In vitro Antioxidant Effects of Porphyra haitanensis Peptides on H2O2-Induced Damage in HepG2 Cells
2021-03-06CHENShengjunYUJiaoHUXiaoYANGXianqingLILaihaoQIBoandDENGJianchao
CHEN Shengjun,YU Jiao,HU Xiao, YANG Xianqing, 2), LI Laihao, 2),QI Bo, and DENG Jianchao
Antioxidant Effects ofPeptides on H2O2-Induced Damage in HepG2 Cells
CHEN Shengjun1), 2), *,YU Jiao1), 3),HU Xiao1), YANG Xianqing1), 2), LI Laihao1), 2),QI Bo1), and DENG Jianchao1)
1),,,,,510300,2),,222005,3),,201306,
In this study, protein fromwas used as raw material to prepare an antioxidant peptide, and its anti- oxidant activity was evaluated. A model of H2O2-induced oxidative damage in HepG2 cells was established, and the effects ofhydrolysates (PHHs) on superoxide dismutase (SOD) activity and malondialdehyde (MDA) content were detected. Finally, the structure of PHHs was identified by ESI-MS/MS. The results showed that the 1,1-diphenyl-2-pyridylhydrazine (DPPH)-free radical-scavenging ability of PHHs was the strongest (59.28% at 1.0mgmL−1) when hydrolyzed with an acidic protease for 4h. PHHs with different concentrations had protective effects on H2O2-induced damage to HepG2 cells, and the protective effect was enhanced with increasing concentrations. When the level was 400μgmL−1, the cell survival rate was as high as 88.62%. More- over, PHHs can significantly reduce oxidative damage to HepG2 cells by H2O2, improve SOD activity, and reduce MDA content. The tetrapeptide Asp-Lys-Ser-Thr, with a molecular weight of 448Da, was identified as an important fraction of PHHs by high-resolution mass spectrometry.
hydrolysates (PHHs); antioxidant peptides; radical-scavenging activity; cell antioxidant capacity; electrospray ionization-mass spectrometry
1 Introduction
Oxidation is an important process during food deterioration that affects the safety, color, taste, and texture of food. Furthermore, oxidation during cellular metabolism can induce DNA mutations, modify the lipid components of cell membranes, and cause denaturation of cell membrane proteins. Antioxidants can prevent or decreasethe harmful effects of free radicals in human body and delay the deterioration of fat, protein, and other food ingredients. Because of their potential teratogenicity, carcinoge- nicity, and mutagenicity, synthetic antioxidants are limit- ed in their application.Numerous researches have been conducted on the preparation of natural and non-toxic anti- oxidants to replace the synthetic ones. These natural anti- oxidants include various plant- and animal-based extracts, such as proteins (Borawska., 2016; Yang., 2016), polysaccharides (Tang., 2016; Xu., 2016), flavonoids (Dulf, 2016; Chaaban., 2017), polyphenols (Shen., 2015; Plaza, 2016), lectins (Wu., 2015), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) (Sahari., 2017).
Antioxidant peptides, which consist of 2–20 amino acids, can be isolated from animal and plant protein by enzymatic hydrolysis. With their properties of easy absorption, intense activity, few side effects, excellent safety, and environment-friendly properties, antioxidant peptides can be used to prepare functional foods, protein supplements, and medications. Many studies have reported that hydrolyzed proteins from various animal and plant sources, such as bean (Marcela e., 2016; Carlos., 2017), corn (Jin., 2016; Wang., 2016), shellfish (Wu., 2016; Jin., 2018), shrimp (Zhou., 2014; Klee- kayai., 2015), fish (Ko., 2013; Qiu., 2014), and algae (Sheih., 2009; Yu., 2016), possess an- tioxidant activity.
Porphyra is one of the most important cultured sea- weeds and a main exported sea product of China (Liu., 2017). The annual Chinese production of porphyra is 0.20 million tons, of whichaccounts for 50% (Department of Fishery and Fishery Administration, Mi- nistry of Agriculture and Rural Areas, 2019). It isrich withproteins, polysaccharides, flavonoids, polyphenols, frames, lectins, DHA, EPA, and other physiologically active com- ponents. The harvest period ofis generally divided into 4–5 parts. The nutritional quality and market price ofdecreases gradually with a prolonged harvest period. In the final harvest period,usually is discarded as a waste, and leads to considerable environmental pollution. Interestingly, this waste can be used as raw material to prepare nutrients and phy- siologically active ingredients such as proteins, polysaccharides, DHA, and EPA. It is of considerable significance to improve the economic value of.
In the present study, the antioxidant activities ofhydrolysates produced with different hydrolysis time was investigated, and its antioxidant mechanism was explored by measuring the radical-scavenging activity of 1,1-diphenyl-2-picrylhydrazyl (DPPH). The antioxidant peptide was separated from the hydrolysate by gel filtration chromatography. The HepG2 cell with oxidative da- mage was used to further evaluate the antioxidant activity of purified PHH and explore the antioxidant mechanism. The peptide sequence was identified using an electrospray mass spectrometer (ESI-MS/MS).
