Coprecipitation of metal ions into calcite:an estimation of partition coefficients based on field investigation
2021-03-03ZhongweiWangJiubinChenHongmingCaiWeiYuanShengliuYuan
Zhongwei Wang•Jiubin Chen•Hongming Cai•Wei Yuan•Shengliu Yuan
Abstract Trace elements(and their isotopes)in carbonates are commonly used to reconstruct paleoenvironment and paleoclimate.Understanding the processes and mechanisms of element incorporation into carbonates is thus crucial for using such geochemical parameters as paleoclimate proxies.In contrast to laboratory-based experimental results,the partitioning of trace metals between solid and solution phases in natural carbonate precipitation systems has rarely been reported.In this study,we investigated the partition coefficients of metal ions between solid and solution in the channel of the natural Baishuitai travertine system,Yunnan,China.Our results show that the partition coefficients of Li+,Na+,Mg2+,Sr2+and Ba2+are<1,that of Ni2+is approximately 1,and those of Co2+,Mn2+,Zn2+and Cu2+are>1,consistent with the results found in previous experimental studies.Although the substitution for Ca2+is likely the main uptake process of these metals into calcite,depending on their ionic radius and charge,trace elements may also be incorporated by adsorption or physical entrapment.Our study shows that unlike laboratory experiments performed under specific conditions,the partitioning of metals between two phases in the natural travertine system could be controlled by several,even multiple,environmental factors(e.g.,carbonate deposition rate,temperature,and pH),which should be taken into account when using trace metals(and their isotopes)in carbonate archives as a paleoclimate proxy.
Keywords Metal ions·Coprecipitation·Calcite·Partition coefficients·Field investigation
1 Introduction
Trace elements in carbonates are widely used for investigating and reconstructing the past surface environment and climate.The process and mechanism of the incorporation of elements into carbonate are the factors restricting the use of such geochemical parameters as paleoclimate proxies.Carbonate minerals mainly consist of calcite,aragonite,and dolomite,with Ca2+and Mg2+as the major cations(Gaffey 1987).As the radius and electrovalence of the cation Sr2+are similar to those of Ca2+,Sr2+can replace Ca2+in carbonates,leading to the enrichment of Sr2+in carbonate minerals(Mucci and Mors,1983).Consequently,Ca2+,Mg2+,and Sr2+,together with elemental ratios such as Sr2+/Ca2+and Mg2+/Ca2+,have received the most attention in the study of inorganic carbonate precipitation and related applications.In addition to containing these alkaline earth metals,carbonate minerals may contain a small amount of metal cations such as Li+,Na+,Co2+,Ni2+,Mn2+,Cu2+,Zn2+and Ba2+(Curti 1999).These elements,together with Ca2+and Mg2+,have been used to trace surface environment evolution and dynamics(Chen et al.2015;Gaillardet et al.1999;Liu et al.2019;White 2011).Moreover,the geochemical characteristics of various chemical components in carbonates have also been of great interest in the study of the geochemistry of sedimentary carbonate deposition and diagenesis(Roberts et al.1998).In addition to the physicochemical characteristics of the elements themselves,external environmental parameters(such as temperature,pH,humidity,salinity,and precipitation rate)determine and control the incorporation of elements into carbonates.External environmental parameters could also restrict the element distribution coefficients between solid and solution phases(Fu¨ger et al.2019).
The study of geochemical indexes related to this kind of mineral started with marine sedimentary carbonates(such as coral,benthic foraminifera,and zooplankton foraminifera)(Carpenter et al.1991;Graham et al.1982).Since then,a number of factors that were not previously considered to be important have been found to exert major influences on carbonate precipitation and related element partitioning.Laboratory experiments have been performed to investigate the impact of these factors on element behaviors during carbonate formation using both natural and synthetic seawater(Katz 1973;Kinsman and Holland 1969;Lorens 1981).It is generally believed that among other factors,seawater temperature and carbonate precipitation rate(depending on the degree of supersaturation of the solution)are the two most likely factors controlling the chemical composition of marine carbonate.Kinsman and Holland(1969)found that the partition coefficient of Sr2+between calcite and aqueous solution decreased linearly with increasing temperature,from 1.17±0.04 at 16 °C to 0.88±0.03 at 80 °C,inconsistent with the results of Katz(1973),which showed only a slight influence.A later study showed that the partition coefficient of Mg2+was closely related to temperature, the coefficients were 0.0573±0.0017 and 0.1163±0.0034 at 25 and 80 °C,respectively(Katz 1973).Both studies found that the presence of other salts(such as NaCl)did not affect the partition coefficients of Sr2+and Mg2+.In addition to temperature,the precipitation rate also impacts the distribution and geochemistry of elements during carbonate precipitation.Lorens(1981)showed that the partition coefficient of Sr2+increases with an increasing calcite deposition rate.Mucci and Morse(1983)measured the contents of Mg2+and Sr2+in calcite precipitated in both natural and synthetic seawater at 25 °C and found that the partition coefficients of Mg2+and Sr2+did not change significantly when the deposition rate changed by an order of magnitude.The same result was observed by Mucci(1987).Recently,Mavromatis et al.(2013)showed that the partition coefficient of Mg2+was positively related to the deposition rate of calcite.In addition to temperature and deposition rate,other factors,such as the partial pressure of carbon dioxide in solution,may also impact the content of Mg2+in carbonates(Burton and Walter 1991).
Apart from Mg2+and Sr2+,other metal ions such as Li+,Na+,Co2+,Ni2+,Mn2+,Zn2+,Cu2+,Cr2+,and Ba2+have also been studied in inorganic carbonate deposition experiments to test the usefulness of these parameters as environmental proxies.The results showed that the partition coefficients of Co2+,Mn2+,and Cd2+decreased with an increased deposition rate of calcite(Lakshtanov and Stipp 2007;Lorens 1981)while those of Li+and Na+increased with increasing deposition rate(Fu¨ger et al.2019).In addition,White(1977)found that the partition coefficient of Na+was related to the Ca2+activity and pH of the reaction solution.
