Facile Synthesis of Fe/Cr-Codoped ZnO Nanoparticles with Excellent Adsorption Performance for Various Pollutants
2021-03-06SUNYongkaiLUOSiqiXINGJingLIZhenjiangandMENGAlan
SUN Yongkai, LUO Siqi, XING Jing, LI Zhenjiang, , and MENG Alan
Facile Synthesis of Fe/Cr-Codoped ZnO Nanoparticles with Excellent Adsorption Performance for Various Pollutants
SUN Yongkai1), LUO Siqi1), XING Jing2), LI Zhenjiang1),*, and MENG Alan3),*
1)College of Electromechanical Engineering, Qingdao University of Science and Technology, Qingdao 266061, China 2) Gaomi Campus, Qingdao University of Science and Technology, Gaomi 266000, China 3) Key Laboratory of Sensor Analysis of Tumor Marker, Ministry of Education, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, China
Adsorption is an effective and low-cost method for removing antibiotics and dyes. However, it remains a challenge to prepare an adsorbent with excellent adsorption properties for both various antibiotics and dyes. Herein, Fe/Cr-codoped ZnO with high adsorption capacity and fast adsorption rate are prepared by an environmentally friendly solvothermal method. Fe/Cr-codope ZnO with a diameter of 10–20nm exhibits superior adsorption capacities for the antibiotics of tetracycline hydrochloride (TC-HCl) (826.45mgg−1) and tetracycline (TC) (331.01mgg−1), and anionic dyes of methyl orange (MO) (1023.88mgg−1), methyl blue (MB) (726.26mgg−1) and direct red (DR) (642.25mgg−1). Meanwhile, it presents fast adsorption rate, it only took 30min to reach more than 90% of the equilibrium adsorption amount for TC-HCl, TC, MO, MB and DR.The adsorption process closely fitted the Langmuir isotherm model and pseudo second-order rate equation. More importantly, simple method has been developed for separating the pollutant from the adsorbent, which not only regenerates the materials, but also completes the recovery of antibiotics and dyes, avoiding the secondary pollution. The broad-spectrum, rapid, environment-friendly and effective adsorption properties make Fe/Cr- codoped ZnO a promising adsorbent for water treatments.
antibiotics; dyes; codoped; adsorption; regeneration
1 Introduction
In recent years, antibiotics are widely used in the treatment of many diseases as well as the production of animal feed. Coincidentally, dyes are also widely applied to many industrial fields (Yao., 2016; Shen., 2018; Zou., 2020). In the past decades, with the widespread utilization and rapid depletion of antibiotics and dyes, a large amount of them have been discharged into the environmental system in the form of waste water or feces. Antibiotics (Zhou., 2013; Zhu., 2013; Li., 2020) and dyes entering the environmental system can bring serious threats to the survival of plants and animals even to human health (Das., 2013; Okesola., 2016; Hu., 2018) and will cause water pollution problems on a massive scale. Therefore, the severe environment pollution and the terrible health threats from antibiotics and dyes effluent will further aggravate the urgent need of developing a highly efficient, environmentally friendly, and cost-effective novel functional material for wastewater treatment.
Accordingly, various wastewater treatment technologies techniques have been proposed, such as adsorption, biodegradation,ion exchange,membrane separation, coagulation and photocatalysis,(Li., 2013; Ge., 2016; Huang., 2017; Lo., 2017; Li., 2018; Qi., 2018). However, due to the wide range and refractory properties of antibiotics and dyes, most of the water treatment methods are not ideal for the wastewater containing antibiotics and dyes. And it is noteworthy that adsorption has been proven to be a well-established and economical approach for the wastewater treatment thanks to its low cost, short equilibrium time and simple operation (Ge., 2016; Chen., 2018; Huang., 2018).
The key problem for the application of the adsorption method is to synthesize adsorbent with high adsorption property (Peng., 2014; Chen., 2016; Zhang., 2018). Different types of adsorbents are developed to remove pollutants from wastewater, such as carbon materials (Perreault., 2015; Musielak., 2019), porous materials (Wu., 2012; Yang., 2014; Wang., 2015), biomaterials, polymers materials (Roosen., 2014; Zheng., 2014; Rekha., 2016; Zhao., 2017). However, practically, those methods are still unable to meet the demand due to their low adsorption capacity, long adsorption time, or second pollution. It is necessary to carry out further research and develop advanced adsorbents with high adsorption capacity and a rapid adsorption rate for antibiotics and organic dyes. Inorganic non-metallic materials have good oxidation resistance, wear resistance and corrosion resistance. They have been attracted widely attention of researchers and are often used as adsorption materials. Although the inorganic nonmetallic material adsorbent has good development prospects, the silica powder is toxic and has potential damage to the human health. Additionally, the preparation technology of carbon-based inorganic non- metallic adsorbent is complex and low yield, so it cannot be widely used in wastewater treatment. Zinc oxide has been widely used in many fields such as light, electricity and catalysis (Izaki., 2013; Zhang., 2014; Hsu., 2017)because of its low cost, stable property and environmental friendliness, but rarely apply to the field of water treatment due to its low adsorption capacity and long equilibrium times. Recently, the pure zinc oxide were modified to overcome its inherent defects, such as doping, (Li., 2008; Song., 2011) making it has practical application value in the field of adsorption. For example,Xiangning Zheng and his colleagues synthesized the magnetic ZnO clay nanocomposite hydrogel with good adsorption property for La (III) (58.8mgg−1) (Zheng., 2014; Perreault., 2015). Despite of the good achievement they have made, it is still meaningful for obtaining ZnO modified by co-doping two metal ions to acquire the excellent adsorption performance, especially, it could adsorb on the various the antibiotics and dyes meanwhile. In addition, the selection of doped elements should meet the following two requirements. First of all, the doped elements can make the adsorbent surface with more positive charge; in the second place, the doped elements should have ionic radius close to that of zinc. Based on the above considerations, we then choose Fe and Cr with higher valence state as the doped elements in this work.