2 Materials and Methods
2.1 Materials and Chemicals
was supplied by the Peilong seaweed cul- ture area in Chenghai District, Shantou City, Guangdong Province, China.was dried at 60℃ for 4h, then ground and filtered through a 100-mesh screen, and finally stored dry. Acid protease (5×104Ug−1) was supplied by Hefei BoMei Biotechnology Incoporation (Hefei, China). HepG2 cells were provided by Guangzhou YeshanBiotechnology Incoporation (Guangzhou, China). All other materials for cell culture were obtained from Hyclone (Lo- gan, Utah, USA). Sephadex G-15 was purchased from Cool Chemical Technology (Beijing, China). Reduced glutathi- one (MW 307.3Da) and bacitracin (MW 1422.69Da) were obtained from Guangzhou Qiyun Biotechnology Incoporation (Guangzhou, China). Aprotinin (MW 6511.83Da) and cytochrome C (MW 12400Da) were obtained from Shanghai Maclean Biochemical Technology Incoporation (Shanghai, China). Glutathione (GSH), H2O2, and L-oxi- dized glutathione (MW 612.63Da) were purchased from Sigma (St. Louis, MO, USA). All chemicals were of analytical grade.
2.2 Protein Extraction from P. haitanensis
The protein was extracted fromusing an ultrasonic cell grinder (JY99LLDN, Ningbo Scientz Biotechnology Company, Jiangsu, China).(1.0g) and 100mL of distilled water were mixed and stirred uniformly, and extracted at a power of 1260W, room tem- perature for 30min, and then centrifuged with 558.95×at 4℃for 10min to remove cell debris. The pH of the su- pernatant was adjusted to an isoelectric point of 4.2.After the solution was kept at room temperture for 1h,it was centrifuged at 558.95×for 10min to remove the supernatant. The precipitate was dissolved in distilled water, ad- justed to neutral pH, and dialyzed in a dialysis bag with a molecular weight cutoff of 100 Da for 24–36h. The protein solution was pre-chilled at −20℃ for 12h, freeze- dried under vacuum at −44℃ for 36h in an Alpha 1-4 lyophilizer (Marin Christ, Germany), and stored dry at room temperture.
2.3 Enzymatic Hydrolysis of P. haitanensis
The protein ofwas hydrolyzed by acid protease for 2.0, 4.0, 6.0, 8.0, and 10.0h at 54.4℃, pH 3.67, with 4240Ug−1of the enzyme. Reactions were stopp- ed by heating at 95℃ for 10min, and samples were centrifuged to remove insoluble material. The degree of hydrolysis (DH) ofproteins was determined using the pH-stat method.hydroly- sates (PHHs) were adjusted to neutral pH and dialyzed for 24–36h in a 100Da dialysis bag. PHHs were concentrat- ed, and freeze-dried under vacuum at −44℃ for 36h in an Alpha 1-4 lyophilizer (Marin Christ, Germany), and stored dry at room temperture.
2.4 Molecular Weight Distribution of PHHs
The molecular weight distribution of PHHs was determined using high-performance size-exclusion chromato- graphy (Sallam., 2018). The column was TSK-GELG2000SWXL (7.8mm×300mm), and the mobile phase was acetonitrile containing 0.1% trifluoroacetic acid and double distilled water containing 0.1% trifluoroacetic acid. The detection wavelength was 214nm, the sample volume was 10μL, the flow rate was 0.5mLmin−1, and the elution length was 60min. A molecular weight standard was dissolved in mobile phase at a concentration of 0.2mgmL−1. By fitting time and molecular weight, a regression equation was obtained: log MW=−0.206+6.8009,2=0.9874. The molecular weight distribution of PHHs was calculated by normalization of the peak area.
2.5 Purification of PHHs
Purification of PHHs was performed using selective gel filtration chromatography (Mei., 1998). After lyophi- lization, the polypeptide was dissolved in deionized water at a concentration of 25mgmL−1. The sample was loaded onto a Sephadex G-15 column (1.6cm×100cm), eluted with deionized water at a flow rate of 0.6mLmin−1, and monitored at 214nm. Fractions with strongest DPPH-free radical-scavenging ability were concentrated, lyophilized, and further analyzed.
2.6 Antioxidant Activity Assays
2.6.1 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity assay
DPPH radical-scavenging activity was measured accord- ing to a previously described method (Jang., 2016) with some modifications. The sample (1.0mL) was combined with 1.0mL of DPPH (0.15mmolL−1 in ethanol). Thesolution was immediately mixed and incubated in the dark for 30min. The absorbance of the resulting mixture was measured at 517nm. The control sample contained ethanol (1.0mL) and DPPH (1.0mL). DPPH free radical-sca- venging activity was calculated as (1−As/Ac)×100%,whereAs and Ac represent absorbance of the sample and control, respectively.