In addition to the geochemistry of a single element,elemental ratios or couplings such as Sr2+/Ca2+,Mg2+/Ca2+,Cd2+/Ca2+,Sr2+/Ba2+,and Zn2+/Ba2+have been widely used for reconstructing past environmental changes,such as temperature and salinity variations(Carpenter et al.1991;Graham et al.1982;Rosenthal et al.1997).For example,the changes in the ion ratios Mg2+/Ca2+and Sr2+/Ca2+in secondary sedimentary carbonate(stalagmite)are influenced by various climatic and environmental factors on a local or regional scale(Gascoyne 1983;Luo et al.2013;Roberts et al.1998)Roberts et al.(1998)found that the annual Mg2+/Ca2+oscillations may be caused by seasonal temperature changes,while the influence of external humidity would be more important for long-timescale research.In Belgium and southern Brazil,Mg2+/Ca2+and Sr2+/Ca2+in stalagmites were suggested to be mostly controlled by changes in precipitation(Roberts and Wright 1993).
Although these previous studies have substantially improved our knowledge of the distribution and geochemistry of alkaline earth elements during the formation of carbonates,they have sometimes reported inconsistent or even contrasting results.In addition,the geochemical behaviors of other elements,such as Li+,Na+,Co2+,Ni2+,Mn2+,Cu2+,and Zn2+,with respect to carbonate precipitation processes remain unclear.Moreover,most of this knowledge was obtained from laboratory experiments,and large uncertainties would exist when applying these results to the reconstruction of the paleoenvironment.Therefore,in order to better understand the incorporation of elements into solids and its related mechanisms,more systematic studies should be performed on the geochemistry of trace elements in natural carbonate archives,such as travertine.This would be useful for the precise application of these parameters in investigating and reconstructing the past surface environment.In this paper,we investigated the distribution of some typical metal elements(Li+,Na+,Co2+,Ni2+,Mg2+,Ca2+,Mn2+,Cu2+,Zn2+,Sr2+,and Ba2+)during surface carbonate precipitation in the natural Baishuitai travertine system,Yunnan,China.The objectives of this work are(1)to investigate the geochemistry of metal elements during travertine formation,(2)to assess the partition coefficients of different metal ions between the solid and liquid phases,and(3)to identify the factors impacting the partition coefficients of metal ions in the natural travertine system.
2 Methods
2.1 Sampling site
The Baishuitai travertine site(27°30′N,100°02′E)is located approximately 103 km south of Shangri-La Town,Yunnan Province,China(Fig.1),with an average elevation of approximately 2380 m above sea level.The area is characterized by a subtropical monsoon climate,with>75%(-750 mm)of the annual precipitation occurring during the rainy season from May to October.The annual mean air temperature is approximately 8 °C(Liu et al.2003).The area features a typical karst landscape and hosts one of the largest travertine deposits in China,the Baishuitai travertine system.The travertine forms during calcite precipitation from supersaturated groundwater.
The endogenic travertine samples in this study were collected along a 2.6 km-long canal.The water in the canal was supplied mainly by a spring,with limited water contribution from the adjacent Baishuitai River.When spring water emerged,a vast quantity of CO2was released to the atmosphere,creating an increase in the saturation state of calcite and thus leading to simultaneous calcite precipitation from the water(Yan et al.2016).
2.2 Sample collection and preparation
In December 2013,a set of surface water and travertine samples were simultaneously collected along the canal(Fig.1).To obtain the in situ formed travertine samples,plexiglass substrate plates(15×15 cm)were placed in the water(8 cm deep,in the middle of the canal)at 11 different sites(numbered 1—11)along the canal.Water samples were collected in 2 L precleaned polypropylene containers(Nalgene,America)and then filtered through 0.45μm Millipore express membrane filters immediately after collection.Solutions for cation concentration measurements were acidified to pH<2 with double-distilled HNO3.An aliquot of the solution was not acidified and was prepared for major anion concentration analysis.The precipitated solid travertine samples on the plexiglass plates were transported to the laboratory in 50 mL polypropylene tubes,freeze-dried,and crushed in a mortar for further treatment.
To measure the trace metal concentration contained only in pure carbonates and avoid the dissolution of other substances,approximately 0.5 g of powdered travertine was dissolved in 20 mL 6%acetic acid(v/v)for approximately 24 h to ensure sufficient time and acid to completely dissolve calcium carbonate(Liu et al.2013).The samples were then centrifuged,and the supernatants were collected with pipettes and transferred to Teflon beakers.Afterevaporation at 100 °C,the residue was dissolved again in 3% HNO3for concentration measurements via inductively coupled plasma-mass spectrometry(ICP-MS).
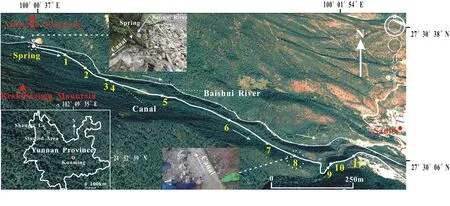
Fig.1 Location of samples in the Baishuitai travertine canal system,Yunnan Province,China
2.3 Chemical parameter analysis
The in situ temperature,pH,dissolved oxygen content,and conductivity of water at each sampling site in the canal were measured using a hand-held multiparameter analyzer(WTW 3430,WTW,Germany),with resolutions of 0.2 °C,0.004,0.01 mg/L and 1μS/cm(micro-Siemens/cm),respectively.The concentrations of Na+,Sr2+,Mg2+,and Ca2+were measured with an inductively coupled plasma optical emission spectrometer(ICP-OES)with a precision generally better than 10%.Selected trace elements were analyzed on a Perkin-Elmer Elan 6000 quadrupole ICP-MS with the addition of an indium solution as an internal standard.The precision of trace element measurements was generally better than 5%.Typical travertine samples were selected for mineral phase analysis by X-ray diffraction(XRD).Scanning electron microscopy(SEM)measurements were also performed to characterize the calcite crystal shapes and habits.All measurements were made at the State Key Laboratory of Environmental Geochemistry of the Institute of Geochemistry,Chinese Academy of Sciences,Guiyang,China.
