulsed laser to fragment and then direct the 2D materials from bulk flakes to assembled nanorod structures. The obtained gra‑phene nanorods, MoS2nanorods, WS2nanorods and BN nanorods suggested the potential in transparent conducting applications.
LAL is summarized to be a “top‑down” or “bottom‑up” physiochemical method, which usually uses the bulk solid targets (PLAL) or a precursor solution and additional metal absorption layer (PLICN) in liquid condition as the precur‑sors. Therefore, the laser parameters required for LAL are in a wide range, which covering the laser wavelengths from ultraviolet (UV), visible (Vis) to near‑infrared (NIR), and the pulse widths from femtosecond, picosecond, nanosec‑ond, microsecond, millisecond, and even extending to the continuous‑wave (CW) range. In general, the visible laser with 532 nm wavelength was frequently utilized to process powder precursors in liquid, while the near‑infrared laser with 1064 nm wavelength was utilized for target precursor. Ultrafast lasers such as fs or ps laser were usually used for synthesis of nanomaterials through laser fragmentation in liquid, which induced the coulomb explosion process. The effects of the precursors and parameters of laser on the com‑position nature of obtained nanomaterials in liquid are sum‑marized in Table 3.
3 Laser as Microfabrication Technique for Application
Laser as a microfabrication technique means that the laser is used as an energy source to precisely focus on desirable positions and create patterns locally without affecting the adjacent area [146]. From the major terms, laser synthesis is involved in the laser microfabrication process. Namely, laser as a microfabrication technique is composed of the simultaneous laser synthesis and patterning during the laser processing. Laser microfabrication technique has the advantages of high production efficiency, low cost, stable and reliable processing quality; therefore, it has the good economic and social benefits. In particular, ultrashort pulse femtosecond laser can produce ultrahigh light intensity, it possesses the preponderances of precision and low damage threshold, ultrasmall heating affected area, and can almost precisely process various kinds of materials. In this section, the as‑patterned materials after microfabrication through the laser are reviewed, which present high performance in energy storage and conversion devices such as batteries, supercapacitors, sensors and electrocatalytic materials [147].
3.1 Light–Thermal Conversion Devices Fabricated by Laser Technology
Solar‑driven water evaporation, wastewater purification and energy conversion are potential green and sustainable technologies; thus, the efficient solar energy harvesting and photothermal conversion play the important role in these applications [148]. In addition, the efficient light‑to‑heat conversion instead of other forms of energy and heat transferring to the applied process also should be satisfied [149]. It is noteworthy that the light‑to‑heat conversion effi‑ciency depends greatly on the absorbing material. Therefore, light-thermal conversion materials with high conversion efficiency and broad solar absorption range have attracted widespread interests, such as carbon‑based materials, semi‑conductors, metals and polymers [149-151].
For the light-thermal conversion materials, not only excellent optical and thermal properties are essential, eco‑nomic practicality and manufacturing simplicity on a large scale should also be taken into account. The laser process‑ing technique provides a reliable and cost‑effective strat‑egy for fabricating nanomaterials with broad‑spectrum solar energy absorption on a large scale. In particular, these laser‑microfabricated materials are widely applied to various photothermal conversion, anti‑reflection and light harvesting applications [152, 153]. By successively constructing micro‑structures and nanostructures via ultrafast laser patterning and subsequent thermal oxidation, an infrared antireflection nanowire array was obtained on a Cu surface [154]. Attrib‑uted to the light trapping by the microstructures, as well as the enhanced photophonon dissipation by the nanowire structure, the ultralow reflectance of 0.6% was achieved for the infrared light with a 17 μm wavelength. Furthermore, Fan et al. also fabricated the cauliflower‑shaped copper nanostructures by laser direct writing, which exhibited extremely high broadband omnidirectional absorption of sunlight with high water evaporation efficiency [155]. And then, a universal strategy for fabricating micro/nanohybrid antireflection structures on different metal surfaces by laser direct‑writing method was proposed by this group (Fig. 10a, b), the minimum reflectance on W, Cu, and Ti substrates at ultraviolet to near‑infrared spectrum reached 2.5%, 1.4%, and 0.29%, respectively [156].
Table 3 Effects of the precursors and parameters of laser on the composition nature of obtained nanomaterials in liquid
Table 3 (continued)
Carbon‑based photothermal materials, including gra‑phene, carbon nanotubes, carbon black and graphite, have been used for highly efficient full‑spectrum solar absorption materials. Creating a nanostructure with a 3D framework of pores and arrays can reduce light reflection and minimize angular dependence of the incident light. Qu et al. prepared an efficient three‑dimensional solar steam generation (SSG) material platform through laser 3D printing technology (Fig. 10c). The as‑fabricated graphene framework with a highly vertically ordered pillar array enlarged both the avail‑able evaporation area and free space within the graphene framework (Fig. 10c), which resulted in an extremely high evaporation rate of 2.10 kg m−2h−1under 1 sun irradiation (Fig. 10d) [157].
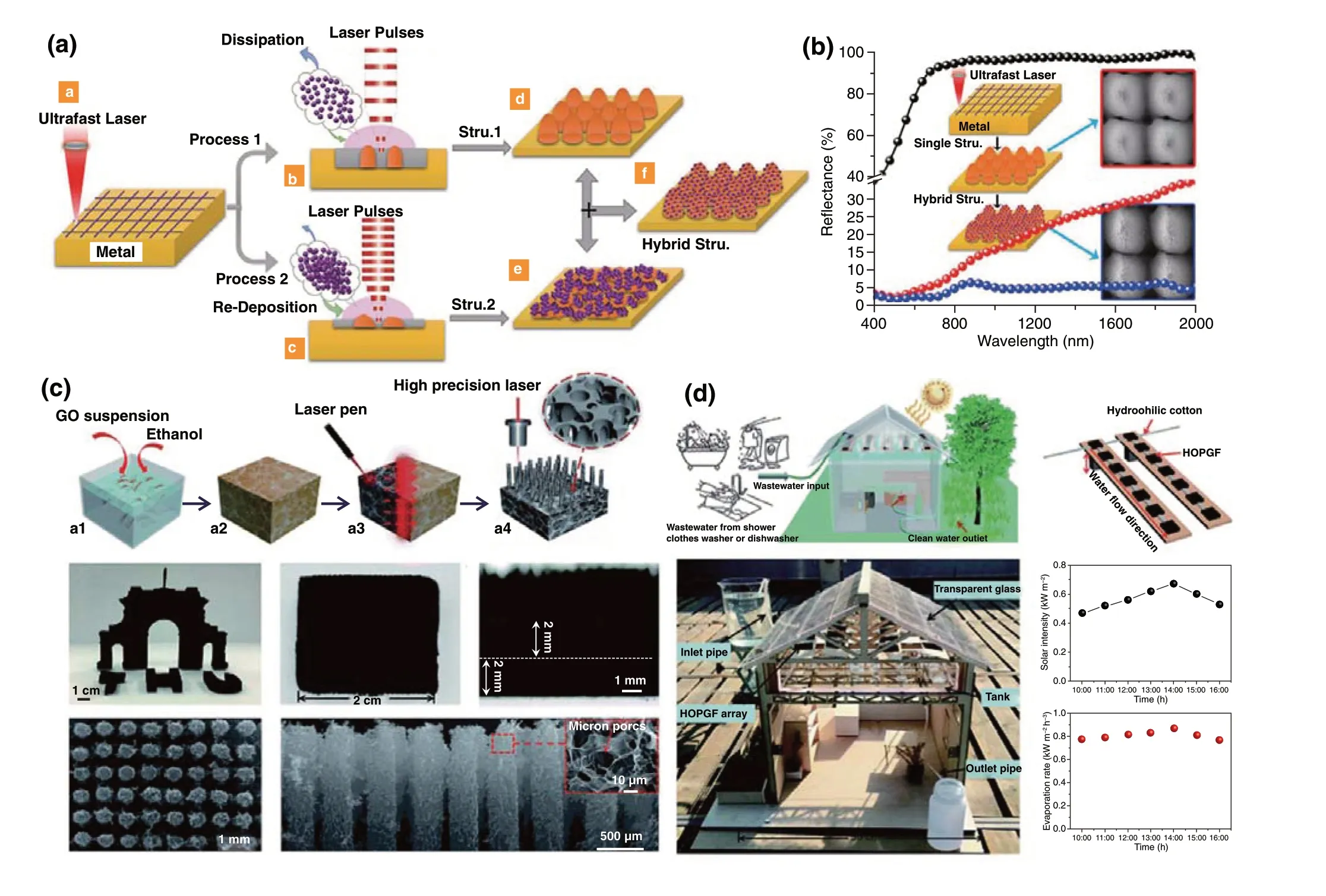
Fig. 10 a Schematic illustrations of the pulse injection controlled ultrafast laser direct‑writing strategy. b Hemispherical reflectance of differ‑ent structures in the UV-Vis-NIR region [156]. c Schematic illustration of the preparation of highly vertically ordered pillar array of graphene framework (HOPGF) and the cross‑sectional scanning electron microscopy (SEM) images of HOPGF. d Schematic illustration of a house sup‑plying clean water based on SSG and the photograph of a laboratory‑made house model under the sunlight at Beijing [157]
By utilizing the photothermal effects, Zheng et al. [158] proposed an optothermally gated photon nudging (OPN) technique under laser processing. By virtue of an optother‑mal surfactant thin layer to regulate the interaction between particles and the substrate, the manipulation of colloidal par‑ticles into any desired pattern positions with optical scatter‑ing force was achieved. However, the orientational control of anisotropic nanoparticles and sub‑20 nm position accuracy remained challenging due to the optical diffraction limit.