2.6.2 Hydroxyl radical-scavenging activity assay
Hydroxyl radical-scavenging activity was measured ac- cording to a previously described method (Chi., 2015) with some modifications. Firstly, 2mL sample was mixed with 0.3mL of 1,10-phenanthroline (5mmolL−1 in ethanol), 0.2mL of 0.15molL−1phosphate buffer (pH 7.4), and 0.3mL of 0.75mmolL−1ferrous sulfate, and the solution was stirred vigorously. Then 0.2mL of 0.1% H2O2was added to the solution and incubated at 37℃ for 60min. Finally, the absorbance of the sample Aswas measured at 510nm. When an equivalent volume of deionized water was used instead of the sample and H2O2solution, the absorbance va- lue Abwas applied as the control. When Ddeionized water was used instead of the sample, the absorbance value A0was applied as the blank. Hydroxyl radical-scavenging ac- tivity was calculated as (1−(AS−A0)/Ab)×100%.
2.7 Antioxidant Activity Analysis in HepG2 Cells
2.7.1 Cell culture
HepG2 cells stored in liquid nitrogen were thawed in a water bath at 37℃. They were cultured in Dulbecco’s Mo- dified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100UmL−1penicillin, and 100µgmL−1streptomycin. Cells were propagated in a 25cm2tissue culture flask, harvested using trypsin during exponential growth, and seeded into 96-well plates at a density of 1×105cellsmL−1. Cells were incubated at 37℃ in 5% CO2, and the medium was changed every 2d.
2.7.2 Cell viability assay
HepG2 cells at Log-phase were seeded into 96-well plates with a density of 1×105cellsmL−1and cultured for 24h at 37℃ in a incubator containing 5% CO2. After the cells were cultured for 24h, 20µL of a 5mgmL−1methyl thiazolyl te- trazolium (MTT) solution was added to each well, and the cells were cultured for another 4h. Then the supernatant was carefully discarded, 200µL of dimethylsulfinic acid was added to each well. After the plate was shaken for 15min,the absorbance value was measured at 490nm. Cell via- bility was calculated based on absorbance (Jang, 2008).
2.7.3 Establishment of the cell model
In drug screening experiments, an induction concentration with a survival rate of 50% to 70% is generally selected as the optimal concentration. When the concentration of inducer is too high, the cells will be susceptible to excessive damage and the survival rate will be very low. HepG2 cells were treated with different concentrations of H2O2solutions (50, 100, 200, 400, 600, and 800μmolL−1) for 4h. Cell viability was determined using the MTT me- thod to determine the appropriate H2O2concentration and treatment time (Jang, 2016).Using the cell survival rate as the indicator, the optimal induction condition to obtain a survival rate of 60% wasculturing the cells in 259μmolL−1H2O2for 4h. HepG2 cells were divided into a blank group, a control group, and a treatment group. The blank group was HepG2cells without H2O2treatment. The control group was HepG2cells treated with 259µmolL−1H2O2for 4h. In the treatment group,the cells were treat- ed with GSH or PHH-III with a concentration of 100, 200, and 400μgmL−1for 24h, respectively, and then were treated with H2O2.These cells were determined as low-, medium-, and high-dose groups.
2.7.4 Determination of superoxide dismutase (SOD) activity and malondialdehyde (MDA) content
HepG2 cells at Log-phase were prepared as single-cell suspensions and seeded into 96-well plates with a con- centration of 1×104cells per 200µL. Following establish- ment of a cell model, different concentrations (100, 200, and 400µgmL−1) of polypeptide and GSH were used to treat oxidatively damaged HepG2 cells, and the MDA con- tent and SOD activity were determined. The SOD activity was determined using the Total Superoxide Dismutase As- say Kit with WST-8 according to the manufacturer’s instructions. The MDA content in the cells was determined using the Lipid Peroxidation MDA Assay Kit according to the manufacturer’s instructions.
2.8 Structure of PHHs
Structure of PHHs was identified according to a previously described method (Lin., 2019) with some mo- difications. PHH-III was dissolved in a mobile phase, fil- tered through a 0.22µm filter, placed in a sample vial, and analyzed using ultra-high-pressure liquid chromatography-high-resolution mass spectrometry. PHH-III were precipi- tatedan Agilent SB-C18 RRHD column (2.1mm×50mm, 1.8µm) with a flow rate of 0.2mLmin−1, 0.1% formic acid as phase A and acetonitrile as phase B, and an in- jection volume of 20.0µL. The gradient elution conditions were as follows: 0–1min, B phase remained unchanged at 15%; 1–4.5min, volume fraction of phase B solution in- creased from 15% to 90%; 4.5–8min, phase B maintain- ed at 90%; and 8.5–10min, phase B decreased from 90% to 15%. Scanning was performed in positive-ion mode with a scan range of m/z 100–1400.