Herein, a mild one-step-solvothermal method was exploited to prepare Fe/Cr-codoped ZnO NPs with splendid adsorption capacity for the various antibiotisc and dyes. Meanwhile, a range of experiments were executed to investigate various factors affecting the adsorbability of adsorbent, such as pH of solution, initial concentration and contact time. Kinetics and isotherms model manifested that the as-prepared sample takes on the fast adsorption rate as well as high adsorption capacity of 826.45mgg−1for tetracycline hydrochloride (TC-HCl), 331.01mgg−1for tetracycline (TC), 1023.88mgg−1for methyl orange (MO), 726.26mgg−1for methyl blue (MB) and 642.25mgg−1for direct red (DR), respectively. The proposed adsorption mechanism reasonably explains the effective removal of the various antibiotics and anion pollutants by Fe/Cr-codoped ZnO NPs. What’s more, the regeneration channel of the adsorbent was acquired, ma- king the pollutant and adsorbent can be separated simply and efficiently.
2 Materials and Methods
2.1 Chemicals and Instruments
The Zn(NO3)2·6H2O, Cr(NO3)3·9H2O, Fe(NO3)3·9H2O, NaOH and ethanol absolute were all purchased from Sinopharm Chemical Reagent Co., Ltd., (China). All the chemicals are analytical grade and without further treatment.
Absorbance spectra of pollutants were measured using a UV-vis spectrophotometer (Perkin Elemr, Lambda 35, USA). The morphology of the as-prepared products was acquired using scanning electron microscope (SEM, JSM- 6460LV) and transmission electron microscope (TEM, JEM-2100). Crystalline structure of adsorbents was characterized using X-ray diffraction (XRD, D/MAX-2500/ PC, CuKa). X-ray Photoelectron Spectroscopy (XPS) pat- tern was conducted on Thermoelectron spectroscopy (ESCALAB 250, Al Ka=1486.6eV, approximately 5× 10−9torr).
2.2 Synthesis of Fe/Cr-Codoped ZnO NPs
Firstly, the Fe/Cr-codoped ZnO NP was synthesized by a solvothermal method in a Teflon-lined stainless autoclave. Specifically, 2.0mmol of Zn(NO3)2·6H2O, 0.5950g Cr(NO3)3·9H2O and a certain amount of Fe(NO3)3·6H2O were absolutely dissolved in 40mL ethanol absolute at room temperature, and the solution was stirred for 30 min. Then, 4.0molL−1NaOH solution (40mL) was dropped into the above solution. After stirring for 1h at room temperature, the mixture was transferred into autoclave, heated 12h at 120℃in an oven. After natural cooled to room temperature, the products were centrifuged, washed, and freeze-dried for 8h.
2.3 Adsorption Experiments
Adsorption experiments of typically antibiotics and dyes wastewater (TC-HCl, TC, MO, MB and DR) were carried out in batch adsorption experiments at room temperature (25℃). In the single wastewater system, 10mg of Fe/Cr-codoped ZnO was added into 30mL of solution containingantibiotics (TC-HCl and TC) or dyes (MO, MB and DR) with different initial concentrations (the initial concentrations of TC-HCl, MO, MB and DR is 100, 200, 300, 400, 600, 800 and 1000mgL−1, respectively, and the initial concentrations of TC is 50, 75, 100, 150, 200, 250, 300mgL−1) at room temperature, and the pH values of the initial solution were adjusted from 6.0 to 11.0 using 0.1molL−1HCl and 0.1molL−1NaOH solution. In the adsorbing process, 3mL of wastewater sample was taken out at definite time up to 12h and then centrifuge. Concentration of the remaining contaminant solution was determined using a UV-vis spectrophotometer.
The adsorption capacities at any timeq(mgg−1), and the adsorption capacities at equilibrium q(mgg−1) of the contaminant on Fe/Cr-codoped ZnO NPs can be calculated using the following Eqs. (1)–(2).
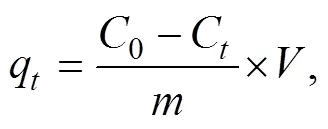
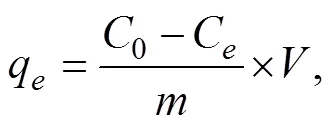
where0andCare the initial and equilibrium concentrations of contaminant in the solution (mgL−1), respectively,qis the quantity of adsorbed contaminant on Fe/Cr- codoped ZnO NPs adsorbent at equilibrium (mgg−1),is the volume of the contaminant solution (L), andis the mass of Fe/Cr-codoped ZnO NPs sample (g). All the adsorption tests were carried out in triplicates, and the data in this paper were the average measurements.
2.4 Regeneration of Fe/Cr-Codoped ZnO NPs
Firstly, 20mL 1molL−1HNO3was added into a beaker containing 0.8g of Fe/Cr-codoped ZnO NPs absorbed contaminant drop by drop until the particles were completely dissolved; secondly, 1molL−1NaOH was added into the above solution until the pH is 8, forming a hydroxide flocculent precipitate; thirdly, the precipitate was obtained by centrifugation; fourthly, the precipitate obtained by centrifugation was washed multiple times, and then dried at 60℃ for 8h; finally, it was calcined at 180℃ for 2h.
3 Results and Discussion
3.1 Material Characterization
XRD measurements were performed to investigate the crystal structure of the samples. XRD pattern of ZnO and Fe/Cr-codoped ZnO in Fig.1 showed similar pattern, and several peaks corresponding crystal plane of (100), (002), (101), (102), (110), (103), (200), (112), (201), respectively, which is consistent with wurtzite-phase ZnO crystal (JCPDS No. 36-1451) (Li., 2018). Moreover, there was no other peaks appeared in XRD pattern of Fe/ Cr-codoped ZnO NPs, indicating that no second phase products were produced after Fe and Cr were doped in ZnO. In addition, it can be seen from the inset of Fig.1 that the peaks of the doped sample deviates slightly to a large angle, suggesting that the doped elements of the small size enter into the zinc oxide lattice to replace some zinc.
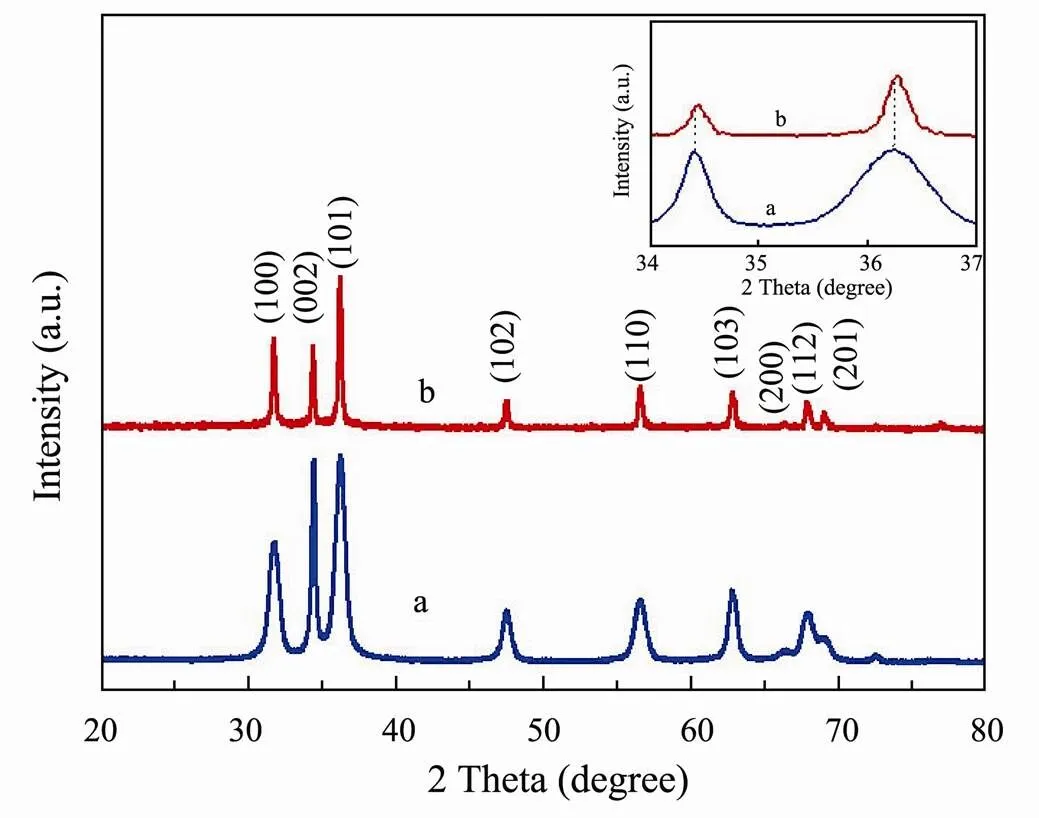
Fig.1 XRD patterns of ZnO (a), Fe/Cr-codoped ZnO (b) NPs and the shift of corresponding diffraction peaks of ZnO and Fe/Cr-codoped ZnO NPs (the inset).
The morphologies of Fe/Cr-codoped ZnO nanoparticles were investigated through SEM and TEM (Fig.2). As can be seen from SEM images (Figs.2A and B), the as-prepared Fe/Cr-codoped ZnO is nanoparticle with a relatively uniform size, whose diameter is approximately 10–20nm. Fig.2C shows TEM image, indicating that Fe/Cr-codoped ZnO has a homogenous particle size with good dispersion. As shown in Fig.2D, the size of the particles is between 10–20nm. The inset of Fig.2D shows HRTEM (Chen., 2000) pattern of the Fe/Cr-codoped ZnO, and the lattice stripes of the nanoparticles are clear- ly visible, indicating that the as-prepared sample has good crystallinity, and the spacing of 0.2800nm correspond to the (100) planes of wurtzite-phase ZnO crystals, which was consistent with the result of XRD.

