Effects of Diet on the Volatile Flavor and Nutritional Ingredients of Common Octopus (Octopus vulgaris)
2021-03-05LUOQihaoWANGWeijunLIZanZHUXinghuaWANGXueZHANGTonghuaXUHeandYANGJianmin
LUO Qihao, WANG Weijun, LI Zan, ZHU Xinghua, WANG Xue,ZHANG Tonghua, XU He, and YANG Jianmin, , *
Effects of Diet on the Volatile Flavor and Nutritional Ingredients of Common Octopus ()
LUO Qihao1), 2), WANG Weijun1), 3), 4), *, LI Zan1), ZHU Xinghua1), 2), WANG Xue1), 2),ZHANG Tonghua3), XU He4), and YANG Jianmin1), 3), *
1),,264025,2),,201306,3).,.,257000,4).,.,222100,
Cephalopods are important economic shellfish that have been developed extensively in the coastal water ofvarious countries.is a large-scaled economic cephalopod that is mainly cultured in South China. This study explored the effect of different diets on the volatile flavor and nutritional ingredients of. Four diets were tested in four groups: Group A (fish ()), Group B (crab (Rathbun)), Group C (clam ()), and Group D (squid ()). Octopus muscles were sampled after 36 days of feeding, and volatile flavor substances (VFSs), fatty acids (FAs), and amino acids (AAs) were detected. Results showed that the VFSs, FAs, and AAs of octopus in the four groups were obviously different. The sum of volatile ketones and aldehydes was higher in Group B than in the other groups, which could present much more flavors. All groups were abundant in unsaturated FAs, including eicosapentaenoic acid (EPA) and docosa- hexaenoic acid (DHA). In terms of content and variety, the FAs in Group B were more beneficial tohuman health than those in the other groups. The content of each AA in Group B was basically higher than those in the other groups and was significantly higher than that in Group D (<0.05). Comparing the VFSs, FAs, and AAs insamples fed with four kinds of diets, the results indicate that using crab to feedcan achieve better effects on volatile flavor and nutritional ingredients.
; volatile flavor substances; fatty acids; amino acids; diet effect
1 Introduction
Cephalopods are a molluscan class composed of Nau- tiloidea and Coleoidea subclasses and more than 800 ex- isting species worldwide (Allcock, 2014). Cephalo- pods (octopus, squid, and cuttlefish) are the most neces- sary commercial marine species today, accounting for ap- proximately 4% of the global marine catch (FAO, 2018).The common octopusis one of the most important commercial cephalopods in the world because of its strong environmental adaptability, rich nutrition, fast growth, wide source of bait, and high conversion rate. Many southern European countries, such as Spain and Por- tugal, regardas an important fishery resource in the local market because of the high versatility in terms of recipes and pleasant sensory properties of this species (Mendes, 2017; Oliveira, 2019). Given its de- licious meat, rich nutrition, high proportion of edible parts,and pharmaceutical value, fresh or frozenhas gradually become one of the best-selling aquatic products worldwide (Barbosa and Vaz-Pires, 2004). At present, stu- dies on cephalopods, especially octopus, have been gra- dually becoming popular, focusing on feeding and breed- ing (Song, 2018; Cerezo Valverde, 2019; Green- well, 2019).
In addition, the relationship between animal diets and transformed product quality has been the focus of research in the world. Dietary lipid nutrition strategy could be formulated to improve the nutritional value and flavor quality of swimming crab () mus- cles (Yuan, 2020). Chen(2019a) used spiru- lina algae as a feed additive to analyze the value and fla- vor components of silkie hens eggs and found that adding 0.3% spirulina to feed could improve the performance of silk-bone chickens and the nutritional value and flavor of eggs. Zhou(2019) evaluated the nutrition and quality of muscles offed with mysid shrimp, miscellaneous fish, and clam meat, and showed that the clam meat group had better nutrition, taste, and quality than the other groups. Li(2020) used broad bean meal in place of different proportions of soybean meal and evaluated its effects on the fatty acid (FA) composition and muscle mass of grass carp () muscle. Many researches havereported the influence of bait on the growth and nutrition of. Morillo-Velarde(2015) formulated semi-humid diets withdifferent cod oil contents forlipid utilization to improve lipid replenishment. Biandolino(2010)studied the effect of natural diet ongrowth and FA composition. Cerezo Valverde(2008) formulated two formulas to feedon a wet diet and studied their growth, feed efficiency, and physical condition.
However, the effect of different diets on the meat flavor and nutritional value ofremains unknown. In this study, four types of natural diets commonly used in the cultivation ofwere compared,including Japanese-Spanish mackerel (), Tianjin thick crab (Rathbun), four-cornered clams (), and Japanese squid (). The aim of this research is to study the effects of baits on the flavor and nutritional con- tent of.
2 Materials and Methods
2.1 Sample Collections
Octopus individuals were collected from the offshore of Xiapu, Fujian, China. The experiment was carried out at Shandong Huachun Fishery Co., Ltd., which is located in Dongying, Shandong, China. The animals were accli- mated to the pond environment for a week, and then 120 individuals with the same size and strong vitality were se- lected and divided into four ponds each with 10 healthyfemales and 20 healthy males. Group A was fed with Japa- nese-Spanish mackerel (, chill- ed bait), Group B was fed with Tianjin thick crab (Rathbun, live bait), Group C was fed with four-cornered clams (, live bait), and Group D was fed with Japanese squid (, chilled bait). The weight of the bait was from 10% to 20% of the weight of the octopus, and the octopuses were fed daily at 5:00 pm for 36d.Tissuesfrom the second wrist on the left side of the male octopus were cut into pieces after peeling treatment and placed in a 2mL cryovial tube. The tube was immediately placed in liquid nitrogen for fast cryopreservation and then stored in −80℃ ultra-low temperature freezer.