2.9 Statistical Analysis
Results are presented as the mean and standard deviation of triplicates. Statistical analysis was performed using SPSS 20.0 software (SPSS, Chicago, IL, USA).
3 Results and Discussion
3.1 Antioxidant Activity and Molecular Weight Distribution of PHHs
PHHs obtained by different hydrolysis times were test- ed for DPPH radical-scavenging activity. As shown in Table 1, PHHs with a hydrolysis time of 4h were found to be more efficient than the others, and the resulting hydro- lysates exhibited higher antioxidant capacity. This may re- sult from hydrolysis producing more small peptides with antioxidant activity because of energetic electrons (Wang., 2013). However, excessive hydrolysis time increases may result in the degradation of these small fragments, which can cause down-rugulated antioxidant activity. PHHs prepared by 4h of hydrolysis were then further isolated for the identification of antioxidant peptides.
The chromatographic data (Table 1) showed that PHHs contained a significant amount (70%–80%) of oligopeptides below 1kDa. The results indicate that the acid proteases are effective at producing short oligopeptides and removing large peptides or undigested proteins. Hydroly- sates with different molecular weights have differences in antioxidant capacity (Li, 2013). Moreover, some re- search findings indicated that small molecular peptides pos-sess more robust free radical-scavenging activity (Zhuang., 2010), further increasing the antioxidant function. Therefore, according to the molecular weight distribution of PHHs, an appropriate method can be selected to purify PHHs and further evaluate their antioxidant capacity.

Table 1 Comparison of DPPH radical-scavenging activity and molecular weight distribution of PHHs at different enzymatic hydrolysis times
Notes: DPPH radical-scavenging capacity is determined at a sample concentration of 1.0mgmL−1. Values are shown as mean±standard deviation of triplicates (=3).
3.2 Purification of Different Fractions of PHHs
Gel filtration chromatography has been used for separation of biological extracts and protein hydrolysates (Knu- ckles., 2006). Considering the molecular weight of PHHs, a Sephadex G-15 column is suitable for further se- paration. PHHs were further separated into three fractions (I–III) by gel filtration chromatography. Each fraction was pooled, lyophilized, and tested for DPPH-scavenging abi- lity. As shown in Fig.1, fraction III exhibited the most vi- gorous DPPH radical-scavenging activity with a rate of 73.32% at 1.0mgmL−1. In this research, we found that the eluted fractions’ antioxidant activity increased with in- creasing retention time. Consequently, the fraction of PHH- III, which demonstrated the most potent activity, was se- lected for further analysis.

Fig.1 (A) Chromatogram of PHH fractions purified by gel filtration column; (B) DPPH radical and hydroxyl radical-sca- venging ability of each fraction (samples at 1.0mgmL−1). Values within the same column, followed by the different letters (a, b, and c), are significantly different (P<0.05).
3.3 Antioxidant Capacities of PHH-III to HepG2 Cells
3.3.1 Cell viability of the antioxidant activity cell model
Oxidative stress refers to cell damage caused by high concentrations of reactive oxygen molecules or chemical derivatives of oxygen to cells. It is an essential mediator of the occurrence and development of obesity, diabetes, li- pid deposition, and chronic inflammation (Skalicky., 2008). H2O2is one of the commonly used substances for establishing oxidative damage in cells and has been used in osteoblasts, nerve cells, vascular endothelial cells, and hepatocytes (Zorov., 2000). HepG2 cellscan fully express antioxidant enzymes and detoxification enzymes as in normal hepatocytes. Accordingly, They are often used for studying cytoprotection against exogenous chemicals and natural antioxidants (Lee., 2012). As shown in Figs.2 and 3, compared with healthy cells (Blank), the vi- ability of HepG2 cells in the injury group (Control) was reduced significantly. Moreover, the injured cells showed inter-adhesion, irregular shapes, and lower cell viability. Different doses of PHH-III in each group can protect the cell and significantly increase cell viability (0.05). To- gether with GSH, PHH-III was better at improving the morphology and cell viability with a concentration of 100µgmL−1. Additionally, the viability of cells cultured with different doses of PHH-III was higher than that withdif- ferent doses ofGSH. The results of these experiments in- dicate that PHH-III can increase the viability of cells da- maged by H2O2.

Fig.2 Effects of different concentrations of PHH-III and GSH on the morphology of HepG2 cells treated with 259µmolL−1 H2O2 for 4h. (A) Blank, (B) Control, (C) 100µgmL−1 GSH, (D) 200µgmL−1 GSH, (E) 400µgmL−1 GSH, (F) 100µgmL−1 PHH-III, (G) 200µgmL−1 PHH-III, and (H) 400µgmL−1 PHH-III. The blank group was HepG2 cells without H2O2 treatment. The control group was HepG2 cells treated with 259µmolL−1 H2O2 for 4h.