Fig.2 Typical SEM (A, B), TEM (C, D) images of Fe/Cr-codoped ZnO NPs.
To furtherly investigate the chemical composition and valence state of the composites, X-ray photoelectron spectroscopy (XPS) analysis of Fe/Cr-codoped ZnO na- noparticles was carried out. The XPS survey spectrum (Fig.3A) clearly indicates that there are peaks of Cr, Fe, Zn, and O elements in Fe/Cr-codoped ZnO. Figs.3B–D shows the high-resolution XPS spectrum of Cr 2p, Fe 2p, and Zn 2p regions, respectively. In Fig.3B, two peaks with binding energies of 577.2 and 586.4eV are assigned to Cr 2p3/2and Cr 2p1/2, respectively, which correspond to Cr3+. In Fig.3C,the high-resolution Fe 2p spectra show two broad peaks of Fe 2p1/2and Fe 2p3/2with binding energies of 725.3 and 711.5eV, respectively, corresponding to Fe3+. The peak at 717.4eV is attributed to the shakeup satellite peak (denoted as ‘Sat.’) of Fe3+(Grosvenor., 2004). The results of XPS can further indicated that Cr3+and Fe3+entered into the lattices of ZnO and substituted the positions Zn2+, which are in accordance with the result of XRD. Peaks at 1022.0 and 1045.1eV, as shown in Fig. 3D, corresponding to Zn 2p3/2and Zn 2p3/2, respectively, can be assigned to the Zn2+species in the ZnO nanomaterial (Song., 2016).