2.2 Determination of VFSs
The gas chromatography-ion mobility spectrometer (GC- IMS, FlavorSpec) of Gesellschaft für Analytische Senso- rysteme mbH (GAS, Dortmund, Germany) was used to de- termine the VFSs of octopus muscles from four groups. Three samples of each group were tested twice through following method: 1g of chopped samples was placed into a 20mL headspace vial and then incubated at 40℃ for 10min. Then, the device automatically drew 1000μL of the sample headspace into the GC-IMS instrument by using a heated syringe (45℃). Subsequently, the sample was blowninto the FS-SE-54-CB-1 capillary column (15m×0.53mm) through the carrier gas (nitrogen) of the syringe and then heated at 60℃ to separate in time. After elution in iso- thermal mode, the analyte was driven into the ionization chamber and then detected through IMS. The molecules were ionized and driven to a drift tube, which was run at a temperature of 45℃ and a drift gas flow rate of 150mLmin−1. The analysis software of the instrument included Laboratory Analytical Viewer, Reporter plug-in, Galleryplug-in, Dynamic principal component analysis (PCA) plug-in, and GC×IMS Library Search, which could perform qualitative and quantitative analyses on samples.
2.3 Determination of FAs
The FA compositions of octopus muscles fed with dif- ferent diets were measured by GC in accordance with GB 5009.168-2016, and three samples per group were used in the analysis. The muscle sample was freeze-dried under vacuum for 35h and ground into a powder. A 0.1000g sample was placed in a 50mL digestive tube, in which 2mL of methanolic hydrochloric acid (acetyl chloride: an- hydrous methanol=1:10) and 1mL of-hexane (chroma- tographically pure) were added. After sealing and shaking, the sample was placed in a metal bath at 80℃ for 2h for transfatting. After cooling to room temperature, 3mL of 6% K2CO3was added and allowed to stand for 5min. The su- pernatant was methylated FA. The supernatant was filter- ed through a 0.22μm filter membrane into an FA determi- nation vial and analyzed on a high-performance gas chro- matograph (GC-2010, Shimadzu, Japan) with an injection volume of 1μL. Finally, the FA species of the sample were selected according to the FA standard peak time, and the relative content was calculated using the corrected area normalization method.
2.4 Determination of AAs
The AA compositions of octopus muscles fed with dif- ferent diets were measured using HPLC in accordance with GB 5009.124-2016. Three samples from each group were measured. First, the sample was pulverized with a grinder and ground into a powder. A quantitative sample (accurate to 0.0001g) was weighed into a hydrolysis tube, added with 6molL−1hydrochloric acid, and then hydrolyzed in a thermostat at 110℃. After 24h, the hydrolysate was con- centrated using a rotary vacuum evaporator to completely remove the solvent. Sodium citrate buffer solution was add- ed to the test tube to dissolve it after drying. After shaking and mixing, the solution through a 0.22μm filter was drawn and transferred to the sample injection bottle of the in- strument. The sample solution was injected into the AA analyzer in the same volume, and the AA concentration in the sample measurement solution was calculated from the peak area by using the external standard method.
2.5 Statistical Analyses
Statistical analysis was performed by SPSS version 22.0 (SPSS Inc., Chicago, IL). Quantitative data were express- ed as mean±standard deviation (mean±SD), and compa- rison between different groups was analyzed by one-way analysis of variance (ANOVA) and Duncan’s multiple com- parisons test. Statistical significance was considered at<0.05. R software version 3.6.1 (MathSoft Inc.) was used for PCA and boxplot analysis, and their corresponding im- ages were drawn.
3 Results
3.1 Effects of Diet on VFSs
The VFSs of themuscles fed with different diets were detected using GC-IMS. A total of 35 flavor com- pounds were identified, which involved six types, includ- ing four acids, six aldehydes, nine ketones, eight esters, six alcohols, and two other chemical components. The finger- print is shown in Fig.1, which could intuitively and quan- titatively compare the differences in VFSs among differ- ent samples. The degree of color depth of the points, re- presenting the octanal and heptanal concentrations in Fig.1a, was in an order of Groups A>B>C>D. In addi- tion, the concentrations of 2-hexanone-M and 2-hexan- one-D in Fig.1b were in an order of Groups B>D>A>C, and the content in Group C was very low. As shown in Fig.1c, Group D had the highest acetoin concentration among the groups, and the content of the other groups was very low. In Fig.1d, the concentrations of linalool and 1-hexanol in Groups A and D were higher than those in Groups B and C, and the concentrations of 2-hexanol and 2-methylbutanol in Group D were the highest. The con- centrations of methyl hexanoate-M, methyl hexanoate-D, and isobutyl acetate in Fig.1e were higher than those in Groups B and D. In Fig.1f, Group D had the highest con- centrations of propyl acetate-D, methyl 2-methylbutano- ate, and ethyl acetate among the different groups.
The peak volume data of all measured substances in each sample were analyzed by one-way ANOVA (Table 1) to explore whether the VFSs in the muscles were signifi- cantly different in each group. Among all the acids, the pentanoic acid content in Group A was significantly lower than those in the other threegroups (<0.05). For alde- hydes, with the results in Fig.1a of the fingerprint, octanal and heptanal showed significant reductions in Groups A, B, C, and D (<0.05). For ketones, the 2-hexanone-M and2-hexanone-D contents in Group B were significantly high- er than those in the other threegroups (<0.05). The ace- toin content in Group D was significantly higher than those in the other threegroups (<0.05). For alcohols, the li- nalool and 1-hexanol contents were significantly higher in Group D than in the other groups; the 1-hexanol content was significantly lower in Group D than in the other groups; and the 2-methylbutanol content was significantly lower in Group A than in the other groups (<0.05). For esters, the methyl hexanoate-M content was significantly the high- est in Group B among the groups (<0.05).