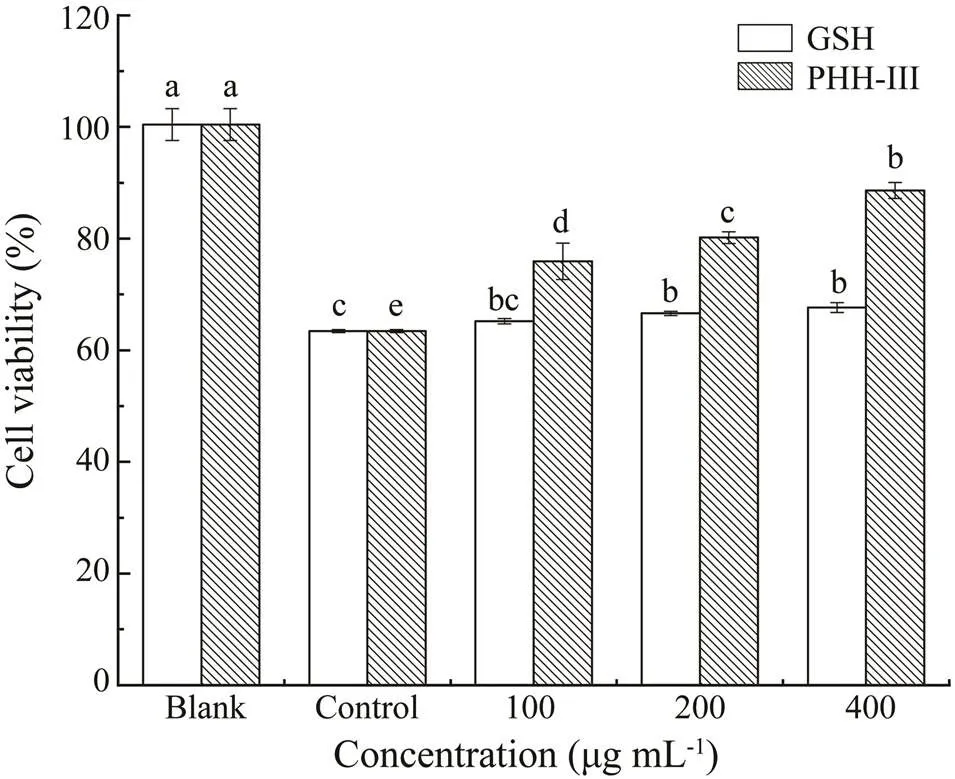
Fig.3 Effects of different concentrations of PHH-III andGSH on the viability of HepG2 cells treated with 259µmolL−1 H2O2 for 4h. The blank group was HepG2 cells without H2O2 treatment. The control group was HepG2 cells treated with 259µmolL−1 H2O2 for 4h. The treatment group was treated with GSH or PHH-III with a concentration of 100, 200, or 400µgmL−1 for 24h, respectively,as the low-, medium-, and high-dose groups. Then the H2O2 treatment was applied. Values within the same column, followed by different letters (a, b, c, d, and e) are significantly different (P<0.05). Values are shown as mean±standard deviations from triplicates (n=3).
3.3.2Superoxide dismutase (SOD) activity and malondialdehyde (MDA) content in HepG2 cells with different treatments
SOD catalyzes the disproportionation of superoxide an- ion to H2O2and O2to scavenge free radicals, and its vita-lity plays an important role in cellular oxidation and an- tioxidant balance (Miller, 2004). As shown in Fig.4, com- pared with healthy cells (Blank), the SOD activity in injured HepG2 cells (Control) was significantly reduced. When medium- or high-dose PHH-III was added in the cul-ture media, the SOD activity in the cells could be increased significantly (0.05), while only high dose GSH could significantly increase the SOD activity(0.05).
MDA is an oxidation end product obtained by free ra- dicals acting on lipids in the body (Bedoya-Ramírez., 2017). The MDA content in liver cells is generally considered to be an essential indicator of the degree of lipid peroxidation (Hu., 2017). As shown in Fig.4B, com- pared with untreated cells (Blank), the MDA content inin- hured HepG2 cells (Control) was significantly increased. The low-, medium-, and high-dose of PHH-III or GSH sig-nificantly inhibited the increase of MDA content in the cells caused by H2O2in a dose-dependent manner (0.05). Compared with GSH, PHH-III was better at reducing the MDA content in cells with low-, medium-, and high-dose.Thus PHH-III can increase the SOD activity of H2O2-in- jured cells, inhibit the increase in MDA content, and en- hance the antioxidant response of HepG2 cells.