3.2 Battery and Supercapacitors Fabricated by Laser Technology
Two kinds of typical electrochemical energy storage (EES) device, including batteries and supercapacitors, have been increasingly developed by researchers. However, the application of EES devices has often been limited by poor mechanical performance, low power density, high cost, and short cycle life of devices [159]. The laser microfabrication technologies provide efficient direct‑writing processed and novel, low‑cost, reliable, environment‑friendly and template‑free patterning methods to design and fabricate high‑perfor‑mance electrodes for high‑quality energy storage devices.
3.2.1 Supercapacitors
Supercapacitors (SCs), also known as electrochemical capacitors, were considered as one of the most promising EES systems due to their fast charge-discharge capacity, long cycle life, high power density and safety [160-162]. In recent years, laser‑based technologies have been used for SCs in laser‑derived graphene microfabrication and prepara‑tion from graphene oxides [163, 164] or polymers [32, 165, 166]. In 2012, using LightScribe DVD burner, Kaner et al. [25] directly laser‑reduced the GO to laser‑scribed graphene (LSG) in one step for scalable fabrication of SCs, which led to the microfabrication of many LSG electrodes and flex‑ible SCs. For example, they also used a LightScribe DVD burner to interdigitate graphene electrodes on the disc, and more than 100 micro‑supercapacitors can be produced on a single disc in 30 min or less [26]. Noteworthily, the pro‑duced devices are extremely thin and are completely flexible. Lamberti et al. [167] reported a type of porous laser‑induced graphene with a large surface area and good electrical con‑ductivity, which was fabricated by a CO2laser direct‑writing PI sheet. As illustrated in Fig. 11a, after transferring the obtained LIG onto PDMS as the stretchable and flexible electrodes to fabricate LIG/PDMS supercapacitors, stable mechanical and electrochemical properties under highly stretching conditions were achieved. Moreover, laser micro‑fabrication technologies also have been utilized to fabricate micro‑supercapacitors (MSCs) with outstanding capaci‑tive performance, which allowed massive construction of microelectrode patterns on a flexible substrate [168, 169]. With this technique, the five parallel rows of six series‑con‑nected MSCs was simultaneously fabricated and displayed the high voltage output of 9.6 V, successfully powering the electronic pen container with music alarm [169] (Fig. 11b, c). It also demonstrated that the compact hybrid 3D MSCs array integrated with solar cells contributed to efficient solar energy harvesting and storage. Besides, carbon-metal oxide nanocomposites, such as MnO2/graphene [170], graphene/RuO2[171], TiO2/graphene [172], LIG/NiO/Co3O4[173], and metal/oxide [170], have been prepared via laser or laser combining with other fabrication processes, which can effi‑ciently improve power density and energy density of SCs. Li et al. [174] demonstrated that the laser microfabrication technique was also widely applied in pseudocapacitive mate‑rials. As shown in Fig. 11d, hybrid composites of pseudoca‑pacitive materials, such as MnO2, FeOOH and polyaniline (PANI) integrated with LIG, were achieved by combining the laser microfabrication for patterns with the subsequent electrodeposition process. At the fixed current density of 0.25 mA cm−2, the volumetric and areal specific capacitance of the LIG‑FeOOH//LIG‑MnO2asymmetric device was cal‑culated to be 5.4 F cm−3and 21.9 m F cm−2, respectively (Fig. 11e). Through comparison, the presence of pseudo‑capacitive materials in MSCs benefits for a greater electro‑chemical performance than some commercial SCs, and even comparable to Li thin‑film batteries (Fig. 11f), which was ascribed to the high capacitance of metal oxides and the elec‑trical conductivity of LIG. In resent report, Wu et al. firstly developed conductive MOF [175] grown on LSG selectively as electrodes for electrochemical micro‑supercapacitors with greatly enhanced performance. Teng et al. [176] combined electrophoresis deposition with laser microfabrication to pattern the activated mesophase pitch carbon integrated with a gel electrolyte on the substrate, which was utilized for the on‑chip assembly of micro‑supercapacitors. In addi‑tion, Wang and Fang et al. [177] fabricated a femtosecond laser‑etched MXene MSCs with double‑side configura‑tion via arbitrary on‑ and through‑substrate connections of MXene MSC units. The MXene double‑side MSC fabricated by the series connection of 12 spiral pattern MXene MSC units with interdigital electrodes of 10 μm width interspace can output a large working voltage of 7.2 V. In contrast to the complexities of necessitating masks and the wet‑etching process for photolithography, laser microfabrication process is low‑cost, quick, and readily scalable.