Fig.1 Fingerprint of diet effects on volatile flavor substances. The X-axis is the characteristic peak identification of each volatile organic compound, and the Y-axis shows the samples from each group. Each column represents the signal peak of the same volatile compound in different samples, and each point represents a type of VFSs. The color represents the con- centration of the substance: white means low concentration, red means high concentration, and darker color means higher concentration.
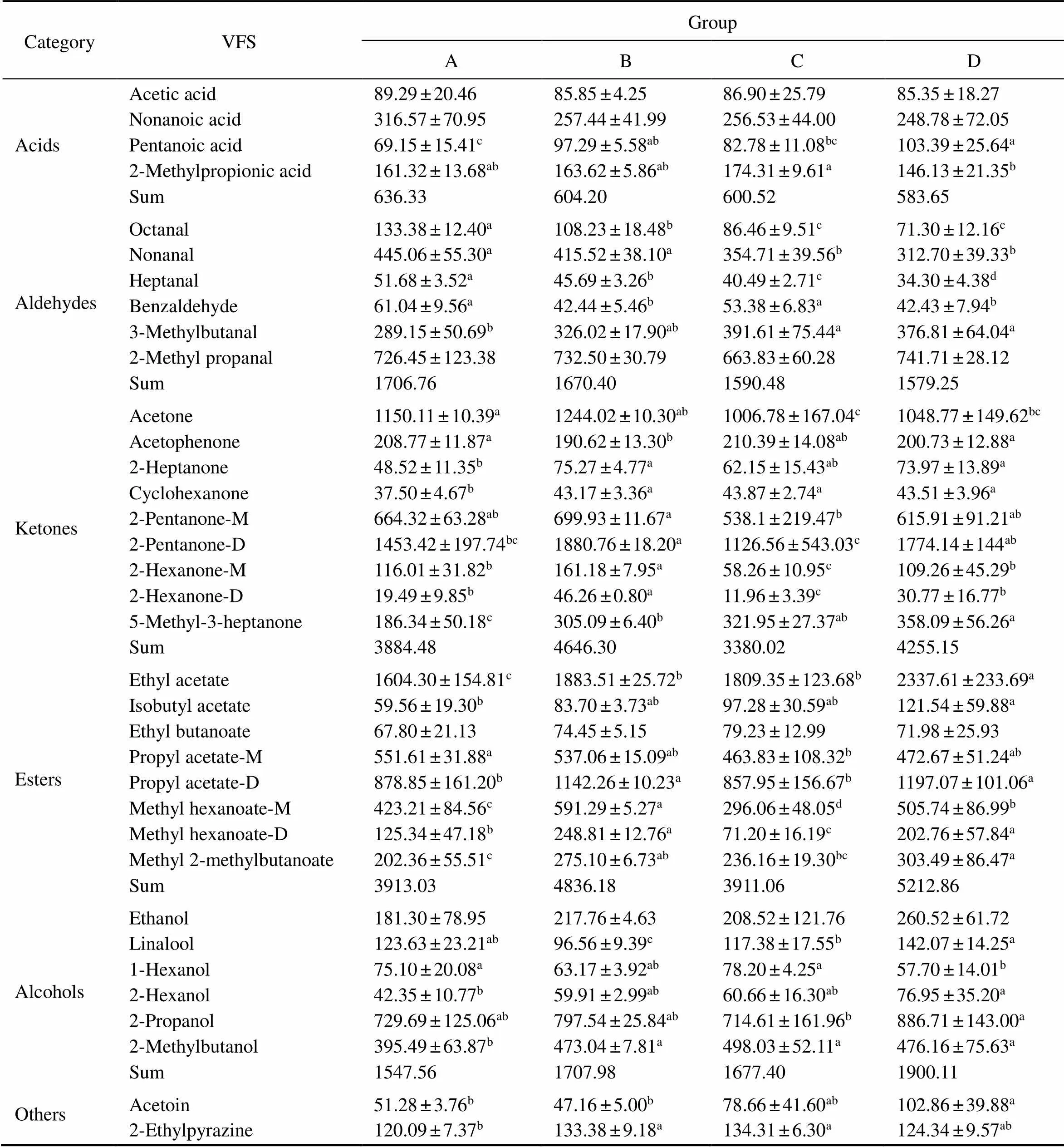
Table 1 Effects of diet on volatile flavor substance compositions (means±SD)
Note: superscript letters in the same row indicate difference between groups (< 0.05).
3.2 Effects of Diet on FAs
The PCA of FAs in the muscles offed with four diets is shown in Fig.2. The contribution ratios of the first principal component (PC1) and the second principal component (PC2) were 80.74% and 8.53%, respectively, and the cumulative contribution ratio of the two components was 89.27%. As shown in Fig.2, Groups A, B, C, and D could be distinguished obviously, suggesting thatfeeding the octopus with different diets can affect the quan- tity and types of FAs in its muscles.