3.3.3 Mass spectrometry identification analysis
Mass spectrometry can accurately determine the mole- cular weight and amino acid sequence of peptides and has potential applications in proteomics analysis (Li., 2007). In order to identify the peptides exhibiting major antioxidant activity, the PHH-III fractions were further se- quenced by ESI-MS/MS. The MS spectrum of this frac- tion is shown in Fig.5A, and the MS/MS spectrum of a single-charged ion with m/z 449Da is shown in Fig.5B. The molecular weight of the peptide in PHH-III was de- termined to be 449Da. Since each peptide matches a specific mass number and corresponding fragment map (Ma., 2012), the amino acid sequence of the peptide in PHH-III was identified as Asp-Lys-Ser-Thr. According to the naming system proposed by Roepstorff and modified by Biemann, the N-terminal fragment ions are represent- ed by the letters a, b, and c, and the C-terminal fragment ions are represented by the letters x, y, and z (Biemann, 1992). Since the amide bond in the peptide is relatively easy to be broken, the b- and y-type fragment series may have a higher frequency of occurrence on the mass spectrum. The y3 (m/z 334), y2 (m/z 205), b2 (m/z 245), and y1 (m/z 120) fragments are produced by cleavage of a peptide bond. The neutral molecule may also lose water or ammonia (Sun., 2008), resulting in a high abundance of m/z 431. It has been reported that residues of acidic amino acids (Asp, Glu,.) contribute to the antioxidant capacity of peptides (Saiga., 2003). Moreover, Lys contributes to the termination of a radical chain reaction, the interaction with free radicals, and the prevention of radical formation. In general, the number, type, and composition of amino acids play an essential role in antioxidative peptides.

Fig.4 (A) Effects of different concentrations of GSH and PHH-III on SOD activity in HepG2 cells treated with 259µmolL−1 H2O2 for 4h, (B) Effects of different concentrations of GSH and PHH-III on MDA content in HepG2 cells treated with 259µmolL−1 H2O2 for 4h. The blank group was HepG2 cells without H2O2 treatment. The control group was HepG2 cells treated with 259µmolL−1 H2O2 for 4h. The treatment group was treated with GSH or PHH-III with a concentration of 100, 200, and 400µgmL−1 for 24h, respectively,as the low-, medium-, and high-dose groups. Then H2O2 treatment, was applied. Values within the same column, followed by different letters (a, b, c, d, and e) are significantly different (P<0.05). Values are shown as mean±standard deviations from triplicates (n=3).

Fig.5 (A) Mass spectrum of the chromatographic peak III; (B) MS/MS spectrum of ion m/z 449.
4 Conclusions
In this study, the DPPH free radical-scavenging ability of PHHs was the strongest (59.28% at 1.0mgmL−1)when hydrolyzed with an acidic protease for 4h. Among the hy- drolyzed products, PHHs with a molecular weight range of less than 1kDa accounted for 70% to 80% of the totalPHHs. Sephadex G-15 was used to separate and purify PHHs, and it was found that the PHH-III fraction had the most vigorous activity. PHH-III could alleviate the oxidative damage of HepG2 cells caused by H2O2, which might be realized through increasing SOD enzyme activity and reducing the production of MDA. The tetrapeptide Asp- Lys-Ser-Thr with a molecular weight of 448Da was identified by high-resolution mass spectrometry in the PHH- III. The specific signaling pathways and targets for the re- gulation of oxidative stress response in cells affected by algae peptides need to be further studied.
Acknowledgements
This work was supported by the National Key R&D Pro- gram of China(No. 2018YFD0901102), the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (No. 2020KJ151), the Spe- cial Scientific Research Funds for Central Non-profit Institutes, Chinese Academy of Fishery Sciences (No. 2020 TD69), and the China Agriculture Research System (No. CARS-50).
Bedoya-Ramírez, D., Cilla, A., Contreras-Calderón, J., and Alegría-Torán, A., 2017. Evaluation of the antioxidant capacity, furan compounds and cytoprotective/cytotoxic effects upon Ca- co-2 cells of commercial Colombian coffee., 219: 364-372.
Biemann, K., 1992. Mass spectrometry of peptides and proteins., 61 (1): 977-1010.
Borawska, J., Darewicz, M., Vegarud, G. E., and Minkiewicz, P., 2016. Antioxidant properties of carp (L.) pro- teinandhydrolysates., 194: 770-779.
Carlos, M. G. A., Walter, M., and Jonh, J. M. A., 2017. Antioxidant potential use of bioactive peptides derived from mung bean hydrolysates ()., 11 (3): 67-73.
Chaaban, H., Ioannou, I., Chebil, L., Slimane, M., Gérardin, C., Paris, C., Charbonnel, C., Chekir, L., and Ghoul, M., 2017. Ef- fect of heat processing on thermal stability and antioxidant activity of six flavonoids., 41 (5): 1-12.
Chi, C. F., Hu, F. Y., Wang, B., Li, T., and Ding, G. F., 2015. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam () muscle., 15: 301-313.