Fig. 11 a Manufacturing and processing of laser‑induced graphene electrodes for highly stretchable supercapacitors [167]. b Galvanostatic charge/discharge curves of the MSC array at the current density of 0.5 mA cm−2. c Electronic pen container with music alarm was powered by the 6S × 5P AMSC array [169]. d Scheme of the fabrication of MSCs with LIG-MnO2, LIG-FeOOH, or LIG-PANI. e Areal and volumetric specific capacitance of LIG-FeOOH//LIG-MnO2 over a current density range of 0.25-10 m cm−2. f Ragone plots of LIG-MnO2‑2.5 h, LIGPANI‑15, and LIG-FeOOH//LIG-MnO2, compared with commercially available energy storage devices [174]. g Schematic to illustrate the pro‑cess flow used to fabricate the nitrogen‑doped 3D graphene directly onto Cu foil through laser scribing. h Galvanostatic charge/discharge profiles of NLSG‑2 electrode at 0.1 A g−1. i Rate performance of LSG, NLSG‑1, and NLSG‑2 electrodes at different current densities [54]
3.2.2 Battery
As well known, batteries with fast surface redox reaction always have high energy density but low power density and poor cycling performance. Such limitations restrict their applications as independent energy storage devices. Recently, the laser microfabrication technology, which attracted special attention, has been deemed promising to minimize the fabrication costs, increase the operational life‑time and enhance the power density performance of batter‑ies [178]. Laser‑assisted chemical vapor pyrolysis (LaCVP) [179] was used to synthesize the active materials in batter‑ies. For instance, Muna et al. [180] reported preparation of the Si‑based nanostructured electrodes using LaCVP as an anode material in advanced lithium‑ion batteries for poten‑tial practical usages, which achieved high performance, dra‑matic cyclability and fast scalable production in high purity. Zhang et al. [181] used the laser‑induced MnO/Mn3O4/N‑doped‑graphene hybrid as binder‑free anodes for lithium‑ion batteries, which performed a high reversible capacity of 992 mAh g−1at 0.2 A g−1and excellent rate capacity of 365 mAh g−1at 2.0 A g−1, as well as a high cycling stabil‑ity. Laser fabrication technologies have also been used for electrochemical storage of Na‑ions batteries. Zhang et al. [182] demonstrated that CO2laser irradiation was a fast and effective way to fabricate hard carbons for application in Na‑ion batteries, where the structural organization degree was increased by laser microfabrication through producing locally ordered turbostratic carbon. Furthermore, Zhang et al. [54] fabricated N‑doped 3D graphene directly bonded to the current collectors as anode for sodium‑ion batteries, which was manufactured through a direct laser‑scribing process under nitrogen gas atmosphere (Fig. 11g). Because of the high concentration of nitrogen doping and high electrochemical surface area of LSG, the laser‑fabricated electrode exhibited excellent electrochemical performance with an initial discharge capacity of 659 mAh g−1and a recovered charge capacity of 485 mAh g−1(Fig. 11h). In addition, Na‑ion capacities up to 425 mAh g−1at 0.1 A g−1and 148 mAh g−1at 10 A g−1were achieved with excellent rate capabilities and cycling durability (Fig. 11i). This work points out a promising route for fabrication of metal‑ion capacitors directly on current collectors via optimized laser conditions. It was noteworthy that, only a few works on the application of laser processing in batteries were reported. Hence, laser processing in batteries, especially for the fab‑rication of active materials directly on current collectors via the optimized laser microfabrication technique should be extensively explored for electrochemical storage of different metal cations in the future.
3.3 Sensor Devices Fabricated by Laser Technology
Because of the instantaneous local high temperature and pressure environment occurred on the surface of materials when irradiation by laser technology, the surface structures of laser‑treated materials are porous and fluffy, which have some unique advantage to be the sensitive layer of pressure or gas sensor. Furthermore, the pattern array device is easy to be obtained by laser microfabrication method. Therefore, most devices fabricated by laser technology are suitable to be used as sensor devices [183]. In this section, different sensor devices fabricated by laser technology were reviewed.
3.3.1 Force Sensor
Force sensors are widely used in various fields, such as smart textiles, wearable electronics, artificial intelligence robots and structural health monitoring [184]. As mentioned in Sect. 2.1, carbon materials were commonly fabricated by laser ablation. Generally, they were also nanomaterials for application in force sensors. Rahimi et al. reported a unidi‑rectional strain sensor based on transfer and embedment of carbonized patterns fabricated by laser microfabrication of thermoset polymers (Fig. 12a), which obtained the stretch‑ability of up to 100%, and sensitivity of up to 20,000. They used this technique for realizing measurement of finger motion in real time (Fig. 12b, c) [185]. Tao et al. fabricated an intelligent artificial throat using a similar method. The graphene foam fabricated through laser pyrolysis was the sensitive layer for AI throat, which could not only gener‑ate sound by thermal acoustic but also detect sound by pie‑zoresistive effect (Fig. 12d, e) [186]. Laser‑induced carbon materials with 3D pattern structures by two‑step fabrication extended their applications in force sensors [187]. For exam‑ple, Tour et al. utilized the 3D patterned graphene foams by laser laminated object manufacturing in strain sensors to monitor the pulse fluctuation frequency [37]. Araromi et al. patterned PDMS‑carbon composite layers by laser ablation, the high‑resolution and mechanically robust electrode was achieved for stretchable silicone elastomer actuators and sensors. They validated that the devices fabricated by laser microfabrication can realize the large area of up to A4‑size and high sensitivity (Fig. 12f) [188]. Luo et al. [27] used the scribing process for the reduction and patterning of graphene oxide film to realize LSG sensor fabrication. The versatility of LSG sensors provided a promising strategy for monitoring and mapping large‑scale deformation and strain distribution of polymeric composites. Nag et al. used the laser‑patterned composite of multi‑walled carbon nanotube (MWCNT) as conductive film and PDMS as substrate for the flexible and wearable sensor application, which realized monitor‑ing of respiration and limb movements [189]. Besides of the composite of MWCNT and PDMS, Nag et al. also tried to use Al film as the conductive layer on PI substrate. The laser microfabrication‑induced aluminum interdigitated electrodes for tactile sensors were sensitive to finger pres‑sure [190].
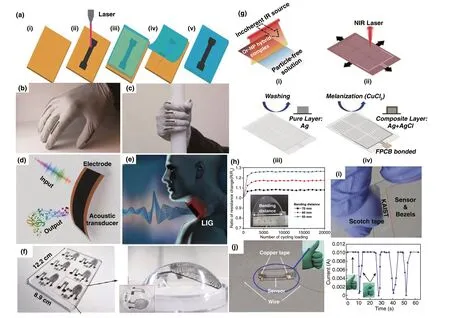
Fig. 12 a Schematic of the fabrication process for stretchable carbon nanocomposite using laser pyrolization of polyimide; b, c Human finger motion detection with stretchable carbon traces [185]. d LIG has the ability of emitting and detecting sound in one device; e Artificial throat can detect the movement of throat and generate controllable sound, respectively [186]. f Interdigitated transducer geometries produced by laser ablation of cast carbon-silicone bonded to an elastomer [188]. g Overall fabrication steps of the touch device using the laser process. h Cycling bending test for the laser‑processed touch sensor, as a function of bending distance. The bending rate was 500 mm min−1. The inset is a photo‑graph of bending test setup. i Peeling test using a conventional scotch tape after 100 times [191]. j Photograph and human motion detection of finger with flexible sensor based on copper electrode [192]
Except for the carbon materials, the patterned non‑car‑bon electrodes by laser microfabrication also have excel‑lent sensitivity for sensors. Son et al. performed organo‑metallic metallization using laser thermochemical treatment (Fig. 12g). Attributed to the advantage of device patterning by laser, a high pressure resolution can be obtained by sen‑sor arrays (Fig. 12h, i) and this laser patterning method was crucial for high‑yield industrial production [191]. Bai et al. prepared the patterned devices based on copper electrode by laser microfabrication, high sensitivity and mechanical robustness were realized when it was applied as a touch switch (Fig. 12j) [192]. Arnaud Spangenberg et al. [193] used the near‑infrared fs laser irradiation to prepare crystal‑lized TiO2and TiO2/C nanocomposite microstructures as microsized pressure sensors. When using the AFM tip to gradually approach the sensor, the pressure sensing exhib‑ited the sensitivity at the microscale from a single line and a repeatable response.
3.3.2 Gas Sensor
Gas sensors, such as CO detectors, humidity sensors or other harmful gas sensors, play an important role in health moni‑toring. Therefore, gas sensors are significant for our daily lives. Laser microfabrication technology also derived many excellent researches in this field [194].