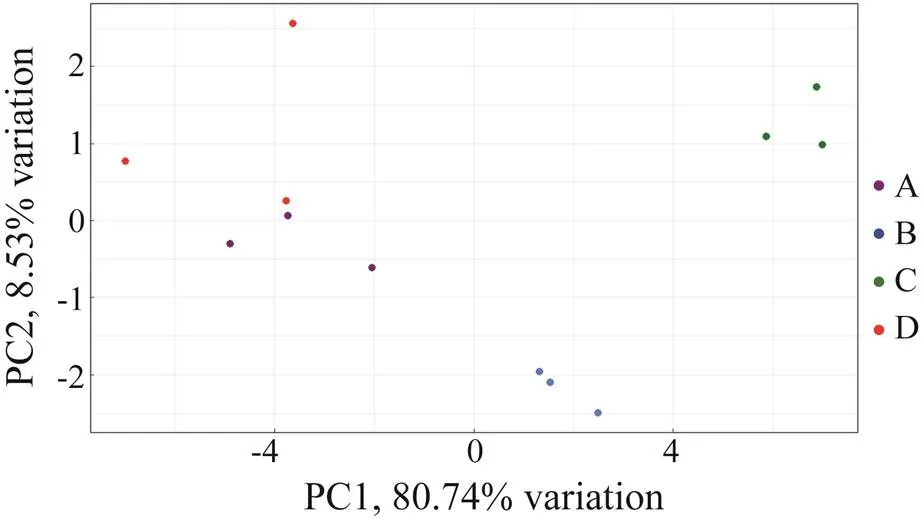
Fig.2 PCA of diet effects on fatty acids.
One-way ANOVA was performed on 25 types of FAsmeasured to explore the differences in FAs in muscles offed with four diets. Table 2 shows the effects of different diets on the FA compositions in octopus meat. The saturated fatty acids (SFAs) content in Group B was significantly higher than that in Group D (<0.05). In addition, C14:1 was detected in Groups B and C but notin Groups A and D. The contents of polyunsaturated fatty acids (PUFAs) was in an order of Groups D>A>B>C, and significant differences were found among each group (<0.05). The content of total fatty acids (TFAs) was in an order of Groups D>A>B>C, and significant differences were found among each group (<0.05).

Table 2 Diet effects on fatty acid compositions (means±SD)
Notes: superscript letters in the same row indicate difference between groups (<0.05). Abbreviations: FA, fatty acids; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; UFA, unsaturated fatty acids; TFA, total fatty acids.
3.3 Effects of Diet on AAs
The distribution characteristics of AAs in the four diet groups are shown by a boxplot (Fig.3). The contents of various AAs in Group D were significantly lowerthan in the other threegroups, and thevalues of Group D. Groups A, B, and C were all less than 0.05 (., 0.0038, 0.0016, and 0.012, respectively).
One-way ANOVA was performed on the 16 AAs to find out the effects of different diets on the AAs of octopus meat (Table 3). The contents of all the AAs in Group D were significantly lower than those in the other threegroups. The contents of total essential amino acids (TEAAs) and total flavor amino acids (TFAAs) were in an order of Groups B>A>C>D.
4 Discussion
4.1 Comparison and Evaluation of VFSs in Different Groups
GC-IMS has the advantages of simple sample prepara- tion, non-destruction, easy management, fast and accurateanalysis. This method can be used to analyze the quality of food and agricultural products; in particular, it can be applied for the rapid detection and identification of orga- nic volatile flavor components (Arroyo-Manzanares, 2018; Chen, 2019b; Jia, 2019; Martín-Gómez, 2019).
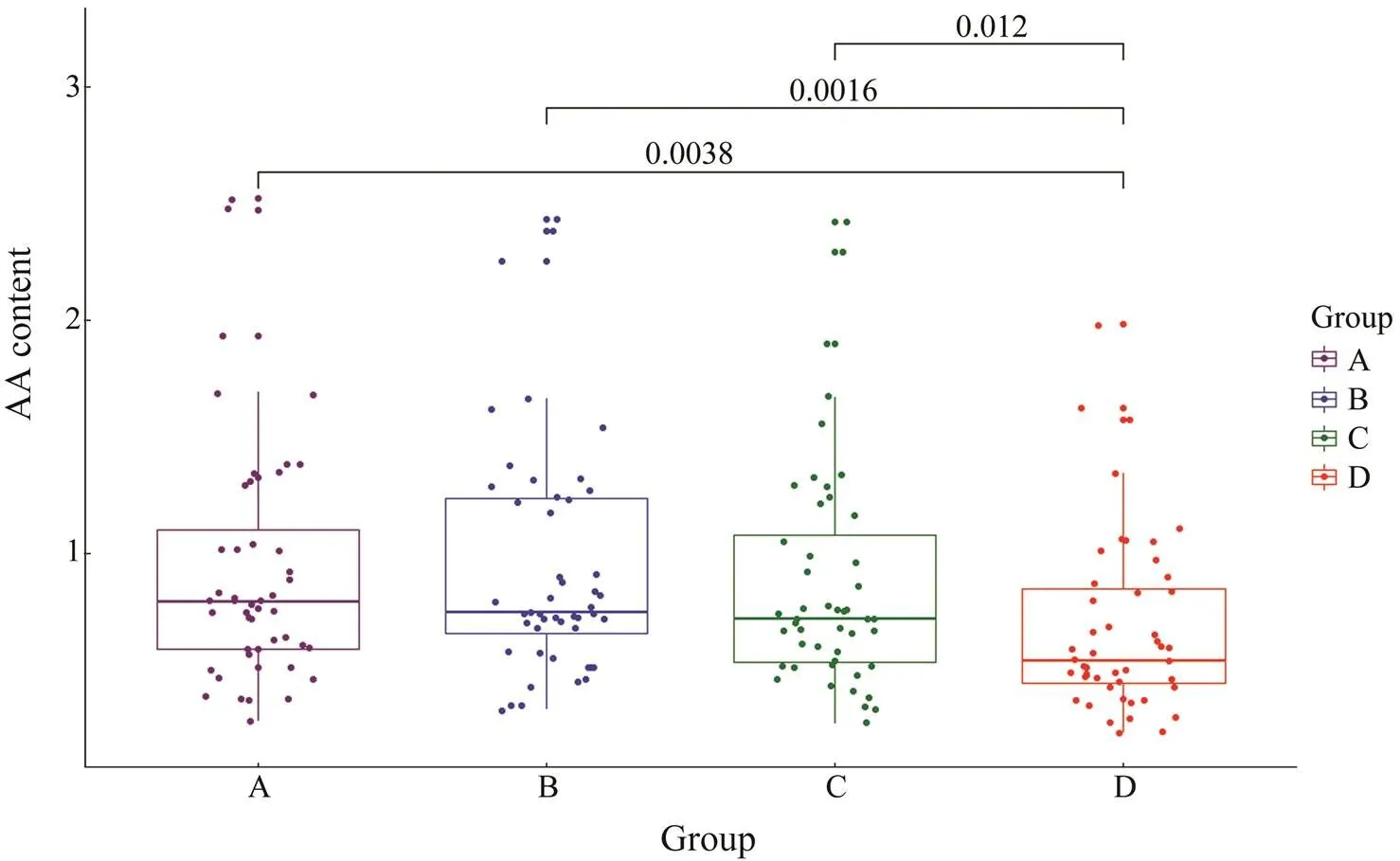
Fig.3 Boxplot of diet effects on amino acid content. The values between the box diagrams represent the P-values between groups. Significant differences in the content of AAs between groups are indicated by P<0.05.