Fig.3 XPS spectra of the Fe/Cr-codoped ZnO NPs. XPS survey spectrum (A); High-resolution XPS spectra of Cr 2p (B); Fe 2p (C) and Zn 2p (D).
3.2 The Effect of Adsorption Conditions on Adsorption Property
To achieve optimistic performance, the effect of adsor- ption conditions, such as pH values of solution, the initial concentration of pollutants and contact time, on adsorption property were systematically examined.
3.2.1 The effect of pH
The pH of the solution is an important parameter influencing the adsorption capacity of adsorbents. Hence,the effect of pH on the adsorption capacity of pollutants (TC-HCl, TC, MO, MB and DR) over Fe/Cr-codoped ZnOare shown in Fig.4. Fig.4(A) displays the adsorption capacity of Fe/Cr-codoped ZnO under different pH conditions. It can be observed that the adsorption capacity of Fe/Cr-codoped ZnO is increased first and then decreased with the pH value changing in the range of 6–11, reaching the maximum value at pH=7. The characterization results in Fig.4(B) explain the reasons for this phenomenon. The effect of pH on adsorbent performance is carried out by affecting the surface zeta potential of the adsorbent. The zeta potential of Fe/Cr-codoped ZnO is positive in the range of pH6–11. The surface of the adsorbent has the greatest positive charge when the pH is 7, so the maximum adsorption capacity was reached at under this condition.
3.2.2 The effect of initial concentration
Different initial concentration of the pollutant may have important effects on the adsorption capacity of the adsorbent. Fig.5A shows the effect of the pollutant initial concentration on adsorption capacity of adsorbent. The curve in the Fig.5A shows that the adsorption capacity of adsorbent in equilibrium gradually increases and finally tends to be stable with the pollutant initial concentration increasing. Specifically, when the initial concentration is within the range of 100–400mgL−1for TC-HCl, MO, MB or DR, the adsorption capacity of adsorbent increases rapidly. While the adsorption capacity changes slowly, when the initial concentration increases within the range of 400–800mgL−1. Similarly, when the initial concentration is within the range of 50–200mgL−1for TC, the adsorption capacity of adsorbent increases rapidly. However, when the initial concentration increases within the range of 200–300mgL−1, the adsorption capacity changes slowly. The reason may be that the increase of the driving force molecules motion with the increase of the pollutant concentration, and then, increasing the chances of collision between pollutant molecules and the active sites on the surface of adsorbent. However, when the initial concentration increasesto a certain degree, the available active sites on the adsorbent surface decrease, reducing the rate of collision between the pollutant molecules and the active sites on the surface of the adsorbent, so the adsorption capacity increases slowly. Finally, the adsorbent reaches adsorption saturation. As a result, the concentration of the pollutants continues to increase but the adsorption capacity of adsorbent is no longer increased.