As mentioned above, the laser‑induced structure of car‑bon nanomaterials is porous and floppy, which means that the devices based on laser‑induced carbon structure are also suitable for gas sensors [34]. For instance, Wu et al. fabricated a graphene‑based NH3sensor by one‑step laser treatment of PI. The response/recovery time of this NH3sensor were 214 and 222 s for detection of 75 ppm NH3, respectively. In addition, the sensitivity and cyclic stabil‑ity were greatly improved under the heating temperature of 70 °C (Fig. 13a, b) [195]. The laser‑fabricated molybdenum carbide/graphene composites mentioned above can not only apply for paper‑based supercapacitors, but also for the gas sensors and electrochemical ion detectors applications [81]. Park et al. used the interdigitated rGO obtained by one‑step laser treatment as a humidity sensor pasted on fingernail (Fig. 13c-f), where wearable monitoring was realized [196]. Drmosha et al. reported a Pt‑loaded rGO/ZnO gas sensor fabricated by laser, the significant selectivity and response of about 99% toward hydrogen with a low concentration of 400 ppm was achieved, which was 5 and 10 times higher than those of the rGO/ZnO composite and pure ZnO, respec‑tively [197]. In addition, Chang et al. precisely fabricated the spiral graphene pattern electrodes as the sensing test region by double scanning of the picosecond laser. The obtained gas sensor had high detection sensitivity to the CO, H2O, and air (Fig. 13g) [198].
3.3.3 Other Devices Fabricated by Laser Technology
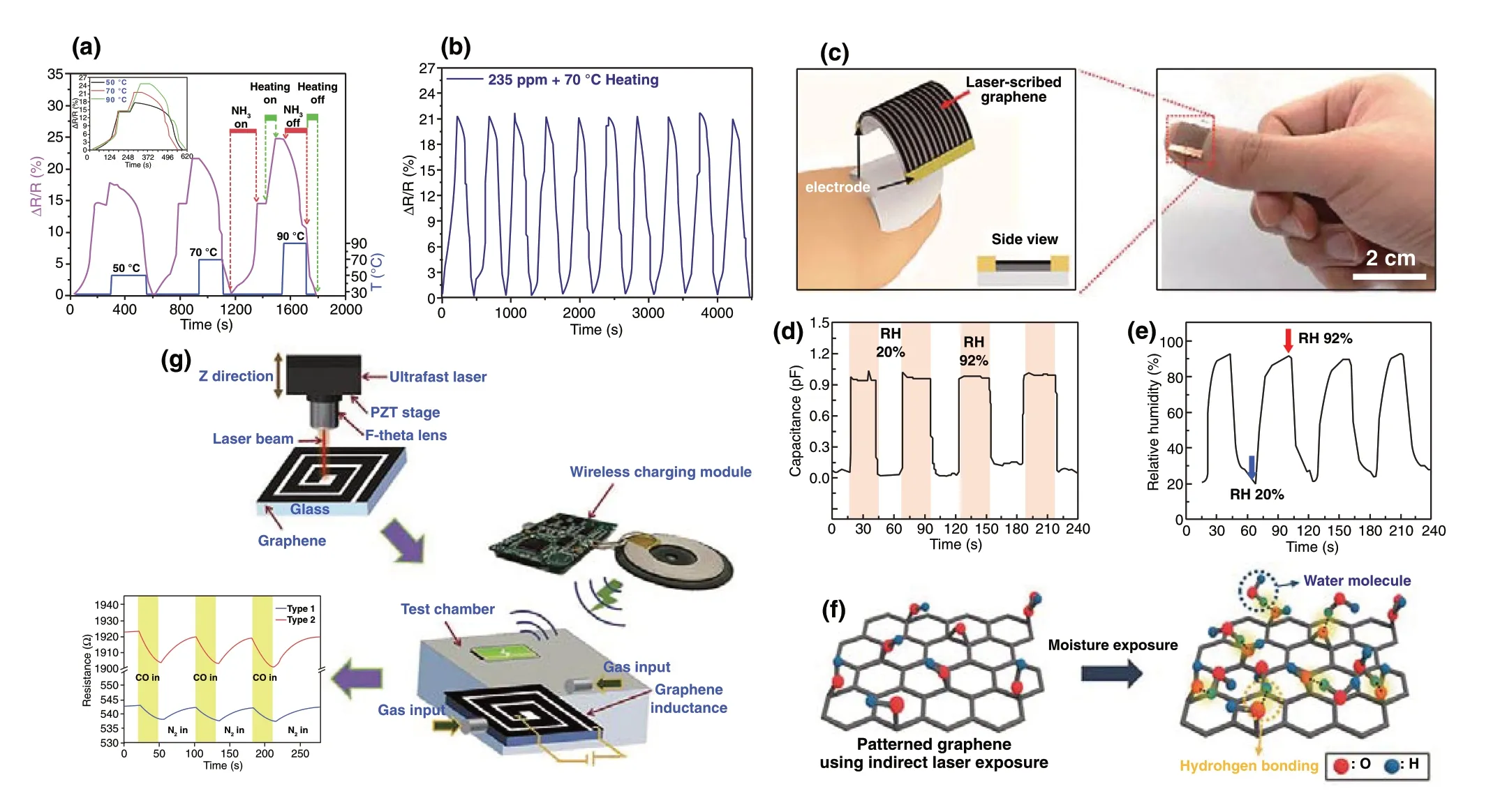
Fig. 13 a Normalized real‑time resistance response/recovery behavior of the sensor to 235 ppm NH3 at various desorption temperature from 50 to 90 °C. b Real‑time cycling response of the sensor to 235 ppm NH3 gas at 70 °C [195]. c Schematic view and digital image of the laser‑scribed rGO‑based flexible humidity sensor attached on a nail. d Real‑time signal responses as measured cyclic capacitance changes from the laser direct‑writing (LDW) GO‑based humidity sensor, exposed to RH in the range of 20-92% at 30 s intervals. e Monitored relative humidity (RH) changes collected from the real‑time data logger correspond to the graph in d. f Schematic drawing of absorption process of water molecules by hydrogen bonding on the partially reduced GO surface after moisture exposure [196]. g Schematic of the gas detection setup [198]
One‑step laser carbonization provided more possibili‑ties for other flexible devices. For example, Yan et al. reported a kind of electrophysiological sensor composed of laser‑patterned porous graphene as the sensing components and sugar‑templated silicone elastomer sponges as the sub‑strates (Fig. 14a). The sensors exhibited much higher water vapor permeability (18 mg cm−2h−1) than those without pores, which solved the problem of limited gas permeability. More importantly, this strategy used simple and effective laser processing instead of thin‑film deposition, photolithog‑raphy, or other complex procedures [199]. Stanford et al. fabricated a high output power triboelectric nanogenerator (TENG) by CO2laser treated polymers (Fig. 14b) [200]. High output power TENG through laser‑treated surface of friction materials was also fabricated by Huang [201].
Most of the sensor devices transformed the strain/pressure or chemical energy to the electric energy [202]. However, the stretchable organic light‑emitting device (OLED) was another sensor device that was mechanically insensitive to fatigue strain. OLED has become a promising alternative in electronic skin and deformable displays. Sun et al. developed a stretchable OLED with an ordered drape pattern through laser‑programmable microfabrication, the stretch‑release cir‑culation can be controlled and the maximum efficiency of 72.5, 68.5, and 70 cd A−1at mechanical strain of 0%, 40%, and 70%, respectively, was achieved [203].
Besides the applications of energy storage and sensors, nanostructures patterned by laser microfabrication tech‑nology for biological applications are receiving increasing attention, especially for the study of adhesive interactions between cells and nanostructured interfaces. Healy et al. fab‑ricated nanocraters in quartz through the pulsed femtosecond laser; then, they studied the effect of spatial distribution and features of the nanocraters on cell migration, morphology, and spatial organization. It demonstrated that this laser surface‑patterning approach provided a simple proposal for controlling cell behavior [204].