Table 3 Diet effects on amino acid compositions (means±SD)
Notes: superscript letters in the same row indicate difference between groups (<0.05). Abbreviations: AA, amino acids; EAA, essential amino acids; FAA, flavor amino acids; TEAA, total essential amino acids; TFAA, total flavor amino acids; TAA, total amino acids.
In this study, 35 flavor compounds were identified by GC-IMS. Volatile aldehydes and alcohols canshow mild, delicate, and pleasant smell or tastes, giving most seafood a common sweetness and plant-like aroma (Turchini, 2004). In this experiment, comparison of the peak areas detected by GC-IMC revealed that the aldehyde content in Group B was higher than those in Groups C and D, and the alcohol content in Group D was the highest among the four groups. Octanal has a mushroom-like fatty taste and can produce a pleasant odor (Zhou, 2020). It is the main volatile active aldehyde in many aquatic animal pro- ducts, such as clams, crabs, and oysters. The octanal con- tent in Group B was significantly higher than those in Groups C and D (<0.05). The heptanal content was sig-nificantly different within each group (<0.05), whereas heptanal has a sweet apricot flavor and nutty aroma. No- nanal can impart crab flavor and strong grassy aroma (Zhuang, 2016). The nonanal content accounted for a large proportion of the aldehydes, and was the repre- sentative odor of aldehydes in octopus.The nonanal con- tent in Group B was significantly higher than those in Groups C and D (<0.05).
The alcohol with the largest difference among the four groups was linalool, and the linalool content in Group D was significantly higher than those in the other three groups (<0.05). Linalool had an admirable aroma of lily of the valley. For ketones, the 2-hexanone-M content in Group B was the highest and can give the octopus muscles a spe- cial spicy flavor.
The content of esters was the highest in all flavored or- ganic species, which is inconsistent with a previous report on fresh cuttlefish (Jin, 2017). The peak area of each sample showed that the ethyl acetate content in Group D was significantly higher than those in the other three groups and that ethyl acetate had a clear, slightly fruity wine aro- ma (<0.05). Propyl acetate-D, which was also very high in each group, had a soft fruit flavor, and its content in Group D was higher than those in the other three groups.
Correlations were found between the effect of different diets on the flavor of muscles and the flavor content of the diets. Three types of VFSs can be found in the mus- cles of Spanish mackerel: aldehydes, ketones, and alco- hols (Fan, 2019). This study showed that the alde- hyde content in Group A was higher than those in the other threegroups. The VFSs of crab meat mainly include aldehydes, alcohols and higher concentrations of methyl hexanoate-M (Yuan, 2020). In the present study, the methyl hexanoate-M content in Group B was significantly higher than those in the other three groups (<0.05).
All of these VFSs have synergistic effects and can co- operate with each other to produce fascinating aromas. The smell of Groups B and D was much more abundant than those of the other groups. In general, Group D had higher contents of esters and alcohols than the other groups and could probably produce a fresh, fruity taste. In addition, the sum of ketones and aldehydes of Group B was higher than those of the other three groups, which could produce an ethereal taste similar to grass.
4.2 Comparison and Evaluation of FAs in Different Groups
FAs are closely related to the formation of food flavor because they are important substrates for lipid oxidation(Xu, 2018).UFAs are closely related to the flavor characteristics of food(Stephan and Steinhart, 2000). In the present study, the UFA content was significantly high- er than the SFA content in all groups, of which eicosa-pentaenoic acid (EPA, C20:5n-3) and docosahexaenoic acid (DHA, C22:6) were the most abundant in all samples, and the result was consistent with previous report (Biandolino, 2010).