Department of Fishery and Fishery Administration, Ministry of Agriculture and Rural Areas, 2019., 23.
Dulf, F. V., Vodnar, D. C., and Socaciu, C., 2016. Effects of so- lid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (L.) by-products., 209: 27-36.
Hu, N., Tu, X. R., Li, K. T.,Liu, Y. K.,Guo, A. M.,Tu, G. Q., and Huang, L., 2017. Changes in antioxidant enzyme activities andmalondialdehyde (MDA) content of rice with blast resistance induced by ag-antibiotic 702., 8 (2): 36-40.
Jang, A., Jo, C., Kang, K. S., and Lee, M., 2008. Antimicrobial and human cancer cell cytotoxic effect of synthetic angioten- sin-converting enzyme (ACE) inhibitory peptides., 107 (1): 327-336.
Jang, H. L., Liceaga, A. M., and Yoon, K. Y., 2016. Purification, characterization and stability of an antioxidant peptide deri- ved from sandfish () protein hydroly- sates., 20: 433-442.
Jin, D., Liu, X., Zheng, X., Wang, X., and He, J., 2016. Preparation of antioxidative corn protein hydrolysates, purification andevaluation of three novel corn antioxidant peptides., 204: 427-436.
Jin, J. E., Ahn, C. B., and Je, J. Y., 2018. Purification and characterization of antioxidant peptides from enzymatically hydro- lyzed ark shell ()., 72: 170-176.
Kleekayai, T., Harnedy, P. A., O’Keeffe, M. B., Poyarkov, A. A., Cunhaneves, A., Suntornsuk, W., and FitzGerald, R. J., 2015. Extraction of antioxidant and ace inhibitory peptides from thai traditional fermented shrimp pastes., 176: 441- 447.
Knuckles, B. E., Fremery, D. D., and Kohler, G. O., 1982. Gel fil- tration chromatography of protein extracts from lucerne ()., 33 (2): 128-132.
Ko, J., Lee, J., Samarakoon, K., Kim, J., and Jeon, Y., 2013. Puri- fication and determination of two novel antioxidant peptides from flounder fish () using digestive pro- teases., 52: 113-120.
Lee, M. S., Kim, J. I., Utsuki, T., Park, N. G., and Kim, H. R., 2012. Cytoprotective effects of phlorofucofuroeckol A isolated fromagainst tacrine-treated HepG2 cells., 83 (6): 1060-1067.
Li, B., Chen, F., Wang, X., Ji, B. P., and Wu, Y. N., 2007. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and elec- trospray ionization-mass spectrometry., 102: 1135-1143.
Li, X. H., Chen, Z. J., Liu, Y. L., Yu, J., Wang, F. X., and Wang, J. H., 2013. Molecular weight and antioxidant activity of enzymatic hydrolysates of silver carp., 34 (17): 28- 32.
Lin, L., Zhu, Q., and Zhao, M., 2019. Preparation of antioxidant peptide fromseeds and its protective effects on oxidatively damaged erythrocytes., 40 (7): 40-46.
Liu, Q. M., Xu, S. S., Li, L., Pan, T. M., Shi, C. L., Liu, H., Cao, M. J., Su, W. J., and Liu, Q. M., 2017.andim- munomodulatory activity of sulfated polysaccharide from., 165: 189-196.
Ma, Z., Zhang, W., Yu, G., He, H., and Zhang, Y., 2012. The pri- mary structure identification of a corn peptide facilitating al- cohol metabolism by HPLC-MS/MS., 37 (1): 138-143.
Marcela, G. M., Eva, R. G., del Carmen, R. R. M., and Rosalva, M. E., 2016. Evaluation of the antioxidant and antiprolifera- tive effects of three peptide fractions of germinated soybeans on breast and cervical cancer cell lines., 71 (4): 368-374.
Mei, W., Dai, J., and Gu, W., 1998. Assay on the molecular weight distribution of oligo peptide in corn protein hydrolysate with gel filtration chromatography.s, 24 (5): 25-27.
Miller, A. F., 2004. Superoxide dismutases: Active sites that save, but a protein that kills., 8 (2): 162-168.
Plaza, M., Batista, A. G., Cazarin, C. B. B., Sandahl, M., Turner, C., Östman, E., and Júniorb, M. R., 2016. Characterization of antioxidant polyphenols frompeel and their effects on glucose metabolism and antioxidant status: A pilot clinical study., 211: 185-197.
Qiu, X., Chen, S., and Dong, S., 2014. Effects of silver carp an- tioxidant peptide on the lipid oxidation of sierra fish fillets () during frozen storage., 38 (2): 167-174.
Sahari, M. A., Moghimi, H. R., Hadian, Z., Barzegar, M., and Mo- hammadi, A., 2017. Physicochemical properties and antioxi- dant activity of α-tocopherol loaded nanoliposome’s contain- ing DHA and EPA., 215: 157-164.