In addition, Jo et al. reported the writing of integrated circuits on a 2D semiconductor using a visible laser. In par‑ticular, by scanning the laser over metal patterns deposited on 2H‑MoTe2semiconductor layers, the spatially resolved doping with controlled dopant profiles and concentrations was achieved. This allowed us to directly write various circuitry in designed patterns, including arrays of n-p-n (p-n-p) bipolar junction transistors (BJTs) and p-n diode discs [205]. Zheng et al. [206] developed a new optother‑moplasmonic nanolithography (OTNL) technique. By vir‑tue of the plasmon‑enhanced light absorption and localized photothermal effect in deposited Au nanoparticles, the on‑demand, low‑power and desired patterning of 2D materials (graphene and MoS2monolayers) was achieved through a continuous‑wave laser irradiation (Fig. 14c). It demonstrated that thermal oxidation for graphene patterns and sublimation for MoS2patterns under the laser‑induced temperature field can lead to direct etching of the corresponding atomic layers.
3.4 Electrocatalytic Materials and Electrodes Fabricated by Laser Technology
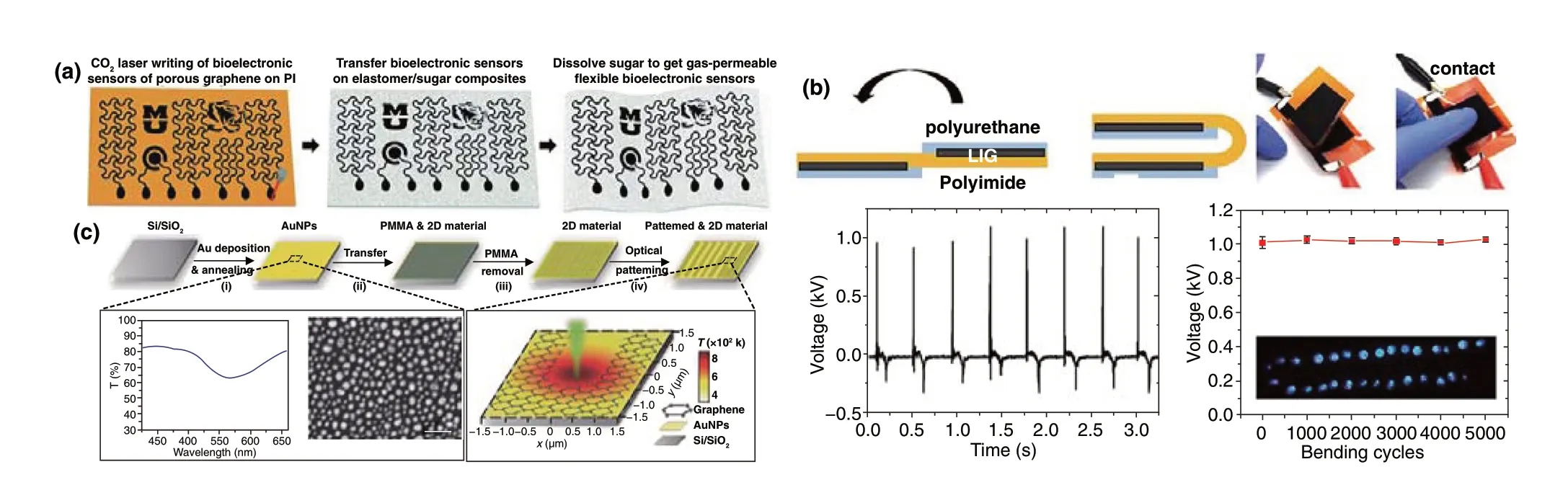
Fig. 14 a Schematic illustration of the fabrication process for bioelectronic sensing systems [199]. b Performance of a flexible metal‑free TENG [200]. c Schematic flowchart of OTNL process for 2D materials patterning [206]
High efficiency of the electrolyzer system is dependent on the electrocatalytic materials with high activity. It is noted that the surface structure and the surface area play an impor‑tant role in improving the activity of electrocatalytic elec‑trode materials for hydrogen evolution reaction (HER), oxy‑gen evolution reaction (OER) or oxygen reduction reaction (ORR). Various techniques have been used to construct the surface structure of electrocatalytic electrodes [207-209], especially by using the template synthesis [209]. How‑ever, the removal of the template was tedious, and the laser microfabrication technique provided an alternative method to construct different surface microstructures of electrocata‑lysts. With regards to the relationship between the high HER performance and laser microfabrication technology, it can be attributed to two factors. On the one hand, powder‑like electrocatalysts prepared by laser ablation in liquid usually possess the high electrocatalytic activity due to the laser‑induced defects and the large specific surface area for expos‑ing more active sites on the electrocatalysts. On the other hand, for the 3D electrocatalytic electrodes fabricated by the laser microfabrication technique, the high performance can be attributed to the different surface microstructures con‑structed by laser. During the laser microfabrication process, the hydrophilic surface, the large specific surface area for exposing more active sites and even the formation of a more active nanomaterials can be achieved, which are all prefer‑able to the improvement of the electrocatalytic performance.
3.4.1 Electrocatalytic Hydrogen Evolution Reaction (HER)
Electrocatalysts with high activity and durability for HER are crucial for the practical demands of electrochemical water splitting. The laser synthesis and microfabrication techniques provide a new avenue toward fabricating nano‑structures with novel properties due to the strong quenching effect [210]. As mentioned in Sect. 2.2.2, Du et al. reported a novel active Ag electrocatalyst [145] and RuAu single‑atom alloy electrocatalyst [111], which was fabricated by laser ablation in liquid. The HER electrocatalytic activities outperformed Pt/C. As reported, the Ag nanoparticle electro‑catalyst exhibited an extremely high activity for HER (a low overpotential of 32 mV at 10 mA cm−2and Tafel slope value of 31 mV dec−1) in acidic electrolyte, and the transformation from inactive to highly active Ag electrocatalysts was attrib‑uted to the improved H absorption energy regulated by laser‑induced stacking faults. The prepared RuAu single‑atom alloys also exhibited high stability and low overpotential of 24 mV at the current density of 10 mA cm−2in alkaline media (Fig. 15a-d). The high HER performance was attrib‑uted to the following factors: Firstly, the strong hydrogen atoms adsorption by Ru matrix can be counteracted by Au with weak hydrogen adsorption. Secondly, the electronic structure of RuAu single‑atom alloys can be regulated by the immiscibility between Ru and Au. Lastly, chemically inert Au favors long‑term stability during the reaction.
Non‑noble metal electrodes with nanostructures micro‑fabricated by laser were also the candidates for effective electrocatalytic electrodes. Röntzsch et al. reported the sur‑face construction of Ni electrodes through the fs pulse laser for highly active hydrogen evolution, which was realized by enlarging the surface area of Ni [211, 212]. An enhance‑ment of HER activity was achieved and the author confirmed that contributions of the microstructure of the redeposited ablation products and the chemical composition could be neglected for the HER activity, only the morphological sur‑face changes with enlarged surface areas contributed to the HER activity. Also utilizing the ultrashort‑pulse laser, the same group reported the laser process‑induced micro‑ and nanostructured Ti surfaces loading Pt by sputter deposition as HER electrode for alkaline water electrolysis [213]. It was demonstrated that the dramatically improved HER per‑formance was ascribed to the significant increase in spe‑cific surface area as well as the rapid gas bubble detachment resulted from superhydrophilic and superwetting properties. In addition, Zheng et al. [214] reported a nickel sulfide nano‑structures prepared by nanosecond pulsed laser ablation of an aqueous precursor solution under ambient condition. The superb electrochemical performances for electrocata‑lytic HER and supercapacitors were achieved (Fig. 15e), which exhibited a low overpotential (− 159 mV vs. RHE at 10 A g−1), lower Tafel slope (218 mV dec−1) and long‑term durability. Another transition metal sulfide, MoS2was regarded as one of the most effective substitutes to the noble metal Pt for HER. Thus, the regulation of MoS2with amor‑phous or crystalline structures and hybrid structures by laser attracted much attention. Tran et al. [215] investigated the crystallization (c‑MoS2) of amorphous molybdenum sulfide (a‑MoSx) induced by laser, which confirmed that a‑MoSxhad a higher catalytic performance for H2evolution. This result displayed that defect‑rich active sites were beneficial to high activity of the HER catalytic performance. The nanosized amorphous molybdenum sulfide (a‑MoSx) with numerous defects and disordered structures by femtosecond laser abla‑tion of ammonium tetrathiomolybdate aqueous solution has been mentioned in Sect. 2.2.2 [132]. It was noteworthy that, large current density of 516 mA cm−2at an overpotential of 250 mV could be achieved by using the optimized a‑MoSxcatalysts (x= 2.73) (Fig. 15f, g). In addition, Wu et al. [216] also prepared MoS2quantum dots with defective structure by ultrafast laser ablation. The enhanced HER electrocatalytic performance was achieved on account of the high conductiv‑ity, abundant exposed active sites and good hydrophilicity resulted from the laser ablation.