2.4 Carbonate deposition rate calculation
In this study,the carbonate deposition rate(R),expressed in mol m-2s-1,was approximately estimated as the amount of travertine deposited in a given time period on the plexiglass plates by subtracting the weight of the plate:

whereWandW0are the weights of the plexiglass substrates after and before each experimental run,respectively.Tis the sample collection duration for each run(30 days),andSis the surface area for precipitation,which was roughly estimated as the total surface area of the substrate(225 cm2).
2.5 Metal ion partitioning between calcite and solution
The definition of the apparent partition coefficientKmeis adopted in this paper,which is applied to coprecipitation with calcite,conforming to what is given in the paper of Doerner and Hoskins(1925):
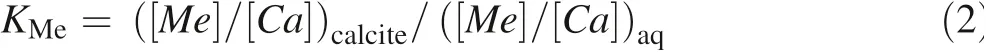
where([Me]/[Ca])calciteis theMe/Camolar ratio of precipitated calcite and([Me]/[Ca])aqis theMe/Camolar ratio of the solution.
3 Result
3.1 Geochemical characteristics of water samples
The geochemical parameters of the 11 water samples are listed in Table 1.The speciation,CaCO3saturation index(SICaCO3)with respect to calcite,CO2partial pressure(PCO2),and ionic strength(I)of the solution is calculated using PHREEQC software with its Visual MINTEQ V4 database and are also listed in Table 1.From sites 1 to 11(with the direction of water flow from upstream to downstream),the pH display a relatively small change between 7.98 and 8.21,but the water temperature gradually decrease from 6.2 to 1.5 °C,the salinity increase from 0.62 to 0.4,andPCO2drop from 349 to 189 Pa.According to the speciation calculation results,Ca2+and HCO3-free ions account for more than 99% of the total ions present in the solution.The logarithmic variation in the deposition rate ranges from-6.00 to-5.09.The results also show that the reactions all occur at a relatively constant ionic strength(I=0.01 mol kg-1).SICaCO3is greater than 0,indicating that calcium carbonate is in a supersaturated state at all sample sites.
3.2 Mineralogy and geochemical composition of the precipitate
The XRD patterns of all solid samples analyzed in this study indicate that calcite is the main phase precipitated in the water flow course(the detection limit of XRD analysis was 2 wt%),which is consistent with previous studies(Liu et al.2006;Yan et al.2016).In accordance with the XRD results,SEM images show that the precipitated solids maintained the rhombic morphology of calcite with overgrowth features on the solid surface(Fig.2).
3.3 Metal ion partitioning between calcite and solution
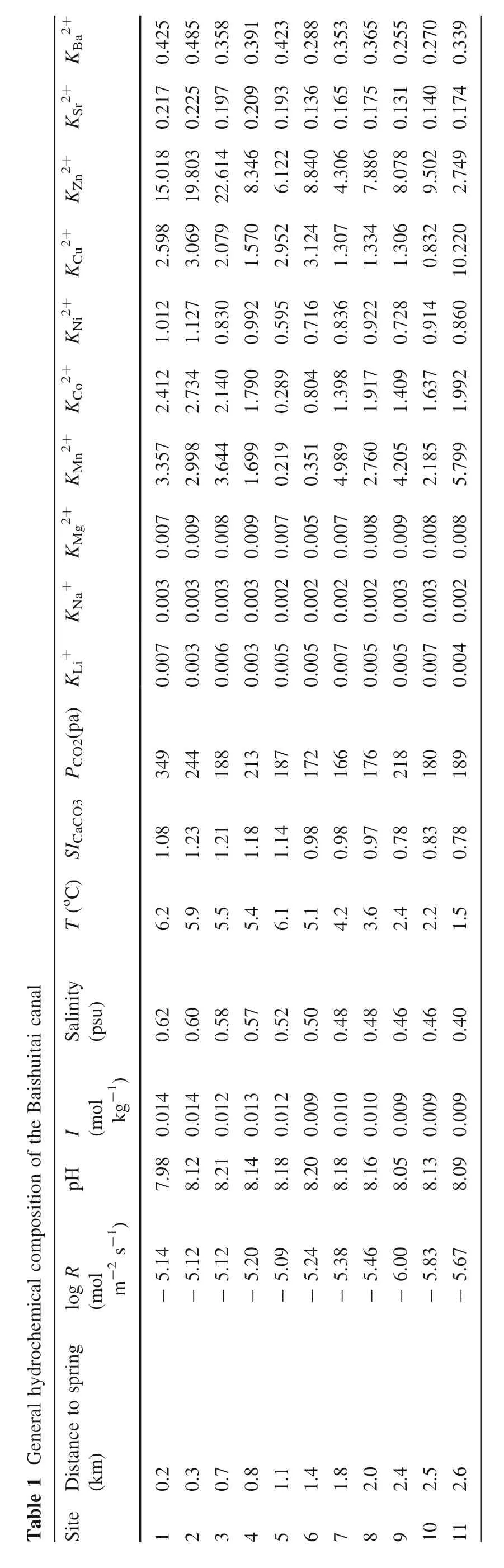
Table 1 General hydrochemical composition of the Baishuitai canal T(oC)SICaCO3 PCO2(pa)KLi+KNa+KMg2+KMn2+KCo2+KNi2+KCu2+KZn2+KSr2+KBa2+Salinity(psu)I(molkg-1)pH log R(molm-2 s-1)Site Distance to spring(km)2.598 15.018 0.217 0.425 3.069 19.803 0.225 0.485 2.079 22.614 0.197 0.358 8.346 0.209 0.391 6.122 0.193 0.423 8.840 0.136 0.288 4.306 0.165 0.353 7.886 0.175 0.365 8.078 0.131 0.255 9.502 0.140 0.270 2.749 0.174 0.339 1.570 2.952 3.124 1.307 1.334 1.306 0.832 0.007 0.003 0.007 3.357 2.412 1.012 0.003 0.003 0.009 2.998 2.734 1.127 0.006 0.003 0.008 3.644 2.140 0.830 0.003 0.003 0.009 1.699 1.790 0.992 0.005 0.002 0.007 0.219 0.289 0.595 0.005 0.002 0.005 0.351 0.804 0.716 0.007 0.002 0.007 4.989 1.398 0.836 0.005 0.002 0.008 2.760 1.917 0.922 0.005 0.003 0.009 4.205 1.409 0.728 0.007 0.003 0.008 2.185 1.637 0.914 0.004 0.002 0.008 5.799 1.992 0.860 10.220 349 244 188 213 187 172 166 176 218 180 189 1.08 1.23 1.21 1.18 1.14 0.98 0.98 0.97 0.78 0.83 0.78 6.2 5.9 5.5 5.4 6.1 5.1 4.2 3.6 2.4 2.2 1.5 0.62 0.60 0.58 0.57 0.52 0.50 0.48 0.48 0.46 0.46 0.40 7.98 0.014 8.12 0.014 8.21 0.012 8.14 0.013 8.18 0.012 8.20 0.009 8.18 0.010 8.16 0.010 8.05 0.009 8.13 0.009 8.09 0.009-5.14-5.12-5.12-5.20-5.09-5.24-5.38-5.46-6.00-5.83-5.67 0.2 0.3 0.7 0.8 1.1 1.4 1.8 2.0 2.4 2.5 2.6 1234567891011