Fig.4 The adsorption capacity (A) and zeta potential (B) of Fe/Cr-codoped ZnO under different pH conditions.

Fig.5 The effect of the initial concentration (A) and contact time profiles (B) of pollutants adsorption on the Fe/Cr-codoped ZnO.
3.2.3 The effect of contact time
Equilibrium time is one of the most important indexes for evaluating the adsorption performance of adsorbent in wastewater treatment systems. In the process of adsorption, the adsorption capacity goes through three stages, from rapid increase to slow increase and finally to equilibrium in Fig.5B. At the first 30min, the adsorption reaction was rapid proceeding, then the process slowed down, and finally the adsorption equilibrium was reached within120min for TC-HCl, MO, MB or DR. Similarly, for TC, during the first 10min of the reaction, the adsorption increased rapidly, then slowly, and finally reached adsorption equilibrium within 60min. The explanation for this phenomenon is as follows: at the initial stage of adsorption, a large number of active sites are available on the surface of adsorbent, so adsorbent can quickly absorb pollutant molecules. Later on, as the adsorption progre- ssed, the available active sites on the surface of adsorbent gradually decrease, thus adsorption becomes slow. Finally, all the active sites were occupied, so the adsorbent no longer adsorbed pollutant molecules, and the adsorbent reached adsorption saturation. Fe/Cr-codoped ZnO will have a promising application prospect in waste-water treatment field due to its fast adsorption rate and large adsorption capacity.
3.3 Adsorption Kinetics
Fig.5B indicates the adsorption rate of Fe/Cr-codoped ZnO for TC-HCl, TC, MO, MB and DR are rapid in the initial short time, then gradually slows. To better clarify the adsorption behavior, the adsorption kinetics of Fe/Cr- codoped ZnO are further evaluated by the following adsorption kinetic equations.
Pseudo first-order equation:

Pseudo second-order equation:

whereinqandqare the adsorption capacity (mgg−1) at timeand the equilibrium, respectively; and1(min−1) and2(g (mgmin)−1) are the adsorption rate constant.
To analyze the adsorption process of Fe/Cr-codoped ZnO, pseudo-first-order (PFO) and pseudo-second-order (PSO) kinetic models were used to fit the experimental data. The results of the kinetic model simulation were shown in Fig.6 and Fig.7, and the relative parameters were tabulated in Table 1 and Table 2. In Fig.6(A), the experimental data of the investigated several adsorbents can be well fitted to the pseudo-second-order kinetic model, and2(correlation coefficient) of PSO for five pollutants are all exceeds 0.9999 (Table 1), indicating that chemisorption is the rate-determining step in the adsorption process (Li., 2019).

Fig.6 Pseudo-second-order adsorption kinetics of pollutants (A) and adsorption isotherm of TC-HCl (B), TC (C), MO (D), MB (E) and DR (F) on Fe/Cr-codoped ZnO.

Table 1 Adsorption kinetic parameters of pollutantson Fe/Cr-codoped ZnO (25℃, pH=7.0)

Table 2 Adsorption kinetic parameters of pollutantson Fe/Cr-codoped ZnO (25℃, pH=7.0)
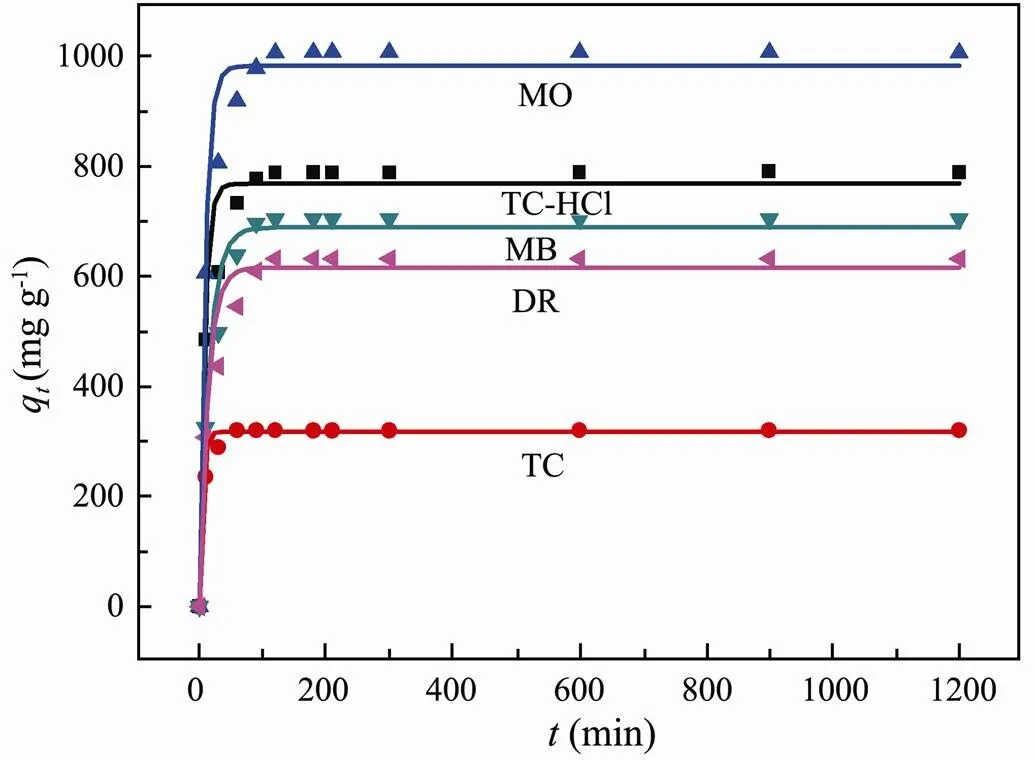
Fig.7 Pseudo-first-order adsorption kinetics of pollutants on Fe/Cr-codoped ZnO.
3.4 Adsorption Isotherms
To quantitatively analyze the adsorption behaviors and mechanism of Fe/Cr-codoped ZnO adsorbent, the adsorptiondata of the adsorbent over the pollutants are fitted by Langmuir and Freundlich isotherm models (Fig.6(B–F)). The Langmuir and Freundlich isotherm model equations are