The electrocatalytic performance of MoS2has also been limited by the poor conductivity. Therefore, the hybrid struc‑ture of MoS2with other conductive materials has been paid attention. Lin et al. [217] presented a laser direct‑writing method to synthesize and pattern MoS2/carbon hybrid mate‑rials as HER electrocatalysts under ambient conditions, where small‑sized MoS2NPs anchored on carbon matrix were in situ synthesized and arbitrary patterns of this hybrid structure on substrates could be designed through computer‑controlled laser beams. Compared with MoS2synthesized by conventional hydrothermal method, the laser direct‑writing method can not only realize the synthesis of MoS2in a short period of time, but also perform superior HER activity for laser‑induced MoS2/carbon hybrid materials (Fig. 15h‑j). Li et al. [218] prepared Pt‑MoS2and Ag‑MoS2composites, which exhibited an enhanced activity for HER. The deco‑rated Pt metal nanoparticles contributed to the high HER activity and was reduced by photogenerated electrons of MoS2nanosheets induced by the femtosecond laser.
3.4.2 Electrocatalytic Oxygen Evolution Reaction (OER)
The most common OER electrocatalysts are regarded as transition metal oxides and hydroxides. By using laser as the power source under ambient atmosphere, laser abla‑tion synthesis of OER electrocatalysts is facile and fea‑sible. Most of the synthesis methods for these OER elec‑trocatalysts were laser ablation in liquid, as mentioned in Sect. 2.2.2, all of them exhibited excellent OER activity for water oxidation [219, 220]. For example, Co3O4nanoparti‑cles with an overpotential of 314 mV at 0.5 mA cm−2[117], CoO-Co2O3-Co(OH)2multiphase nanoparticles with an overpotential of 100 mV lower than that of the CoO submi‑cron precursor particles [221], NiFe layered double hydrox‑ides with a lowest overpotential of 260 mV at 10 mA cm−2[118], CoO nanoclusters/CoFe LDHs hybrid with an overpo‑tential of 254 mV at 10 mA cm−2[222] and oxygen vacancy‑modified CoOOH requiring an overpotential of 330 mV to reach 10 mA cm−2[223]. The performance of most of the OER electrocatalysts synthesized by LAL even outperforms RuO2.
Besides the transition metal oxides and hydroxides, Tour et al. [224] used laser microfabrication to prepare oxidized laser‑induced graphene (LIG‑O) as an efficient metal‑free OER electrocatalyst (Fig. 16a). The laser‑induced graphene was highly porous to expose more active sites for OER. Furthermore, the oxidation of LIG by O2plasma to form LIG‑O boosted the activity for OER, exhibiting a low onset potential of 260 mV with a low Tafel slope of 49 mV dec−1(Fig. 16b, c). The same group also prepared Co3O4in gra‑phene [80] and NiFe/LIG catalysts [225] for oxygen elec‑trocatalysis, just by depositing the different metal precursors on a preformed LIG surface followed by laser scribing. On account of the addition of transition metal precursors, the OER activity significantly improved.
3.4.3 Electrocatalytic Overall Water Splitting
As depicted above, Tour et al. [226] has made great efforts on oxygen evolution reaction electrocatalysts based on LIG, this group also paid a lot of attention on laser‑induced gra‑phene process to fabricate active catalytic electrodes for overall water splitting to generate both O2and H2. Firstly, they fabricated an overall water splitting device with HER and OER electrocatalytic electrodes on opposing faces of a commercial polyimide (PI) sheet. The HER active species were formed in situ in the LIG forming process by impreg‑nating the PI film in a Pt‑ion solution, and the OER active species were formed by subsequent electrodeposition of CoP/Co3(PO4)2or NixFey(OH)2x+3yonto the LIG. As dem‑onstrated, the LIG electrolyzer composed of LIG‑Co‑P as cathode and LIG‑NiFe as anode was employed in 1 M KOH, delivering 1.66 V at the current density of 10 mA cm−2with efficient HER and OER activity. In addition, this group used CO2laser scribing to transform wood into hierarchical porous graphene [227] (Fig. 17a). By varying the electro‑deposition materials on the LIG with Co‑P or NiFe LDHs, the laser‑induced graphene patterned on the wood surfaces could be readily fabricated into HER and OER electrodes for overall water splitting with high reaction activity at low overpotentials (Fig. 17b, c).
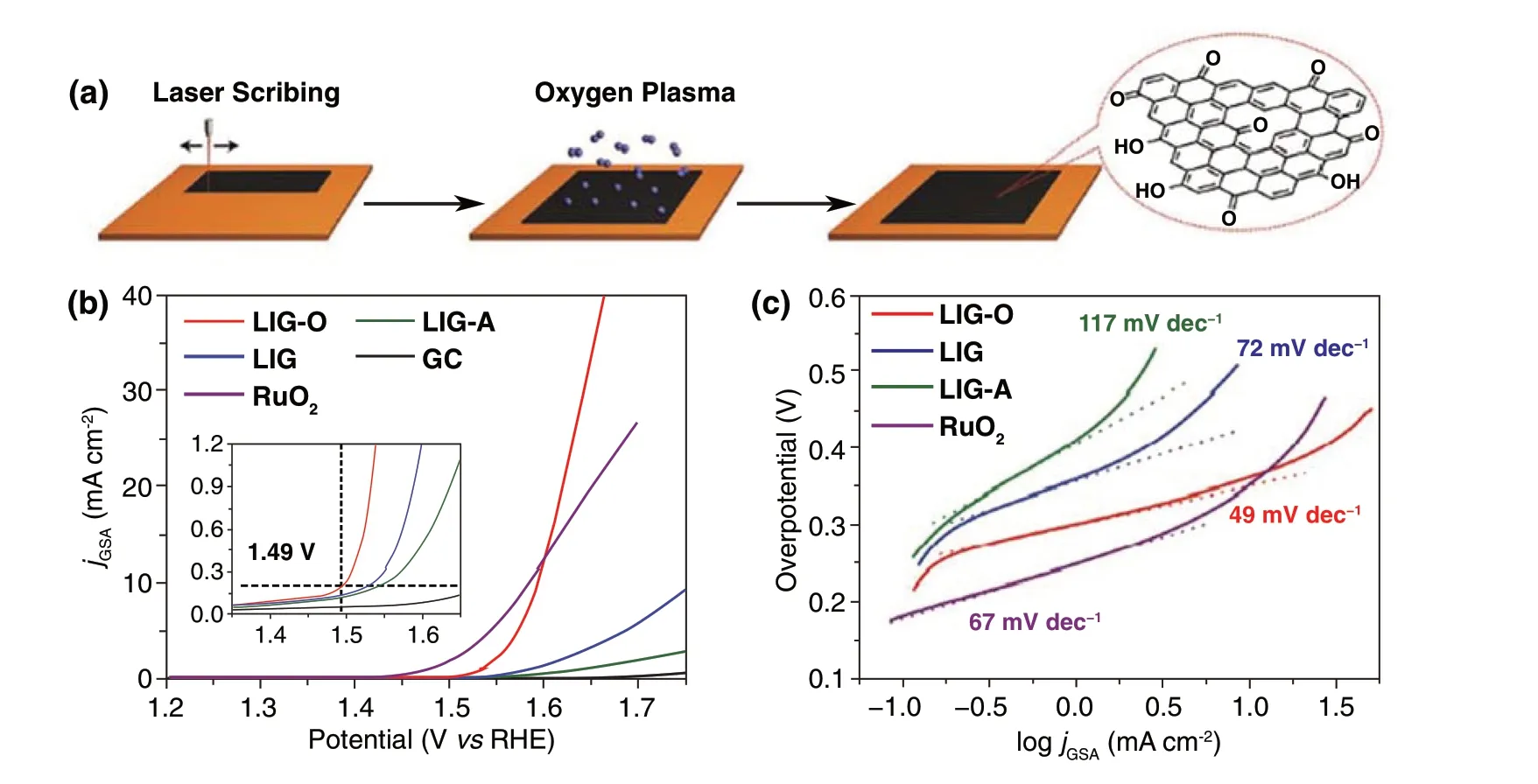
Fig. 16 a Preparation of LIG‑O. b LSV curves of LIG‑O, LIG, annealed LIG (LIG‑A), and a glassy carbon (GC) electrode recorded in 1 m KOH at a scan rate of 2 mV s−1. c Tafel plots calculated from panel b [224]