In Table 1,we list the calculated partition coefficients for elements that have been investigated in previous studies(Crocket and Winchester 1966;Curti 1999;Doerner and Hoskins 1925;Elzinga et al.2006;Fu¨ger et al.2019;Gascoyne 1983;Lorens 1981;Mavromatis et al.2013;Mucci and Morse 1983;Okumura and Kitano 1986;Pingitore and Eastman 1984,1986;Reeder et al.1999;Rimstidt et al.1998;Tesoriero and Pankow 1996).The partition coefficients are relatively smaller for Li+,Na+,Mg2+,Sr2+,and Ba2+,varying from 0.003 to 0.007,from 0.002 to 0.003,from 0.005 to 0.009,from 0.131 to 0.225 and from 0.255 to 0.485 respectively.However,those for Mn2+(from 0.219 to 5 0.799),Co2+(from 0.289 to 2.734),Ni2+(from 0.595 to 1.127),Cu2+(from 0.832 to 10.220)and Zn2+(from 2.749 to 22.614)are bigger and show large variation.The partition coefficients of the ions Li+,Na+,Mg2+,Sr2+,and Ba2+are inferior to 1,the partition coefficient of Ni2+is approximately 1,and the partition coefficients of Co2+,Mn2+,Zn2+,and Cu2+are higher than 1.Notably,these values for the natural travertine system are generally in agreement with those for experimental simulations,as summarized in Table 2(Crocket and Winchester 1966;Dromgoole and Walter 1990;Katz 1973;Kitano et al.1980;Lorens 1981;Mucci and Morse 1983;Pingitore and Eastman 1986;Tesoriero and Pankow 1996;Zhong and Mucci 1995).More precisely,the partition coefficients of Li+,Na+,Co2+,Ni2+,Mg2+,Zn2+,and Sr2+are in the same range as or largely overlapped with published experimental data;the partition coefficients of Mn2+and Cu2+are slightly lower than laboratory simulation results;the partition coefficient of Ba2+is higher than that of the laboratory simulation but consistent with the field observations by Drake et al.(2017).
4 Discussion
4.1 Occurreance or absence of thermodynamic equilibrium
We first evaluated whether the travertine precipitation is equilibrium.Establishing equilibrium conditions is the key point for assessing the distribution characteristics of elements between water and carbonate.The calcites are precipitated from oversaturated water with a relatively high flowrate(approximately 1 ms-1),which would not allow for a complete exchange of metal ions between solution and solid phases,suggesting that an equilibrium state was unlikely.This conclusion could be further confirmed by the fact that only a small proportion of aqueous metal ions are precipitated or incorporated into the solid phase during travertine formation(e.g.,<0.03% for Mg2+and<8%for Zn2+).Moreover,according to the thermodynamic model,at 298 K and 1 bar,a linear relationship should exist between lnKMe(the natural logarithm of the partition coefficient)and the free energy difference(ΔG0Ca--ΔG0Me)for equilibrium precipitation(Sverjensky 1984).Assuming that the reaction betweenMein solution and Ca2+in the solid phase reaches chemical equilibrium,we should be able to predict the linear correlation between the partition coefficient of Me in the carbonate-solution system and the difference in the standard Gibbs free energies of Me and Ca2+.As shown in Fig.3,lnKMeversusthe free energy difference between Ca2+and other ions(Me)displays no obvious correlation(Fig.3).
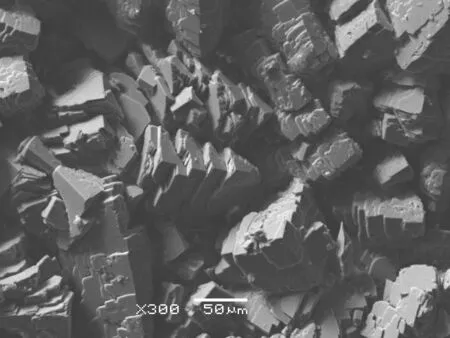
Fig.2 Scanning electron microphotographs of final overgrown precipitates showing a mainly calcite crystal structure
Previous experimental studies of carbonate precipitation in the laboratory show that equilibrium between the solution and the precipitated carbonate is difficult for metal ions to attain even at a long time scale(i.e.,with a much slower precipitation rate)(Morse and Bender 1990;Sverjensky 1984).We hypothesize that no equilibrium state is attained in our travertine precipitation system.Figure 3 demonstrates that ions such as Sr2+,Ba2+,and Mg2+,which possess free energies similar to that of Ca2+,have a partition coefficientKMe<1,whereas ions such as Cu2+,Mn2+and Ni2+with free energies drastically different from that of Ca2+,hadKMe>1.This trend is similar to the features of metal partitioning observed in carbonate precipitation systems in laboratory experiments(Sverjensky 1984).However,the relationship between the partition coefficient and the free energy difference is not consistent with the prediction of equilibrium(Fig.3).Therefore,the equilibrium exchange of metal ions between solution and carbonates would rarely be attained for either laboratory simulations or natural systems.