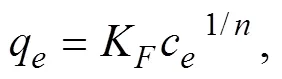
wherecis the concentration (mgL−1) of the pollutant solution when the adsorption reaches equilibrium;qis the equilibrium adsorption capacity (mgg−1);qis the maximum adsorption capacity (mgg−1);Kis the Langmuir isothermal constant (Lmg−1);Kis the Freundlich isothermal constant (mgg−1) (L mg)1/n; andis the factor relating to the adsorption intensity.
The Langmuir isotherm model supposes that adsorption occurs on a uniform surface with homogeneous active sites through monolayer adsorption. Hence, it can reach saturation. Whereas, the Freundlich isotherm adsorption model assumes that multilayer adsorption occurs on heterogeneous surfaces.The isotherms of TC-HCl, TC, MO, MB and DR adsorption onto Fe/Cr-codoped ZnO are shown in Fig.6(B–F), and the relative parameters are listed in Table 3. By comparing the2of five pollutants, it is indicated that Langmuir model has better relativity than Freundlich model. This suggests that the adsorption behavior occurs in the form of monolayer on the heterogeneous surfaces of Fe/Cr-codoped ZnO nanoparticles. Furthermore, the saturated adsorption capacity of Fe/Cr- codoped ZnO for various anionic organic pollutants were obtained, which are 826.45mgg−1for TC-HCl, 331.01mgg−1for TC, 1023.88mgg−1for MO, 726.26mgg−1for MB, 642.25mgg−1for DR, respectively. These data are close to the actual adsorption capacity calculated by Eq. (2) (806.37mgg−1for TC-HCl, 320.56mgg−1for TC, 1008.56mgg−1for MO, 706.18mgg−1for MB, 632.68mgg−1for DR, respectively). These reported results were significantly higher than those of many reported adsorbents (Table 4).
In order to show the effect of Fe/Cr-codoped on improving ZnO adsorption capacity for the various organic pollutants, the comparison of adsorption capacity on ZnO and Fe/Cr-codoped ZnO for the various organic pollutants were presented in Table5.It can be seen that the adsorption capacity of Fe/Cr-codoped ZnO is significantly improved compared with that of ZnO.
Based on the above characterization and experimental results, an adsorption mechanism of Fe/Cr-codoped ZnO for the anionic organic pollutants was proposed, as shown in Fig.8. When Cr3+enters into ZnO lattice to replace Zn2+, the surface of Cr-doped ZnO will have positive charges. However, our previous study showed that when excessive Cr3+was added, a secondary phase (ZnCrO4) would be formed, leading the adsorption capacity decreased. So there is a limit on the doped amount of Cr3+. Therefore, the second metal ionic Fe3+was selected as the dopant to doped ZnO. On the basis of Cr3+doping, some Fe3+entered into the lattice instead of Zn2+(as shown in XPS), making the surface of Fe/Cr-codoped ZnO have more positive charges, and there’s no secondary phase (as shown in XRD) after adding a moderate amount of Fe3+. More positive charge on the surface of dopant would increase the adsorption capacity of the adsorbent for anionic organic pollutant.

Table 3 Fitting parameters of Langmuir and Freundlich adsorption isotherms for pollutants adsorption (25℃, pH=7.0)

Table 4 Comparison of the adsorption capacity for the various adsorbents

Table 5 Comparison of the adsorption capacity on ZnO and Fe/Cr-codoped ZnO for the various anionic organic pollutants

Fig.8 Schematic diagram of the adsorption mechanism of Fe/Cr-codoped ZnO NPs for anionic organic pollutant.
The above analysis was confirmed by the results of pH and Zeta potential affecting on the adsorption capacity. It can be seen from the Zeta potential characterization that the surface of Fe/Cr-codoped ZnO has positive charge when pH is within the range of 6–11 in Fig.4. Besides, according to the effect of pH on the adsorption capacity of five pollutants, the more positive charge on the surface of the absorbent, the greater the adsorption capacity is (For example, when pH=7, Zeta potential is the highest, and the adsorption capacity is also the largest.), indicating that electrostatic interaction is the main adsorption approach. In Fig.8, MO is the representative of three dyes, and TC is the representative of two antibiotics in this work. So, based on the above analysis, Fe/Cr-codoped ZnO NPs can efficient remove anionic organic pollutant by electrostatic interaction.
3.5 Desorption of Pollutant
In practical application, it is very importance for adsorbent to be simple, efficient and non-polluting desorbed and regenerated. In this paper, the separation of adsorbent and pollutant was realized by acid dissolution, alkali precipitation, and finally calcining. Because Fe/Cr-codoped ZnO is soluble in strong acid, it can be dissolved by add- ing HNO3, and iron, chromium and zinc will exist in the solution as the ions. When the appropriate amount of NaOH is added to the above solution, the Zn2+, Fe3+, and Cr3+will form hydroxide precipitation. As we all know, the Ksp(Zn(OH)2)=1.2×10−17,Ksp(Cr(OH)3)=6.3×10−31,andKsp(Fe(OH)3)= 4×10−38, so when the pH reaches 8, the Zn2+, Fe3+, and Cr3+in the solution can be completely precipitated. Then, the adsorbent is separated from the contaminant by centrifugation. Finally, the hydroxide precipitates are calcined to obtain their corresponding oxides. The product was characterized by XRD (shown in Fig.9), and it was proved to be ZnO. There is no peak of iron and chromium, which may due to low levels of iron and chromium.