Besides the LIG‑based electrocatalytic electrode, laser‑induced hierarchical oxide nanostructures were also can‑didates for electrocatalytic overall water splitting. Zhong and Wu et al. [228] manufactured a series of metal oxides (MOx, M = Ti, Mn, Fe, Co, Ni, Cu, Mo, Ag, Sn, W, and NiFe) with a hierarchical nanostructure on corresponding metal substrates through laser ablation. In particular, the NiO/Ni plate electrocatalysts exhibited the HER overpo‑tential of 121 mV at 10 mA cm−2, much lower than that of 279 mV for Ni before laser ablation. Meanwhile, a decreased OER overpotential of 294 mV at 10 mA cm−2than 403 mV for Ni suggested the dramatically enhanced OER activity after laser ablation. Yang et al. [229] developed a surface laser modification approach for fabricating NiCo2O4‑dwith higher Ni3+/Ni2+ratio and abundant oxygen vacancies (Vo··) from NiCo2O4. The laser‑modificated NiCo2O4‑dexhibited a higher electrocatalytic activity for overall water splitting compared with the initial NiCo2O4. Du et al. [119] used laser‑synthesized porous Co0.75Ni0.25(OH)2nanosheets to catalyze the overall water splitting (Fig. 17d-f), which exhib‑ited a high performance for HER (95 mV@10 mA cm−2) and OER (235 mV@10 mA cm−2). When using it as both cath‑ode and anode catalysts, an external voltage of only 1.56 V was needed to reach a current density of 10 mA cm−2, much lower than that of commercial Ir/C‑Pt/C couple (1.62 V). These works demonstrated that using laser ablation to cre‑ate novel nanostructures with highly active sites for overall water splitting has great application prospects.
3.4.4 Electrocatalytic Oxygen Reduction Reaction (ORR)
As mentioned in Sect. 2.1.2, Tour’s group [79] scribed a CO2laser on a metal‑complex containing polyimide film and dif‑ferent metal oxide nanoparticles, Co3O4, MoO2, and Fe3O4embedded in porous graphene were formed, which were highly active in electrocatalytic ORR. However, it was noted that when the as‑prepared metal oxide nanoparticles embed‑ded into porous graphene was applied in ORR, annealing process at 750 °C for 30 min was performed in order to improve the degree of crystallization while maintaining the same crystal phases of metal oxides.
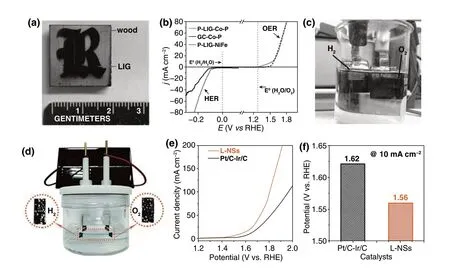
Fig. 17 a A photograph of LIG patterned into a letter R on pine wood. b HER and OER windows (iR compensated) of P‑LIG‑70 deposited with Co‑P or NiFe in 1 M KOH aqueous solution. c A photograph shows hydrogen and oxygen bubbling over the P‑LIG‑Co‑P (left electrode) and P‑LIG‑NiFe (right electrode) surfaces powered by two 1.5 V batteries in series [227]. d Optical image of overall water splitting driven by a 1.51 V solar cell. e LSV curves of Co0.75Ni0.25(OH)2 nanosheets and commercial Pt/C‑Ir/C couple in 6 m KOH for overall water splitting. f Potential values at 10 mA cm−2 [119]
3.5 Other Applications of Materials Fabricated by Laser Technology
3.5.1 Corrosion Protection Application
Corrosion of metals has been studied for its disadvantages of causing tremendous disasters and economic loss. Because of the unique high conductivity, impermeability and chemi‑cal inertness properties, the graphene coating has become a promising choice for anticorrosion application. However, graphene is easily fabricated on copper or nickel substrates while being difficult to grow on carbon steels. Therefore, Zhong et al. grew graphene by laser microfabrication on the solid carbon coating nickel surface [48] and on normal carbon steel by introducing Ni element into the carbon steel surface [230], which exhibited excellent anticorrosion prop‑erty. This strategy contained the advantages of fast growth, large‑area growth and arbitrary pattern design for graphene synthesis and manufacturing, overcoming the limitation of the CVD method. However, it was noted that both of the wide laser beam size and high laser power density were essential during the graphene synthesis process.
3.5.2 Hydrophilic and Hydrophobic Applications
The exciting functionalities of natural superhydrophobic and superhydrophilic surfaces inspired a variety of biomimetic designs. Particularly, the micropatterns combined with wet‑ting states resulted in invigorating applications. By using a fs‑laser microstructuring technology, Kostal et al. [231] mimicked the Namib Desert beetle’s elytra, the double hier‑archical surface structures with superhydrophilic character (water contact angle < 10°) on glass were firstly fabricated through laser. After a Teflon coating to change the wetting state from superhydrophilic to superhydrophobic (water contact angle > 150°) was applied, selective laser ablation was then utilized to locally expose the superhydrophilic pat‑tern. The fabricated micropatterns exhibited a significantly enhanced fog collection efficiency of 60% higher than blank glass, it was demonstrated that efficient drop accumulation and fast drop removal contributed to this enhancement. In addition, Chen et al. presented the fs laser on the thermal‑responsive shape‑memory polymer to fabricate hierarchical micropillars, the laser‑induced micropillars exhibited super‑hydrophobicity performance and the switchable wetting state was achieved [232]. Professor Tour’s group changed the dif‑ferent controlled atmospheres of laser‑induced graphene pro‑cess to regulate the wetting state of the LIG surfaces [233]. When varying the atmosphere from oxidizing to reducing condition, a great switch in the water contact angle of the LIG surfaces from 0° (superhydrophilic, O2or air) to > 150° (superhydrophobic, Ar or H2) was observed. The atmos‑phere‑mediated superhydrophobicity of micro/nanostruc‑tures by laser processing Cu and the subsequent adsorption of hydrophobic volatile organics was also reported [152]. In conclusion, the chemical composition and surface mor‑phology of micropatterns contributed to different wetting properties, which had significance for guiding application researches in electrochemical catalysis, energy storage and electronic devices.