As a result,the geochemical behaviors and element partitioning could not be predicted and simply explained by the thermodynamic model.Practically,the understanding of partition coefficients is complicated by the fact that true thermodynamic equilibrium is rarely if ever,obtained under near-Earth-surface conditions.Partition coefficients are not equivalent to thermodynamic constants,generally represent phenomenological measurements under certainly given sets of conditions,and are influenced by other factors,including both internal ion physicochemical characteristics and external environmental parameters.
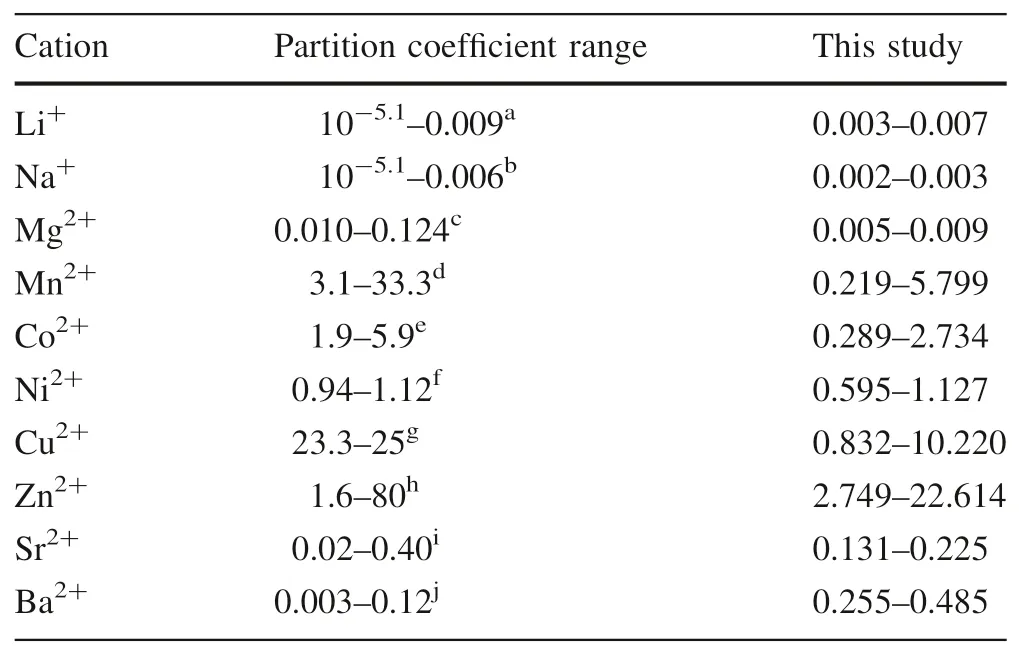
Table 2 Compilation of literature data on the coprecipitation of metals in calcite
4.2 Incorporation of metal ions into calcite
To better define paleoenvironment evaluations using geochemical proxies in surface carbonate archives,the most important consideration is the incorporation mechanism of metal ions into carbonates(Reeder et al.1999).The mechanism is controlled by both the internal physicochemical characteristics of these ions and external environmental factors.
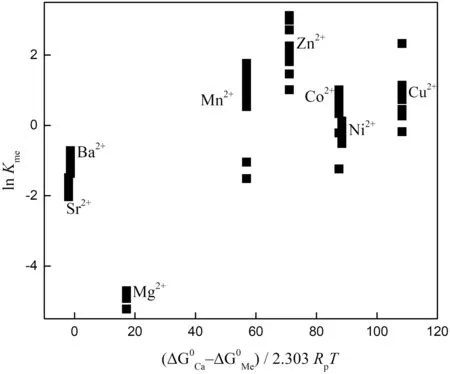
Fig.3 Calculated partition coefficients Kme for certain metal cations between calcite and aqueous solution as a function of the free energy difference between Ca2+and the metal ion Me2+(where Me2+is Co2+,Ni2+,Mg2+,Mn2+,Cu2+,Zn2+,Sr2+and Ba2+).Rp-=0.008306 kJ/K·mol,T=298 K
It is thought that metal ions can exist in carbonate minerals in various ways,including adsorption,coprecipitation,precipitation,and mechanical entrapment(Gaskova et al.2009).Several studies have shown that in addition to the substitution of Ca2+ions in the crystal lattice,other incorporation mechanisms(e.g.,mechanical entrapment,the physical process of occlusion of adsorbates)may be operative in the sequestration of divalent trace metals by calcite,depending on reaction variables such as metal type and concentration,calcite saturation state,reaction time,and pH(Elzinga et al.2006).During incorporation into carbonate crystal lattices,metal ions could eventually be adsorbed on the solid surface.Taking into account the rapid deposition of calcite from water,a certain proportion of metal ions would also be taken up into calcite through mechanical entrapment(Elzinga et al.2006).However,considering the relatively small free spaces in the solid phase,this proportion of metal ions(absorbed in or occupying the spaces)would likely be very limited.Although we could not identify the exact speciation of metals in the calcites at this stage,the substitution of cations(Ca2+)could be the dominant incorporation mechanism.Spectroscopic techniques have provided direct confirmation that metal ions with sizes either significantly smaller or larger than that of host Ca2+can substitute Ca2+in the calcite structure with varying amounts of local distortion(Elzinga et al.2006).In fact,the sizes and charges of coprecipitates are the most important parameters in predicting the partitioning of elements between the two phases(dissolved and solid)and the relative impacts of chemical parameters(Curti 1999).Ions with a radius similar to that of the replaced ion(Ca2+)and the same charge would be more effectively bound in the lattice than would ions with different sizes and charges.Thus,cations with a sharp difference in radius from that of Ca2+would usually have a lower content(and thus a smaller partition coefficient)in carbonate because they are either too large or too small to occupy the octahedral lattice as Ca2+does in calcite(Curti 1999).