UFAs are critical for the development and regulation of physiological processes in vertebrates,which can effective- ly prevent human coronary heart disease (Mozaffarian, 2005). They are related to some serious physiological and process physiological syndromes and also affect the status of some diseases (Nettleton, 2017). In particular, EPA and DHA, which have strong physiological activities, canpromote blood circulation; improve serum fat quality; pro- mote the formation, growth, development of brain cells; and enhance memory (Nichols, 2014). Fish and mol- lusks are rich in omega-3 essential oils (EPA and DHA) (Turchini, 2009). In the present study, UFA/TFA had no significant difference among Groups A, B, and C (>0.05); that is, the UFA/TFA content of each group account- ed for 80.50% evenly. The EPA and DHA contents in GroupB with crab as bait were 10.12 and 20.61, respectively. Mo- zaffarian(2005) studied diet and 15-year mortality in seven countries and found that MUFAs in food signifi- cantly prevent cardiovascular heart disease. In the present study, the MUFA/TFA of Group B was 35.08%.
Compared with the other groups, Group D which fed with squid had a higher content of PUFAs, which might be due to the lipid composition of the bait itself (Almansa, 2006). Clam meat contains higher contents of SFAs than MUFAs and PUFAs and had less fat content (Liu,2019). In the present experiment, the PUFA content in Group C fed with clams was significantly lower than those in the three other groups (<0.05).
In general, UFAs accounted for more than 80% of TFAs in the meat detected in the four groups. The total UFA con- tent in Group D was significantly higher than that in the other groups. Methyl myristate (C14:1) was present in Group B but absent in Group D. Some reports suggested a limit to the daily intake of UFAs because overdosage might lead to cardiovascular disease (Moreira, 2001). Given the variety and content of UFA, Group B was more in line with human nutritional standards.
4.3 Comparison and Evaluation of AAs in Different Groups
Studies showed thatis rich in high-quality protein (Ozogul, 2008). The nutritional value of pro- tein in food mainly depends on the composition and con- tent of AA. In the present study, 16 common AAs were de- tected in the octopus meat fed by four groups of different diets. Eight essential amino acids (EAAs) and eight flavor amino acids (FAAs) were detected in the samples of the four groups. The content and composition characteristics of EAAs were the most important indicators for deter- mining the nutritional value of protein in food. The total essential amino acid (TEAA) content of Group B was the highest among the groups, and the EAA content in Group B was significantly higher than that in Group D (<0.05). Each EAA can have a great effect on the human body and increase the richness of the food itself. Leucine can easily convert to glucose, which helps regulate blood sugar levels and provides energy to body tissues. Phenylalanine and ty- rosine are bitter-tasting EAAs contributing to the salty tasteof soy sauce in addition to glutamate; in addition, they playan important role in improving umami of the food (Lioe, 2004).
Umami substances, especially some FAAs, have a neu- tral flavor between sweetness and saltiness and can en- hance the thickness and complexity of other flavors (Fuke and Ueda, 1996). In this study, the TFAA content in Group B was higher than those in the other groups, and the con- tent in Group D was the lowest (<0.05). The glutamate content in Group B was the highest, reaching 2.35g (100g)−1. Glutamate, defined as a special umami, and mono- sodium glutamate are therefore linked (Yamaguchi, 1971). Glycine and alanine, especially in large quantities, contribute pleasing sweetness to seafood, such as octopus, squid, clams, and scallops (Fuke and Konosu, 1991). The glycine content was the highest in Group B, and the ala- nine contents in Groups A, B, and C were basically simi- lar and were all significantly higher than that in Group D (<0.05). Arginine in Group B accounted for 8.74% of the total amino acids (TAAs). It is widely found in many sea- food, and it gives these products a pleasing overall per- formance as a preference (Spurvey, 1998).
AAs are more abundant in squid than clams (Krishnan, 2019), but Group D fed with squid showed a low level of amino acid concentration, which may be related to the absorption efficiency of AA by.
In general, no significant difference in the type of AAs was found among the four groups, but a certain difference in content was observed. The AA content in Group B was almost the highest among the groups, but no significant dif- ference was found among Groups A, B, and C (>0.05). The AA content in Group D was significantly the lowest among the groups (<0.05). Thus, Group B exhibits better octopus muscle nutrition in AAs and has pleasant uma- mi taste.
5 Conclusions
Feeding with different diets can exert flavor and nutri- tional effects on the muscles of. Groups fed with squid or crab can produce much more flavors than those fed with fish and clam. Each group ofwas rich in beneficial UFAs, including EPA and DHA. Baits can affect the content of FA, andfed with crab is more beneficial to the human health and can produce more pleasant umami and nutrition value compared withthe other baits. Comprehensive comparison of the VFSs,FAs, and AAs of the four diets ofrevealed that using crab to feedcan achieve better effects on volatile flavor and nutritional ingredients.
Acknowledgements
This research was supported by the earmarked fund for the Modern Agro-Industry Technology Research System (No. CARS-49), the Natural Science Foundation of Shan- dong Province (No. ZR2018BC052), the Entrepreneurship and Innovation Talents programme of Jiangsu Province of China (2020–2023), and the Huaguo Mountain Talent Pro- gramme of Lianyungang City Jiangsu Province (2019– 2022).
Allcock, A.L., Lindgren, A., and Strugnell, J.M., 2014. The con- tribution of molecular data to our understanding of cephalopod evolution and systematics: A review., 49 (21-24): 1373-1421, DOI: 10.1080/00222933.2013.825342.
Almansa, E., Domingues, P., Sykes, A., Tejera, N., Lorenzo, A., and Andrade, J.P., 2006. The effects of feeding with shrimp or fish fry on growth and mantle lipid composition of juvenile and adult cuttlefish ()., 256 (1-4): 403-413, DOI: 10.1016/j.aquaculture.2006.02.025.
Arroyo-Manzanares, N., Martin-Gomez, A., Jurado-Campos, N., Garrido-Delgado, R., Arce, C., and Arce, L., 2018. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace-gas chromatography-ion mobility spectrometry., 246: 65-73, DOI: 10.1016/j.foodchem.2017.11.008.