Fig.9 The XRD pattern of the product obtained after the process desorption.
This strategy is as follows:

The as-obtained ZnO can be used in other industrial production or synthesis, and the separated pollutants can also be used in industry. Therefore, the whole process will not produce secondary pollution. This kind of adsorbent, which has excellent adsorption performance for anionic organic pollutant and can be reused through simple processing, will become one of the indispensable adsorbents.
4 Conclusions
In summary, nano-sized Fe/Cr-codoped ZnO particles with excellent adsorption performance for the various anionic organic pollutants have been successfully synthesized. The surface of Fe/Cr-codoped ZnO has a large amount of positive charges, which makes it has excellent adsorption performance to the anionic pollutants. The adsorption capacities of the as-prepared adsorbent are up to 826.45, 331.01mgg−1for the antibiotics of TC-HCl and TC, and 1023.88, 726.26 and 642.25mgg−1for anionic dyes of MO, MB and DR, respectively. The adsorption of various anionic organic pollutants on Fe/Cr- codoped ZnO NPs was fast, it only took 30min to reach more than 90% of the equilibrium adsorption amount for TC-HCl, MO, MB and DR, and for TC, it can exceed 90% of its equilibrium adsorption capacity within 10min. The results of the kinetics study showed that the adsorption of the various anionic organic pollutants followed pseudo-second-order model, and the adsorption process agreed well to Langmuir isotherm. Most importantly, the desorption of pollutant from the surface of adsorbent was realized by a simple and economic methods. From the above, Fe/Cr-codoped ZnO is an adsorbent with extensive application potential in the field of efficient wastewater treatment.
Acknowledgements
The work was supported by the National Natural Science Foundation of China(Nos.51672144, 51572137, 51702181), the Natural Science Foundation of Shandong Province (Nos. ZR2017BB013, ZR2019BEM042), the Higher Educational Science and Technology Program of Shandong Province (Nos. J17KA014, J18KA001, J18KA033), the Taishan Scholars Program of Shandong Province (No. ts201511034) and the Overseas Taishan Scholars Program. We express our grateful thanks to them for their financial support.
Cao, J., Yang, Z. H., Xiong, W. P., Zhou, Y. Y., Peng, Y. R., Li, X., Zhou, C. Y., Xu, R., and Zhang, Y. R., 2018. One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis., 353: 126- 137.
Chen, B., Zhao, H. N., Chen, S. J., Long, F. X., Huang, B., Yang, B. Q., and Pan, X. J., 2019. A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater., 356: 69-80.
Chen, D. Z., Shen, W. S., Wu, S. L., Chen, C. Q., Luo, X. B., and Guo, L., 2016. Ion exchange induced removal of Pb(II) by MOF-derived magnetic inorganic sorbents., 8: 7172-7179.
Chen, L., Chen, N. N., Wu, H. M., Li, W. X., Fang, Z., Xu, Z. W., and Qian, X. M., 2018. Flexible design of carbon nanotubes grown on carbon nanofibers by PECVD for enhanced Cr(VI) adsorption capacity., 207: 406-415.
Chen, X. L., Cao, Y. G., Lan, Y. C., Xu, X. P., Li, J. Q., Lu, K. Q., Jiang, P. Z., Xu, T., Bai, Z. G., Yu, Y. D., and Liang, J. K., 2000. Synthesis and structure of nanocrystal-assembled bulk GaN., 209: 208-212.
Fronczak, M., Krajewska, M., Demby, K., and Bystrzejewski, M., 2017. Extraordinary adsorption of methyl blue onto sodium-doped graphitic carbon nitride., 121: 15756-15766.
Gan, Q. Y., Shi, W. L., Xing, Y. J., and Hou, Y., 2018. A polyoxoniobate/g-C3N4nanoporous material with high adsorption capacity of methylene blue from aqueous solution., 6: 7, https://doi.org/10.3389/fchem.2018.00007.
Ge, X., Song, X. Y., Ma, Y., Zhou, H. J., Wang, G. Z., Zhang, H. M., Zhang, Y. X., Zhao, H. J., and Wong, P. K., 2016. Fabrication of hierarchical iron-containing MnO2hollow microspheres assembled by thickness-tunable nanosheets for efficient phosphate removal., 4: 14814-14826.
Grosvenor, A. P., Kobe, B. A., Biesinger, M. C., and Mclntyre, N. S., 2004. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds., 36: 1564-1574.
Haque, E., Lo, V., Minett, A. I., Harris, A. T., and Church, T. L., 2014. Dichotomous adsorption behaviour of dyes on an amino-functionalised metal-organic framework, amino-MIL- 101 (Al)., 2: 193-203.
Hsu, C. L., Lin, Y. H., Wang, L. K., Hsueh, T. J., Chang, S. P., and Chang, S. J., 2017. Tunable UV- and visible-light photoresponse based on p-ZnO nanostructures/n-ZnO/glass peppered with Au nanoparticles., 9 (17): 14935-14944.
Huang, J. N., Li, Y. H., Cao, Y. H., Peng, F., Cao, Y. G., Shao, Q., Liu, H., and Guo, Z. H., 2018. Hexavalent chromium removal over magnetic carbon nanoadsorbents: Synergistic effect of fluorine and nitrogen co-doping., 6: 13062-13074.
Huang, R. Y., Wu, M. J., Zhang, T., Li, D. Q., Tang, P. G., and Feng, Y. J., 2017. Template-free synthesis of large-pore-size porous magnesium silicate hierarchical nanostructures for high-efficiency removal of heavy metal ions., 5: 2774-2780.
Hu, X. S., Liang, R., and Sun, G. X., 2018. Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution.,6: 17612-17624.
Izaki, M., Chizaki, R., Saito, T., Murata, K., and Shinagawa, T., 2013. Hybrid ZnO/phthalocyanine photovoltaic device with highly resistive ZnO intermediate layer., 5 (19): 9386-9395.
Jin, J. H., Yang, Z. H., Xiong, W. P., Zhou, Y. Y., Xu, R., Zhang, Y. R., Cao, J., Li, X., and Zhou, C. Y., 2019. Cu and Co nanoparticles co-doped MIL-101 as a novel adsorbent for efficient removal of tetracycline from aqueous solutions., 650: 408-418.
Kang, D. J., Hu, C. Q., and Zhu, Q. S., 2018. Morphology controlled synthesis of hierarchical structured Fe2O3from natural ilmenite and its high performance for dyes adsorption.,459: 327-335.
Kasperiski, F. M., Lima, E. C., Reis, G. S. D., Costa, J. B. D., Dotto, G. L., Dias, S. L. P., Cunha, M. R., Pavan, F. A., and Correa, C. S., 2018. Preparation of CTAB-functionalized aqai stalk and its efficient application as adsorbent for the removal of Direct Blue 15 and Direct Red 23 dyes from aqueous media., 205: 1520- 1536.
Li, C. R., Zhuang, Z. Y., Huang, F., Wu, Z. C., Hong, Y. P., and Lin, Z., 2013. Recycling rare earth elements from industrial wastewater with flowerlike nano-Mg(OH)2., 5: 9719-9725.
Li, H., Bao, H. Q., Song, B., Wang, W. J., and Chen, X. L., 2008. Observation of ferromagnetic ordering in Ni-doped AlN polycrystalline powders., 148: 406- 409.
Li, M. R., Wei, D., Liu, T., Liu, Y. R., Yan, L. G., Wei, Q., Du, B., and Xu, W. Y., 2019. EDTA functionalized magnetic biochar for Pb(II) removal: Adsorption performance, mechanism and SVM model prediction., 227: 115696.
Li, S. J., Chen, J. L., Hu, S. W., Jiang, W., Liu, Y. P., and Liu, J. S., 2020. A novel 3D Z-scheme heterojunction photocatalyst: Ag6Si2O7anchored on flower-like Bi2WO6and its excellent photocatalytic performance for the degradation of toxic phar- maceutical antibiotics., 7: 529- 541.
Li, T., Ma, S., Yang, H., and Xu, Z. L., 2019. Preparation of carbonized MOF/MgCl2hybrid products as dye adsorbent and supercapacitor: Morphology evolution and Mg salt effect., 58 (4): 1601-1602, DOI: 10. 1021/acs.iecr.8b06437.
Li, X., Liu, S. W., Fan, K., Liu, Z. Q., Song, B., and Yu, J. G., 2018. MOF-based transparent passivation layer modified ZnO nanorod arrays for enhanced photoelectrochemical water spli- tting., 8: 1800101.
Li, Z. J., Sun, Y. K., Xing, J., Xing, Y. C., and Meng, A. L., 2018. One step synthesis of Co/Cr-codoped ZnO nanoparticle with superb adsorption properties for various anionic organic pollutants and its regeneration., 352: 204-214.
Li, Z. J., Xing, Y. C., Fan, X. Y., Lin, L. G., Meng, A. L., and Li, Q. D., 2018. rGO/protonated gC3N4hybrid membranes fabricated by photocatalytic reduction for the enhanced water desalination., 443: 130-136.
Livani, M. J., and Ghorbani, M., 2018. Fabrication of NiFe2O4magnetic nanoparticles loaded on activated carbon as novel nanoadsorbent for Direct Red 31 and Direct Blue 78 adsorption., 39: 2977-2993.