Table 3 summarizes the effective ionic radii of the ions Li+,Na+,Co2+,Ni2+,Mg2+,Ca2+,Mn2+,Cu2+,Zn2+,Sr2+and Ba2+in six-fold coordination.In Fig.4,the relationship between the partition coefficients and the ionic radii of the coprecipitation ions does not show a good correlation as a whole.However,the ions withKMe>1 whose ionic radius is smaller than that of Ca2+had a positive correlation between the partition coefficient and ionic radius.This correlation is consistent with the predicted behavior;that is,the closer the radius of the ion is to that of the substituted ion Ca2+,the more easily it can replace Ca2+in carbonate,generally resulting in a greater partition coefficient.However,this trend does not apply for ions with aKMeless than 1.It is interesting to note that although Zn2+(KMe>1)and Mg2+(KMe<1)possess the same charge and similar ionic radius,they have different partition coefficients.In fact,the high hydration energy and a strong affinity with H2O molecules in an aqueous solution of Mg likely contribute to the peculiar behavior of this element(Mavromatis et al.2013).Furthermore,transition metals such as Cu2+,Zn2+,and Ni2+(KMe>1)have unique electrons in the d-orbitals,leading to a much lower ligand stabilization energy(LSE).Thus,these transition metals more readily form stronger complexes with oxygen donor ligands than do alkaline earth metals such as Sr2+,Ba2+,and Mg2+(KMe<1)(Schott et al.2014;White 2011).This property may be one of the reasons for the difference in partition coefficients between transitional elements and alkali metal elements in calcium carbonate.However,whether the partitioning of these metals between water and calcite is in equilibrium and the related controlling factors need to be further systematically investigated through both field and laboratory studies.
Therefore,the incorporation ofMe+ions into the calcite lattice is primarily controlled by their ionic radii and charge.Other environmental factors(e.g.,temperature)may also impact the distribution of metal ions during travertine precipitation,especially for ions withKme<1.
4.3 Partitioning of metals between water and carbonate
The partition coefficient is a key parameter for describing the partitioning of metal ions between water and solid in the travertine system.This parameter is controlled by element physicochemical characteristics and external environmental parameters.In the following,we investigate the effects of parameters such as the temperature,carbonate deposition rate,and pH on the partition coefficients of ions in our travertine system.
4.3.1 Possible effects of temperature and pH
Some previous experimental studies show that the partition coefficients of metal ions might be affected by geochemical parameters such as the temperature and pH of water solutions under a given set of conditions.Fu¨ger et al.(2019)reported that the partition coefficients of Li+and Na+incarbonate are related to the activity of Ca2+ions in the reaction solution and the pH.For example,KLiexhibits a strong decrease from 10-3.3to 10-5.1as the pH increase from 6.3 to 9.5.The distribution coefficient of Mg shows a positive correlation with temperature,with values of 0.0121±0.0013 at 5 °C,0.0172±0.0022 at 25 °C,and 0.0271±0.0013 at 40 °C(Mucci and Morse 1983).However,the partition coefficient of Zn2+in carbonate deposition is negatively related to the temperature(and salinity)of the solution(Tsusue and Holland 1966).Notably,these results are mainly obtained from laboratory experiments with a relatively large variation range in geochemical parameters,and the effects of small variations in temperature and pH in a natural system remain unclear.In this study,we do not observe any correlations between these two parameters with the partition coefficients of the metals K+,Na+,Mg2+,and Zn2+.In fact,the pH of the water solution in our travertine system is quasi-constant(between 7.98 and 8.21),and the temperature also displays only a small variation range(gradually decreasing from 6.2 to 1.5 °C).This relative consistency may be one of the reasons why the effects of temperature and pH on metal distribution would likely be limited.
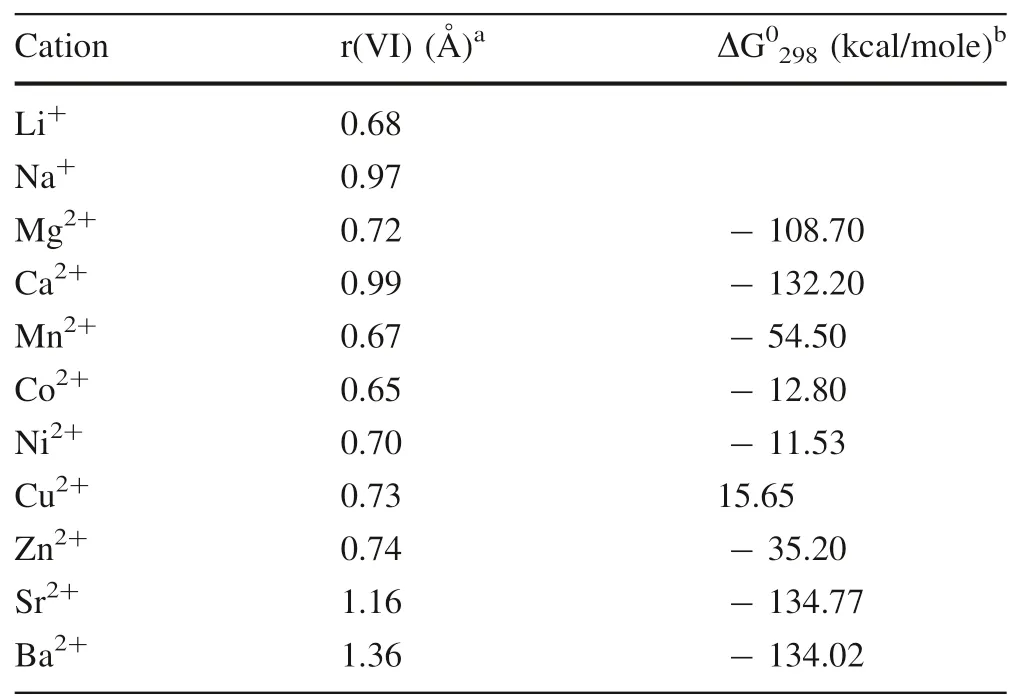
Table 3 Effective ionic radii in six-fold coordination and standard free energies(at 298 K and 1 bar)for ions considered in this paper
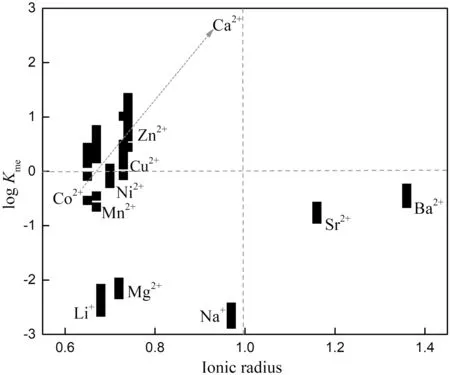
Fig.4 Graphical representation of the partition coefficients of selected metal cations between calcite and solution as a function of the ionic radius of the coprecipitated ion
4.3.2 Effect of calcite deposition rate
Figure 5 indicates the partition coefficients as a function of the deposition rate of calcium carbonate.It should be noted that we were not able to exactly measure the seed crystal surface area at this stage for a given degree of solution saturation;the deposition rate calculated by Eq.1 is not the true calcium carbonate precipitation rate.We could assume a close proportional relationship between the deposition rate calculated by Eq.1 and the true precipitation rate.