Barbosa, A., and Vaz-Pires, P., 2004. Quality index method (QIM)development of a sensorial scheme for common octopus ()., 15 (3): 161-168, DOI: 10.1016/S0956-7135(03)00027-6.
Biandolino, F., Portacci, G., and Prato, E., 2010. Influence of na- tural diet on growth and biochemical composition ofCuvier, 1797., 18 (6): 1163-1175, DOI: 10.1007/s10499-010-9331-x.
Cerezo Valverde, J., Hernández, M.D., Aguado-Giménez, F., and García García, B., 2008. Growth, feed efficiency and condition of common octopus () fed on two formulated moist diets., 275 (1-4): 266-273, DOI: 10.1016/j.aquaculture.2008.01.012.
Cerezo Valverde, J., Rodríguez-González, T., GraneroFernández, M.D., Aguado-Giménez, F., and García García, B., 2019. Suc- cessful rearing of common octopus () fed a formulated feed in an offshore cage., 50 (3): 968-972, DOI: 10.1111/are.13955.
Chen, G.S., Cai, Y., Su, Y.Y., Gao, B.L., Wu, H.B., and Cheng, J.Y., 2019a. Effects ofalgae as a feed supplement on nutritional value and flavour components of silkie hens eggs., 103 (5): 1408-1417, DOI: 10.1111/jpn.13125.
Chen, K., Yang, X., Huang, Z., Jia, S., Zhang, Y., Shi, J., Hong, H., Feng, L., and Luo, Y., 2019b. Modification of gelatin hy- drolysates from grass carp () scales by Maillard reaction: Antioxidant activity and volatile com- pounds., 295: 569-578, DOI: 10.1016/j.foodchem.2019.05.156.
Fan, Z.Y., Zhang, L., Yuan, K., Wang, X.C., Li, Y.J., and Liu, Y.P., 2019. Research on flavor characteristics offish protein isolates., 3: 206-214 (in Chinese with English abstract).
FAO,2018. Fishery and Aquaculture Statistics 2016. FAO Year- book, Food and Agriculture Organization of the United Na- tions, Rome, Italy, http://www.fao.org/3/i9942t/I9942T.pdf (ac- cessed on 30April 2019).
Fuke, S., and Konosu, S., 1991. Taste-active components in some foods: A review of Japanese research., 49 (5): 863-868, DOI: 10.1016/0031-9384(91)90195-T.
Fuke, S., and Ueda, Y., 1996. Interactions between umami and other flavor characteristics., 7 (12): 407-411, DOI: 10.1016/S0924-2244(96)10042-X.
Greenwell, C.N., Loneragan, N.R., Tweedley, J.R., and Wall, M., 2019. Diet and trophic role of octopus on an abalone sea ranch., 26 (6): 638-649, DOI: 10.1111/fme.12381.
Jia, S., Li, Y., Zhuang, S., Sun, X., Zhang, L., Shi, J., Hong, H., and Luo, Y., 2019. Biochemical changes induced by dominant bacteria in chill-stored silver carp () and GC-IMS identification of volatile organic compounds., 84: 103248, DOI: 10.1016/j.fm.2019.103248.
Jin, Y., Bu, T.T., Li, M., Zheng, L., Song, Z.G., and Li, H.S., 2017. Analysis of the volatile components of dried cuttlefish by electronic nose combined with HS-SPME-GC-MS., 38 (15): 189-195, DOI: 10.7506/spkx1002-6630-201620013(in Chinese with English abstract).
Krishnan, S., Chakraborty, K., and Vijayagopal, P., 2019. Nutri- tional profiling of selected species of edible marine molluscs from the south-west coast of India., 66 (1): 56-63, DOI: 10.21077/ijf.2019.66.1.80079-08.
Li, X.X., Chen, S.J., Sun, J.J., Huang, X.D., Tang, H.J., He, Y.H., Pan, Q., and Gan, L., 2020. Partial substitution of soybean meal with faba bean meal in grass carp () diets, and the effects on muscle fatty acid composition, flesh quality, and expression of myogenic regulatory factors., 51 (5): 1145-1160, DOI: 10.1111/jwas.12671.
Lioe, H.N., Apriyantono, A., Takara, K., and Wada, K., 2004. Low molecular weight compounds responsible for savory taste of Indonesian soy sauce., 52 (19): 5950-5956, DOI: 10.1021/jf049230d.
Liu, C.S., Li, T.Y., Liu, E.T., Li, C.L., Wang, A.M., and Gu, Z.F., 2019. Proxeviate composition, amino acid content, andfatty acid profile of the adductor muscle and mantle from two species of the giant clamsand., 38 (3): 529-534, DOI: 10.2983/035.038.0303.
Martín-Gómez, A., Arroyo-Manzanares, N., Rodríguez-Estévez, V., and Arce, L., 2019. Use of a non-destructive sampling me- thod for characterization of Iberian cured ham breed and feed- ing regime using GC-IMS., 152: 146-154, DOI: 10.1016/j.meatsci.2019.02.018.
Mendes, R., Vieira, H., Pereira, J., and Teixeira, B., 2017. Water uptake and cooking losses induring indus- trial and domestic processing., 78: 8-15, DOI: 10.1016/j.lwt.2016.11.087.
Moreira, A.B., Visentainer, J.V., Souza, N.E., and Matsushita, M., 2001. Fatty acids profile and cholesterol contents of three Brazilianfreshwater fishes., 14 (6): 565-574, DOI: 10.1006/jfca.2001.1025.
Morillo-Velarde, P.S., Cerezo Valverde, J., and García García, B., 2015. Utilization of diets with different fish oil content in com- mon octopus (Cuvier, 1797) and resulting changes in its biochemical composition., 46 (12): 2871-2884, DOI: 10.1111/are.12439.