Saiga, A., Tanabe, S., and Nishimura, T., 2003. Antioxidant acti- vity of peptides obtained from porcine myofibrillar proteins by protease treatment., 51 (12): 3661-3667.
Sallam, S., Dolog, I., Paik, B. A., Jia, X., Kiick, K. L., and Wes- demiotis, C., 2018. Sequence and conformational analysis of peptide-polymer bioconjugates by multidimensional mass spec- trometry., 19 (5): 1498-1507.
Sheih, I. C., Wu, T. K., and Fang, T. J., 2009. Antioxidant pro-perties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems., 100 (13): 3419-3425.
Shen, Y., Zhang, H., Cheng, L., Wang, L., Qian, H., and Qi, X., 2015.andantioxidant activity of polyphenols extracted from black highland barley., 194: 1003-1012.
Skalicky, J., Muzakova, V., Kandar, R., Meloun, M., Rousar, T., and Palicka, V., 2008. Evaluation of oxidative stress and infla- mmation in obese adults with metabolic syndrome., 46 (4): 499-505.
Sun, S. W., Yu, C. G., Qiao, Y. T., Lin, Y., Dong, G. J., Liu, C. N., Zhang, L. F., Zhang, Z., Cai, J. J., and Zhang, H., 2008. Deri- ving the probabilities of water loss and ammonia loss for ami- no acids from tandem mass spectra., 7 (1): 202-208.
Tang, W., Lin, L., Xie, J., Wang, Z., Wang, H., and Dong, Y., 2016. Effect of ultrasonic treatment on the physicochemical proper- ties and antioxidant activities of polysaccharide from., 151: 305-312.
Wang, B., Li, L., Chi, C., Ma, J., Luo, H., and Xu, Y., 2013. Pu- rification and characterisation of a novel antioxidant peptide derived from blue mussel () protein hydrolysate., 138 (2-3): 1713-1719.
Wang, L., Ding, L., Yu, Z., Zhang, T., Ma, S., and Liu, J., 2016. Intracellular ROS scavenging and antioxidant enzyme regu- lating capacities of corn gluten meal-derived antioxidant pep- tides in HepG2 cells., 90: 33-41.
Wu, H., Jin, W., Sun, S., Li, X., and Zhu, B., 2015. Identifica- tion of antioxidant peptides from protein hydrolysates of scal- lop () female gonads., 242: 713-722.
Wu, M., Tong, C., Wu, Y., Liu, S., and Li, W., 2016. A novel thy- roglobulin-binding lectin from the brown algaand its antioxidant activities., 201: 7-13.
Xu, Y., Cai, F., Yu, Z., Zhang, L., Li, X., Yang, Y., and Liu, G., 2016. Optimisation of pressurised water extraction of polysa- ccharides from blackcurrant and its antioxidant activity., 194: 650-658.
Yang, S., Tang, Z., Tang, S., Zhang, T., Tang, F., Wu, Y., Wang, Y., Wang, L., and Liu, G., 2016. Purification and characterization of an antioxidant protein from fertilized eggs., 36: 791-798.
Yu, J., Hu, Y., Xue, M., Dun, Y., and Zhao, S., 2016. Purification and identification of antioxidant peptides from enzymatic hy- drolysate of spirulina platensis., 26 (7): 1216-1223.
Zhou, A., Feng, Z., Feng, Y., Liu, X., Xin, L., and Chen, Y., 2014. Research of antioxidant peptides produced from the waste of white shrimp ()., 14: 85-91.
Zhuang, Y., Sun, L. P., Zhao, X., Hou, H., and Li, B., 2010. In- vestigation of gelatin polypeptides of jellyfish () for their antioxidant activity., 48 (2): 222-228.
Zorov, D. B., Filburn, C. R., Klotz, L. O., Zweier, J. L., and Sol- lott, S. J., 2000. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes., 192 (7): 1001-1014.
April 3, 2020;
May 26, 2020;
November 25, 2020
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
. E-mail: chenshengjun@scsfri.ac.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Characteristics of Atmospheric Rivers over the East Asia in Middle Summers from 2001 to 2016
- Facile Synthesis of Fe/Cr-Codoped ZnO Nanoparticles with Excellent Adsorption Performance for Various Pollutants
- Experimental Investigation of Wave Load and Run-up on the Composite Bucket Foundation Influenced by Regular Waves
- Development of a Microfluidics-Based Quantitative Real-Time PCR to Rapidly Identify Photobacterium damselae subsp. damselae with Different Pathogenicity by Detecting the Presence of mcp or dly Gene
- Molecular Cloning, Expression and Characterization of Peroxisome Proliferators-Activated Receptors Gamma in the Sea Urchin (Strongylocentrotus intermedius)
- Otolith Shape Analysis as a Tool to Identify Two Pacific Saury (Cololabis saira) Groups from a Mixed Stock in the High-Seas Fishing Ground