The overall logarithmic variation in the deposition rate ranges from-6.00 to-5.09,and the deposition rate exhibits a linear correlation with the SICaCO3saturation degree of the solution with respect to calcite.For ions withKMe<1,the partition coefficients of Sr2+and Ba2+increase with increasing calcium carbonate deposition rate,but correlations for Li+,Na+,and Mg2+are not obvious.In addition,for ions withKMe>1,there is no similar relationship,except that the partition coefficient of Mn2+decreases with increasing calcium carbonate deposition rate.
The effects of growth rate during calcium carbonate mineral precipitation have been documented for a large number of traces/impurities Lorens(1981).indicated that the partition coefficient of Sr2+increased with the calcite deposition rate,while those of Co2+,Mn2+,and Cd2+decreased.Mavromatis et al.(2013)and Fu¨ger et al.(2019)observed that the partition coefficients of Mg2+,Li+,and Na+increased with increasing calcite deposition rate.However,Mucci and Morse(1983)measured the contents of Mg2+and Sr2+during calcite precipitation from natural and synthetic seawater at 25 °C and found that when the deposition rate of calcite changed by an order of magnitude,the contents of Mg2+and Sr2+did not vary significantly,indicating that there was no significant effect of this parameter on the distribution of Mg2+and Sr2+.Therefore,the precipitation rate has displayed controversial effects on the partition coefficients of metal ions in surface carbonate systems(Morse and Bender 1990).In fact,kinetic influences could cause both increases and decreases in the partition coefficients of metal elements between solid and solution phases.At high precipitation rates,there is even a chance for the physical process of occlusion of adsorbates to occur.However,as described by Rimstidt et al.(1998),with increasing growth rate,elemental partitioning during incorporation into a carbonate mineral phase tends towards unity.Consequently,this behavior iesimplies that the partition coefficients of ions withKMe<1(such as Ba2+,Sr2+,Li+and Na+)increase with the calcite deposition rate,while the partition coefficients of ions withKMe>1(such as Co2+,Mn2+and Cd2+)decrease with the calcite deposition rate.Because the partition coefficient of Ni2+is approximately 1,the change in the Ni2+partition coefficient with the deposition rate of calcite is not obvious.
The impact of deposition rate on metal ion partitioning between calcite and water could be explained by the growth entrapment model(GEM)developed by Watson and coworkers(Fu¨ger et al.2019;Mavromatis et al.2013;Watson 1996,2004).Over the last decades,GEM has been successfully applied to many cases to describe elemental cation partitioning between carbonate minerals and fluids.According to the model,a trace element incorporated in a growing crystal could be either enriched or depleted in the near-surface layer because this region is structurally distinct from the bulk lattice.Cations such as Ba2+or Sr2+,which are relatively incompatible in the calcite lattice(KMe<1),would be enriched in the distorted and hydrated thin layer(<1 nm thick)present at the crystal surface.Rapid growth rates would favor the entrapment of the enriched trace elements in the overgrowth region by increasing the diffusion distance.Accordingly,the partition coefficient of such elements increases with increasing calcite deposition rate.For elements withKMe>1,GEM predicts the depletion of trace metals in the near-surface region and thus inefficient diffusion due to a fast growth rate,leading to growth exclusion and nonequilibrium partitioning.As a result,the partition coefficients of these elements decrease with increasing calcite deposition rate.