Mozaffarian, D., Bryson, C.L., Lemaitre, R.N., Burke, G.L., and Siscovick, D.S., 2005. Fish intake and risk of incident heart failure., 45 (12): 2015-2021, DOI: 10.1016/j.jacc.2005.03.038.
Nettleton, J.A., von Schacky, C., Brouwer, I.A., and Koletzko, B., 2017. International society for the study of fatty acids and li- pids 2016 debate: For science-based dietary guidelines on fats, meta-analysis and systematic reviews are decisive., 71 (1-2): 26-30, DOI: 10.1159/000478794.
Nichols, P., McManus, A., Krail, K., Sinclair, A., and Miller, M., 2014. Recent advances in omega-3: Health benefits, sources, products and bioavailability., 6 (9): 3727-3733.
Oliveira, H., Muniz, J.N., Bandarra, N.M., Castanheira, I., Coel- ho, I.R., Delgado, I., Gonçalves, S., Lourenço, H.M., Motta, C., Duarte, M.P., Nunes, M.L., and Gonçalves, A., 2019. Ef- fects of industrial boiling on the nutritional profile of common octopus ()., 8 (9):411, DOI: 10.3390/foods8090411.
Ozogul, Y., Duysak, O., Ozogul, F., Ozkutuk, A.S., and Tuereli, C., 2008. Seasonal effects in the nutritional quality of the body structural tissue of cephalopods., 108 (3): 847-852, DOI: 10.1016/j.foodchem.2007.11.048.
Song, M.P., Wang, J.H., and Zheng, X.D., 2018. Prey pre- ference of the common long-armed octopus(Ce- phalopoda: Octopodidae) on three different species of bivalves., 37 (5): 1595-1603, DOI: 10.1007/s00343-019-8217-7.
Spurvey, S., Pan, B.S., and Shahidi, F., 1998. Flavour of shell- fish. In:.Shahidi, F., ed., Blackie Academic and Professional, London, 159-196.
Stephan, A., and Steinhart, H., 2000. Bitter taste of unsaturated free fatty acids in emulsions: Contribution to the off-flavour of soybean lecithins., 212 (1): 17-25, DOI: 10.1007/s002170000216.
Turchini, G.M., Mentasti, T., Caprino, F., Panseri, S., and Mo- retti, V.M., 2004. Effects of dietary lipid sources on flavour volatile compounds of brown trout (L.) fillet., 20 (1): 71-75, DOI: 10.1046/j.0175-8659.2003.00522.x.
Turchini, G.M., Torstensen, B.E., and Ng, W., 2009. Fish oil replacement in finfish nutrition., 1 (1): 10-57, DOI: 10.1111/j.1753-5131.2008.01001.x.
Xu, Y., Li, L., Regenstein, J.M., Gao, P., Zang, J., Xia, W., and Jiang, Q., 2018. The contribution of autochthonous microflora on free fatty acids release and flavor development in low-salt fermented fish., 256: 259-267, DOI: 10.1016/j.foodchem.2018.02.142.
Yamaguchi, S., Yoshikawa, T., Ikeda, S., and Ninomiya, T., 1971. Measurement of the relative taste intensity of some l-alpha-amino acids and 5’-nucleotides., 36 (6): 846-849, DOI: 10.1111/j.1365-2621.1971.tb15541.x.
Yuan, Y., Wang, X., Jin, M., Jiao, L., Sun, P., Betancor, M.B., To-cher, D.R., and Zhou, Q., 2020. Modification of nutritional values and flavor qualities of muscle of swimming crab): Application of a dietary lipid nutrition strategy., 308: 125607, DOI: 10.1016/j.foodchem.2019.125607.
Zhou, T.Y., Liu, H., Wu, Q.Q., Hao, L., Pan, D.D., and Dang, Y.L., 2020. The flavor quality of driedwith dif- ferent species and drying methods (charcoal roasting and natu- rally drying)., 14 (1): 613-622, DOI: 10.1007/s11694-020-00377-5.
Zhou, Y.H., Zhou, W.Y., and Pang, G.P., 2019. Different diets feeding:Effect on nutritional quality of., 35 (21): 137-140 (in Chi- nese with English abstract).
Zhuang, K., Wu, N., Wang, X., Wu, X., Wang, S., Long, X., and Wei, X., 2016. Effects of 3 feeding modes on the volatile and nonvolatile compounds in the edible tissues of female Chinese mitten crab ()., 81 (4): S968-981, DOI: 10.1111/1750-3841.13229.
March 19, 2020;
May 18, 2020;
September 25, 2020
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
. E-mail: wwj2530616@163.com
E-mail: ladderup@126.com
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Elevated Ducts and Low Clouds over the Central Western Pacific Ocean in Winter Based on GPS Soundings and Satellite Observation
- Control System Design and Implementation at Flexible, Distributed Offshore Sensor Test Sites in the Yangtze Estuary Area
- Global-Scale Diversity and Distribution Characteristics of Reef-Associated Symbiodiniaceae via the Cluster-Based Parsimony of Internal Transcribed Spacer 2 Sequences
- A Preliminary Investigation of Arctic Sea Ice Negative Freeboard from in-situ Observations and Radar Altimetry
- Comparison of the Pore Structure of Ultralow-Permeability Reservoirs Between the East and West Subsags of the Lishui Sag Using Constant-Rate Mercury Injection
- The Stable Carbon Isotopic Compositions of n-Alkanes in Sediments of the Bohai and North Yellow Seas: Implications for Sources of Sedimentary Organic Matter