3.5.3 Laser Streaming
Transforming a laser beam into a mass flow derived addi‑tional applications in laser propulsion, microfluidics and laser surgery and cleaning [234]. Wang et al. firstly proposed the optofluidic concept, demonstrating the successful gen‑eration of high‑speed liquid flow by pulsed laser streaming on a glass window after surface decorating with high‑density Au nanoparticles [235]. The optofluidic mechanism was demonstrated that plasmonic nanoparticles generated ultra‑sonic waves via the photoacoustic effect when a pulsed laser was incident, and then, the ultrasonic waves pushed their surrounding media to generate laser streaming. Then after using the gold‑implanted plasmonic quartz plate to replace the glass cuvette with Au nanoparticles, similar photoa‑coustic laser streaming occurred and the mechanism of the photoacoustic effect and vibration propagation was verified [236]. This laser‑driven optofluidic strategy will provide an opportunity for versatile microfluidic applications.
Therefore, the laser microfabrication process, especially the “cold microfabrication” process, allows precise control of the processing sites. Therefore, laser microfabrication realizes mask‑free patterning, which benefits for the con‑struction of hierarchical structures on flexible substrates. It is noted that although the laser microfabrication process is significantly fast and time saving when line‑by‑line scan‑ning, the laser punch treatment or point‑by‑point exposure treatment is time consuming, because that laser process is not a parallel processing technique compared with UV lithography. Alternatively, the use of a patterned laser source or multi‑laser beams can substantially enhance the process‑ing efficiency. In summary, the laser technology is expected to contribute to the advancing fabrication of different elec‑tronic devices, such as supercapacitors, batteries, sensors, electrocatalytic electrodes and other application systems.
4 Outlook and Future Challenges
Laser techniques operating with pulsed or continuous‑wave modes covering the wavelength ranges of ultraviolet, visible, and infrared have been employed in synthesis or microfab‑rication of materials. In terms of the synthesis of nanoma‑terials by laser, the basic processing principle is that the absorption of laser irradiation causes the photothermal or/and photochemical effects, which drive the conversion and crystallization of the precursor materials. The demanded thermal or chemical effect can be tuned by varying the laser process parameters, such as the scanning rate, pulse width and laser intensity. Thus, the choice of laser wave‑length is related to the light absorption properties of the precursor materials. If photothermal effects predominate during the synthesis process, laser with a long wavelength is essential, such as the CO2laser, especially for the laser synthesis of carbon materials. In terms of microfabrication of nanostructures by laser technology, two kinds of micro‑fabrication styles are included, one is primarily based on the laser‑induced photothermal effect through synthesis of the purpose materials at a localized position to construct the patterned nanostructures. The other is primarily based on the sputtering etching effect to obtain the desired nanostructure pattern. The feature of localized synthesis to construct the patterned nanostructures is similar to that of laser synthesis, and the only difference is that the formation of the pattern is strongly dependent on the laser process parameters, espe‑cially the pulse width, while the sputtering etching effect to obtain the desired nanostructured pattern is a so‑called cold microfabrication process, which needs highly focused energy to avoid the burrs induced by photothermal effects. Therefore, the ultrafast laser is in ascendancy. Because that the ultrafast laser possesses a high intensity and ultrashort pulse width, thermal side effects are minimized and nonlin‑ear absorption can take place in almost any material. As a result, brittle and transparent materials such as glasses can be processed, and because there is no heat exchange during the ultrafast pulsed laser processing, collateral damage and thermal stress are minimized. Thus, a more precise patterned nanostructure can be obtained.
As an alternative to the conventional annealing treatment, laser processing offers promising advantages. Firstly, this process is significantly fast and time saving. As experimen‑tally verified, laser processing brings the heating and cooling rates of > 106 °C s−1, which is orders of magnitude higher than that of the conventional thermal treatment. Therefore, rapid fabrication of materials with minimal energy losses becomes possible. Secondly, the laser microfabrication process, especially the “cold microfabrication” process, allows precise control of the processing sites. Therefore, laser microfabrication realizes mask‑free patterning, which benefits for the construction of hierarchical structures on flexible substrates. Thirdly, laser processing is adaptable for large size and roll‑to‑roll manufacturing and compatible with a variety of materials. The roll‑to‑roll manufacturing can be realized by integrating and digitizing the laser pro‑cess parameters with computer design and a manufacturing system. Lastly, due to the advantage of precise control of the laser process, the in situ synthesis or in situ sputtering etching, which retaining the precursor morphology after laser processing, can be tailored by tuning the laser focus distance. Hence, laser synthesis and microfabrication are promising protocols for developing nanomaterials and nano‑structures for various applications.
In spite of the advantages discussed above, laser process‑ing of nanomaterials still confronted with several limitations. In terms of laser microfabrication for patterning, although this process is significantly fast and time saving when line‑by‑line scanning, the laser punch treatment or point‑by‑point exposure treatment is time consuming, because that laser process is not a parallel processing technique compared with UV lithography. In addition, 3D nanostructures are difficult to construct through laser processing. Alternatively, the use of a patterned laser source or multi‑laser beams can substan‑tially solve both of the above‑mentioned issues. For practi‑cal production, the processing efficiency can be enhanced by adapting holographic laser processing or spatial light modulators. In the future, with the rapid development of parallel laser processing techniques or by combining 3D printing program, laser fabrication of 3D nanostructures can be completed in a more efficient manner. In addition, although various nanostructure patterns can be fabricated through the laser process, more nanostructure styles have not been realized compared with those constructed by wet chemical methods. Therefore, in situ transformation from the preconstructed nanostructures through the thermal effect of the unfocused laser is also a potential proposal for expanding the application of laser process.
Laser as a synthetic technique and/or microfabrication technique is not limited to the aforementioned applications. Laser synthesis and microfabrication have great potential for many other development directions. For instance, more compound species can be controllably synthesized by laser ablation under different atmospheres instead of only air or Ar atmosphere, such as the synthesis of nitrides under the N2or NH3atmosphere, synthesis of sulfides under the H2S atmosphere, synthesis of carbides under the CH4atmosphere and even synthesis under the CO2atmosphere for oxides or carbides with unique properties. In addition, the limited resolution of the laser process in nanomaterial process‑ing should be improved. By introducing different optical schemes together with the integration with other systems, controlled laser fabrication with higher precision can be achieved. Besides, the combination of laser cold processing and laser thermal synthesis is conducive to the realization of more sophisticated electronics with precise patterns and fine functionalization. In consequence, with the rapid develop‑ment of laser processing technology, additional nanomate‑rial‑based applications will be explored. With the continuous efforts that are being devoted to this dynamic field, laser as a synthetic technique and/or microfabrication technique will have broad application prospects.
AcknowledgementsThis work was supported by Taishan Scholars Project Special Funds (tsqn201812083), Natural Science Founda‑tion of Shandong Province (ZR2019YQ20, 2019JMRH0410, ZR2019BB001) and the National Natural Science Foundation of China (51972147, 51902132, 52022037).
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or for‑mat, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by stat‑utory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creat iveco mmons .org/licen ses/by/4.0/.
杂志排行
Nano-Micro Letters的其它文章
- Band Engineering and Morphology Control of Oxygen‑Incorporated Graphitic Carbon Nitride Porous Nanosheets for Highly Efficient Photocatalytic Hydrogen Evolution
- A Bulk‑Heterostructure Nanocomposite Electrolyte of Ce0.8Sm0.2O2‑δ–SrTiO3 for Low‑Temperature Solid Oxide Fuel Cells
- Present and Future of Phase‑Selectively Disordered Blue TiO2 for Energy and Society Sustainability
- Lotus Leaf‑Derived Gradient Hierarchical Porous C/MoS2 Morphology Genetic Composites with Wideband and Tunable Electromagnetic Absorption Performance
- Efficient Catalytic Conversion of Polysulfides by Biomimetic Design of “Branch‑Leaf” Electrode for High‑Energy Sodium–Sulfur Batteries
- Magnetic Array Assisted Triboelectric Nanogenerator Sensor for Real‑Time Gesture Interaction