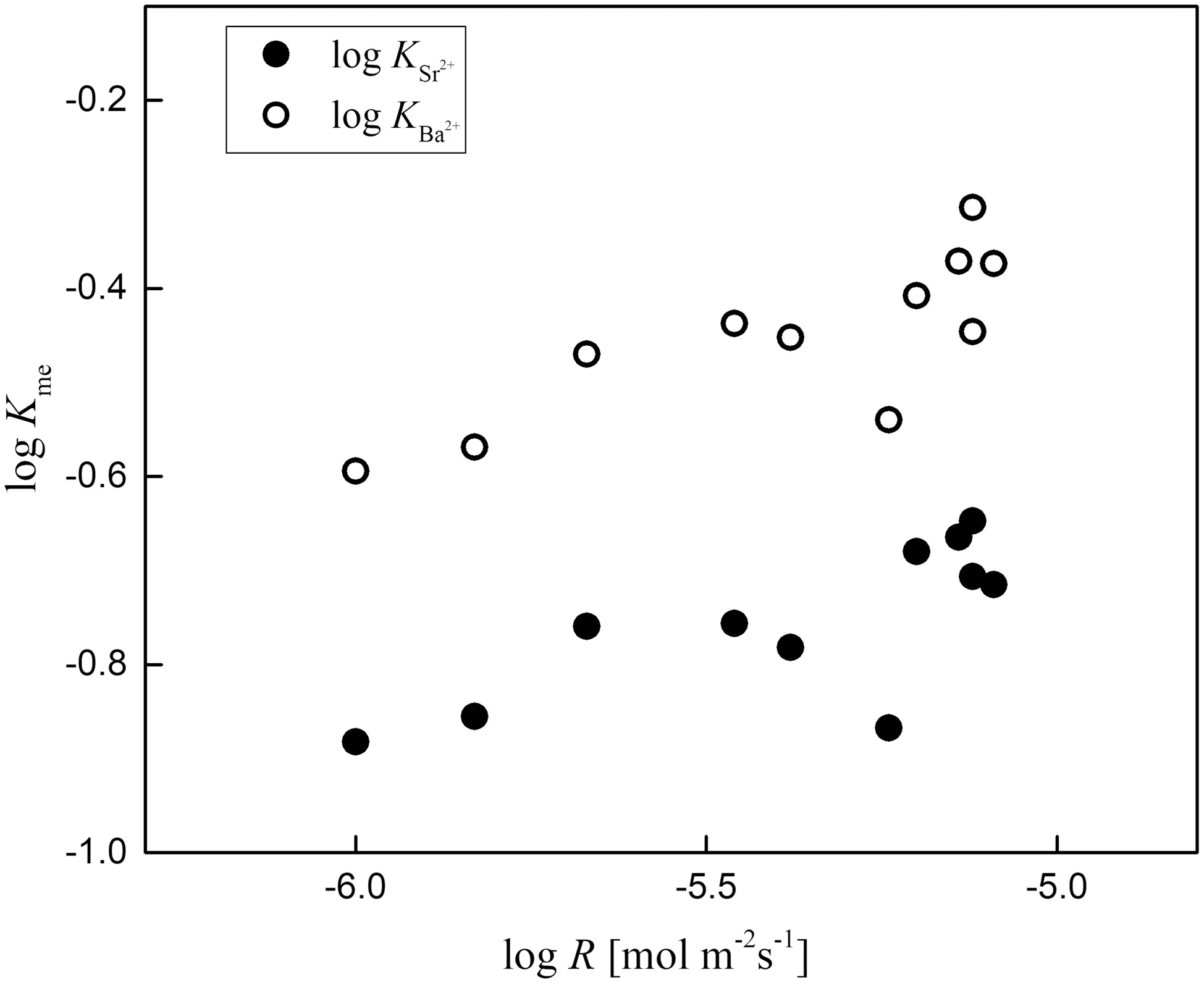
Fig.5 Kme of Mg2+and Sr2+as a function of calcite deposition rate
The partition coefficients of the cations Sr2+and Ba2+exhibit stronger correlations with the calcite growth rate than do those of other impurities in calcite,as demonstrated in Fig.5.This result is consistent with the observed variations in trace element uptake with a growth rate under abiogenic conditions in the laboratory.Consequently,the concentrations of Sr2+and Ba2+in calcite are likely very sensitive indicators of calcite mineral growth,and the partitioning of Sr2+and Ba2+in natural calcite could be used as a potential tool for estimating the formation rate.
However,the partition coefficients of other metals withKMe<1 do not show any correlation with deposition rate,indicating that the kinetic formation rate is not the main factor controlling the partitioning of these metal ions;other parameters would also play an important role.Moreover,the partition coefficients of all metals withKMe>1(Zn2+,Co2+,Mn2+,Cd2+,etc)also display no correlation with the deposition rate,implying no influence of this factor on the partitioning of these metals between the two phases.Above all,these results suggest that the partitioning of most metal ions between solid and water phases in a natural travertine precipitation system would be controlled by multiple parameters other than a single factor.This finding should be taken into account when reconstructing the paleoenvironment or paleoclimate using trace element proxies in natural carbonate archives.
5 Conclusion
Metal partitioning between solid and solution in the natural travertine system showed a large variation in partition coefficients,withKMe<1 for Li+,Na+,Mg2+,Sr2+and Ba2+,KMe>1 for Co2+,Mn2+,Zn2+and Cu2+,and aKMeof approximately 1 for Ni2+,consistent with previous laboratory experiments.The distributions of metals between the two phases were entirely consistent with the predicted behavior of the thermodynamic model,suggesting that the partitioning of metal ions into calcite under surface conditions was not,in general,an equilibrium process.Therefore,in addition to the inherent ionic characteristics of metals,such as the ionic radius and charge,external environmental parameters such as temperature,pH,and carbonate precipitation rate would also substantially affect metal distribution.Our results demonstrate that the partitioning of the metal cations Sr2+and Ba2+was mainly controlled by the calcite precipitation rate but the distributions of other metal ions were likely impacted by several,or even multiple,environmental parameters.As a result,one should pay attention to the specific incorporation mechanism and the corresponding controlling factors when applying metal proxies in surface carbonate archives to reconstruct past environments.The results also imply that further systematic studies are needed to better constrain the trace metal distribution in both natural carbonate systems and laboratory experiments with regard to paleoenvironmental reconstruction.Such research work would also help reduce the uncertainty that arises in interpreting natural carbonate composition data and in paleoclimate reconstruction models employing the partitioning of elements as a proxy.
AcknowledgementsThis work was financially supported by the National Key Research and Development Program of China(2019YFC1804400),the National Natural Science Foundation of China(U1612442,41961144028,41625012,41830647),‘‘Ten Thousand Talent’’project of Ministry of Science and Technology of the People’s Republic of China.
杂志排行
Acta Geochimica的其它文章
- Early Silurian Wuchuan-Sihui-Shaoguan exhalative sedimentary pyrite belt,South China:constraints from zircon dating for K-bentonite of the giant Dajiangping deposit
- Niobium-tantalum oxide minerals in alluvial placer deposits from the Ngoura area,East-Cameroon
- Development of a 100 MPa water-gas two-phase fluid pressurization device
- Helium and argon isotope geochemistry of the Tibetan Qulong porphyry Cu-Mo deposit,China
- Translocation and distribution of mercury in biomasses from subtropical forest ecosystems:evidence from stable mercury isotopes
- Neoproterozoic highly fractionated I-type granitoids of Shillong Plateau,Meghalaya,Northeast India:geochemical constraints on their petrogenesis