Lo, K., and Irene, M., 2017. Removal of ionizable aromatic pollutants from contaminated water using nano γ-Fe2O3based magnetic cationic hydrogel: Sorptive performance, magnetic separation and reusability., 322: 195- 204.
Ma, C. C., Huang, H., Gao, X., Wang, T., Zhu, Z., Huo, P. W., Liu, Y., and Yan, Y. S., 2018. Honeycomb tubular biochar from fargesia leaves as an effective adsorbent for tetracyclines pollutants., 91: 299-308.
Musielak, M., Gagor, A., Zawisza, B., Talik, E., and Sitko, R., 2019. Graphene oxide/carbon nanotube membranes for highly efficient removal of metal ions from water., 11: 28582-28590.
Okesola, B., and Smith, D., 2016. Applying low-molecular wei- ght supramolecular gelators in an environmental setting-self- assembled gels as smart materials for pollutant removal., 45: 4226-4251.
Pellicer, J. A., Rodríguez-López, M. I., Fortea, M. I., Hernandez, J. A. G., Lucas-Abellan, C., Mercader-Ros, M. T., Serrano- Martinez, A., Nunez-Delicado, E., Cosma, P., Fini, P., Franco, E., Garcia, R., Ferrandiz, M., Perez, E., and Ferrandiz, M., 2018. Removing of direct red 83:1 using α-and HP-α-CDs polymerized with epichlorohydrin: Kinetic and equilibrium studies., 149: 736-746.
Peng, Q. M., Guo, J. X., Zhang, Q. R., Xiang, J. Y., Liu, B. Z., Zhou, A. G., Liu, R. P., and Tian, Y. J., 2014. Unique lead adsorption behavior of activated hydroxyl group in two-dimen- sional titanium carbide., 136: 4113-4116.
Perreault, F., Fonseca de Faria, A., and Elimelech, M., 2015. Environmental applications of graphene-based nanomaterials., 44: 5861-5896.
Qi, C. Y., Zhao, L. Q., Lin, Y., and Wu, D. Y., 2018. Graphene oxide/chitosan sponge as a novel filtering material for the removal of dye from water., 517: 18-27.
Rekha, P., Muhammad, R., Sharma, V., Ramteke, M., and Mohanty, P., 2016. Unprecedented adsorptive removal of Cr2O72−and methyl orange by using a low surface area organosilica., 4: 17866-17874.
Roosen, J., and Binnemans, K., 2014. Adsorption and chromatographic separation of rare earths with EDTA- and DTPA- functionalized chitosan biopolymers., 2: 1530-1540.
Shah, J., Jan, M. R., Jamil, S., and Haq, A. U., 2014. Magnetic particles precipitated onto wheat husk for removal of methyl blue from aqueous solution., 96: 218-226.
Shen, X. C., Ma, S., Xia, H., Shi, Z., Mu, Y., and Liu, X. M., 2018. Cationic porous organic polymers as an excellent platform for highly efficient removal of pollutants from water., 6: 20653-20658.
Song, B., Chen, X. L., Han, J. C., Wang, G., Bao, H. Q., Zhu, K. X., Li, H., Zhang, Z. H., Wang, W. Y., Wang, W. J., Zhang, X. H., and Meng, S. H., 2011. Raman scattering and magnetizations studies of (Al, Cr)-codoped 4H-SiC., 323: 2876-2882.
Song, Q. Q., Fang, Y., Liu, Z. Y., Li, L. L., Wang, Y. R., Liang, J. L., Huang, Y., Lin, J., Hu, L., Zhang, J., and Tang, C. C., 2017. The performance of porous hexagonal BN in high adsorption capacity towards antibiotics pollutants from aqueous solution., 325: 71-79.
Song, Y. L., Wang, X. J., Zhang, X. Q., Qi, X. D., Liu, Z. G., Zhang, L. L., Zhang, Y., Wang, Y., Sui, Y., and Song, B., 2016. Colossal dielectric permittivity in (Al+Nb) co-doped rutile SnO2ceramics with low loss at room temperature., 109: 142903.
Tanhaeia, B., Ayatia, A. A., Lahtinenb, M., and Sillanpaa, M., 2015. Preparation and characterization of a novel chitosan/ Al2O3/magnetite nanoparticles composite adsorbent for kinetic, thermodynamic and isotherm studies of methyl orange adsorption., 259: 1-10.
Wang, X. Y., Cai, J. H., Zhang, Y. J., Li, L. H., Jiang, L., and Wang, C. R., 2015. Heavy metal sorption properties of magnesium titanate mesoporous nanorods., 3: 11796-11800.
Wang, Z., Guo, J., Ma, J., and Shao, L., 2015. Highly regenerable alkali-resistant magnetic nanoparticles inspired by mussels for rapid selective dye removal offer high-efficiency environmental remediation., 3: 19960-19968.
Wu, Z. X., Li, W., Webley, P. A., and Zhao, D. Y., 2012. General and controllable synthesis of novel mesoporous magnetic iron oxide@carbon encapsulates for efficient arsenic removal., 24: 485-491.
Xiong, W. P., Zeng, Z. T., Li, X., Zeng, G. M., Xiao, R., Yang, Z. H., Zhou, Y. Y., Zhang, C., Cheng, M., Hu, L., Zhou, C. Y., Qin, L., Xu, R., and Zhang, Y. R., 2018. Multi-walled carbon nanotube/amino-functionalized MIL-53 (Fe) composites: Re- markable adsorptive removal of antibiotics from aqueous solutions., 210: 1061-1069.
Yang, J., Zhang, H. W., Yu, M. H., Emmanuelawati, I., Zou, J., Yuan, Z. G., and Yu, C. Z., 2014. High-content, well-dispers- ed γ-Fe2O3nanoparticles encapsulated in macroporous silica with superior arsenic removal performance., 24: 1354-1363.
Yao, L., Zhang, L. Z., Wang, R., Chou, S. R., and Dong, Z. L., 2016. A new integrated approach for dye removal from waste- water by polyoxometalates functionalized membranes., 301: 462-470.
Yu, X. Y., Xu, R. X., Gao, C., Luo, T., Jia, Y., Liu, J. H., and Huang, X. J., 2012. Novel 3D hierarchical cotton-candy-like CuO: Surfactant-free solvothermal synthesis and application in As(III) removal., 4: 1954-1962.
Zeng, Z. T., Ye, S. J., Wu, H. P., Xiao, R., Zeng, G. M., Liang, J., Zhang, C., Yu, J. F., Fang, Y. L., and Song, B., 2019. Research on the sustainable efficacy of g-MoS2decorated biochar nanocomposites for removing tetracycline hydrochloride from antibiotic-polluted aqueous solution., 648: 206-217.
Zhang, H., Sun, J. M., Dagle, V. L., Halevi, D., Datye, A. K., and Wang, Y., 2014. Influence of ZnO facets on Pd/ZnO catalysts for methanol steam reforming., 4 (7): 2379-2386.
Zhang, L., Wang, J., Ren, X. Y., Zhang, W. T., Zhang, T. S., Liu, X. N., Du, T., and Wang, J. L., 2018. Internally extended growth of core-shell NH2-MIL-101(Al)@ZIF-8 nanoflowers for the simultaneous detection and removal of Cu(II)., 6: 21029-21038.
Zhang, S. P., Li, Y. K., Shi, C. H., Guo, F. Y., He, C. Z., Cao, Z., Hu, J., Cui, C. Z., and Liu, H. L., 2018. Induced-fit adsorption of diol-based porous organic polymers for tetracycline remo- val., 212: 937-945.
Zhao, R., Li, X., Sun, B., Li, Y. Z., Li, Y. M., Yang, R., and Wang, C., 2017. Branched polyethylenimine grafted electrospun polyacrylonitrile fiber membrane: A novel and effective adsorbent for Cr(VI) remediation in wastewater., 5: 1133-1144.
Zheng, X. N., Wu, D. B., Su, T., Bao, S., Liao, C. A., and Wang, Q. G., 2014. Magnetic nanocomposite hydrogel prepared by ZnO-initiated photopolymerization for La(III) adsorption., 6: 19840-19849.
Zhou, L. J., Ying, G. G., Liu, S., Zhang, R. Q., Lai, H. J., Chen, Z. F., and Pan, C. G., 2013. Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China., 444: 183-195.
Zhu, Y. G., Johnson, T. A., Su, J. Q., Qian, M., Guo, G. X., Stedtfeld, R. D., Hashsham, S. A., and Tiedje, J. M., 2013. Diverse and abundant antibiotic resistance genes in Chinese swine farms., 110: 3435-3440.
Zou, J., Wu, K., Wu, H. D., Guo, J., and Zhang, L. F., 2020. Synthesis of heterostructure d-MnO2/h-MoO3nanocomposite and the enhanced photodegradation activity of methyl orange in aqueous solutions.,55: 3329- 3346.
May 29, 2020;
October 20, 2020;
October 29, 2020
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
. E-mail: zhenjiangli@qust.edu.cn
E-mail: alanmengqust@163.com
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Corrosion Mechanism of 5083 Aluminum Alloy in Seawater Containing Phosphate
- In vitro Antioxidant Effects of Porphyra haitanensis Peptides on H2O2-Induced Damage in HepG2 Cells
- Molecular Characterization and Expression Analysis of SKIV Infection of Interferon-Induced Protein with Tetratricopeptide Repeats 1 (IFIT1) in Epinephelus lanceolatus
- Characteristics and Influencing Factors of the Microbial Concentration and Activity in Atmospheric Aerosols over the South China Sea
- Characteristics of Atmospheric Rivers over the East Asia in Middle Summers from 2001 to 2016
- Otolith Shape Analysis as a Tool to Identify Two Pacific Saury (Cololabis saira) Groups from a Mixed Stock in the High-Seas Fishing Ground
