Translocation and distribution of mercury in biomasses from subtropical forest ecosystems:evidence from stable mercury isotopes
2021-03-03YiLiuCheJenLinWeiYuanZhiyunLuXinbinFeng
Yi Liu •Che-Jen Lin•Wei Yuan•Zhiyun Lu•Xinbin Feng
Abstract To understand its source,distribution,storage,and translocation in the subtropical forest ecosystems,mercury(Hg)concentrations and stable isotopes in forest biomass tissues(foliage,branch,bark,and trunk)were investigated at Ailao Mountain National Nature Reserve,Southwest China.The total Hg(THg)concentrations in the samples show the following trend:mature foliage(57±19 ng g-1)>bark (11±4.0 ng g-1)>branch(5.4±2.5 ng g-1)>trunk(1.6±0.7 ng g-1).Using the measured THg concentrations and the quantity of respective biomasses,the Hg pools in the forest are:wood(60±26μg m-2)>bark(51±18μg m-2)>foliage(41±11μg m-2)>branch(26±8.3μg m-2).The tree biomasses displayed negativeδ202Hg(-1.83‰ to-3.84‰)andΔ199Hg(-0.18‰ to-0.62‰).The observedΔ200Hg(-0.08‰ to 0.04‰)is not significantly from zero.AΔ199Hg/Δ201Hg ratio of 1.05 was found in tree biomasses,suggesting that mercury has undergone Hg(II)photoreduction processes.A Hg-isotope based binary mixing model suggests that Hg in the tree biomasses mainly originated from foliage uptake of atmospheric Hg0,constituting 67%,80%,and 77% of Hg in wood,branch,and bark,respectively.Our study sheds new light on the transportation and sources of Hg in the subtropical forest ecosystems.
Keywords Hg·Subtropical forest ecosystem·Hg stable isotope·Hg-isotope based binary mixing model
1 Introduction
Mercury(Hg)in the atmosphere mainly exists(>90%)in the form of gaseous elemental Hg(GEM)(Gratz et al.2010),whose atmospheric lifetime(0.5-1 year)allows long-range transport globally(Selin 2009).Forest ecosystems play an important role in the biogeochemical cycling of Hg on a global scale.Oxidation of Hg0followed wet deposition(e.g.,precipitation and/or throughfall),or Hg0uptake by vegetation followed by litterfall deposition are the main pathways for Hg deposition.Recent studies suggested that the forest ecosystems act as a major sink of atmospheric Hg0through litterfall Hg deposition(Johnson and Lindberg 1995;Laacouri et al.2013;Louis et al.2001;Wang et al.2017).However,the post-depositional biogeochemical processes controlling the storage,accumulation,and sequestration of Hg remain not well understood(Gu et al.2011;Jiang et al.2015;Jiskra et al.2015;Wang et al.2003;Yu et al.2016;Yuan et al.2019).
The Hg distribution in forest ecosystems highly depends on the processes governing the translocation of Hg after vegetative uptake,while the translocation and distribution result in the storage of Hg in the forest.Previous studies reported the levels of THg in plants(Ericksen et al.2003;Kang et al.2019;Yang et al.2018),suggesting that a dual mechanism controlled Hg uptake in tree tissues with some uptake from the soil by the roots and the majority of uptake from atmospheric sources by the leaves.A recent study reported that the Hg pool size in tree woody biomass can be 1–3 times higher than that in foliage at four forested sites in the northeastern USA,which highlighted the role of woody biomass in Hg cycling in forest ecosystems(Yang et al.2018).
Hg isotopic signatures provide a valuable fingerprint for tracing source and process(Bergquist and Blum 2009;Blum et al.2014;Yin et al.2014).The isotopic composition of Hg in foliage in the forest ecosystem has been investigated extensively(Demers et al.2013a;Yu et al.2016;Yuan et al.2019;Zheng et al.2016),explaining Hg in the foliage was mainly sourced from GEM.However,data suggesting the sources and net reserves of Hg in biomass tissues of trees other than foliage are still relatively rare,which limits the understanding of Hg cycling in forest ecosystems.
The objective of this study is to provide a comprehensive description of the distribution,storage,and translocation of Hg in biomass in a subtropical forest ecosystem,the Ailao Mountain National Nature Reserve,Southwest China.We performed measurements on THg concentrations,and its associated isotopic compositions in foliage,branch,bark,and trunk biomasses and estimate the Hg storage pools in each of the biomass compartments.These measurements aim to gain a better understanding of the distribution and sources of Hg in the forest ecosystem.
2 Materials and methods
2.1 Study area,sampling and sample preparatio n
The Xujiaba region(24°32′N,101°01′E)is located in the Ailao Mountain National Nature Reserve,Southwest China,at an altitude of 2400–2650 m(You 1983)(Fig.1).It is a subtropical evergreen broad-leaved forest characterized with abundant rainfall(1840 mm per year)(Song et al.2015)with three dominant evergreen tree species,includingLithocarpus xylocarpus(LX),Castanopsis wattii(CW),andSchima Noronhae(SN)at the proportion of 16.3%,16.3%,and 23.3%,respectively(Xie et al.1996).The frost-free period lasts about 180 days annually(Tan et al.2011).Influenced by the Indian Ocean southwest monsoon,the forest is cool and humid,with an annual average temperature and relative humidity at 11.3 °C and 84%,respectively.In the forest area,the soil is primarily yellow–brown with a pH of 4.4–4.9(Liu et al.2002).
Samples were collected at three elevations(S1:N24.5398°,E101.0278°,2450 m a.s.l;S2:N24.53817°,E101.02968°,2550 m a.s.l;S3:N24.53629°,E102.03129°,2650 m a.s.l).Three dominant tree species as previously mentioned were selected for biomass sampling.Mature foliage,branch,bark,and wood were sampled in October 2016.The foliage and branch samples were sampled at three canopy heights(i.e.,upper,middle,and lower canopy)at three heights(20,21,and 22 m),and then mixed as a composite sample.The bark and wood samples from each selected tree species were collected at the chest height(~1.5 m)at three replicates.Bedrock samples were sampled from a depth of 20 m underground using a drilling machine.
All the samples were placed in clean polyethylene bags and taken back to the laboratory for analysis.During the each sampling,new polyethylene gloves were worn to avoid cross-contamination.Each sample was washed by DDW and freeze-dried at-60° to constant weight,then ground to fine powder.
2.2 Sample analysis
2.2.1 THg concentration
THg concentration in tree biomass samples was measured by the Lumex RA-915+(Lumex Analytics,Russia,detection limit:1 ng g-1)atomic absorption spectrometric analyzer(Wang et al.2010).The method recovery was 4.6±3.5 %(n=12,1σ),96.3±5.4 %(n=10,1σ),97.2±4.6 %(n=10,1σ)for GBW10049(GSB-27),BCR-482(Lichen)and GBW10020(GSB-11)respectively.
2.2.2 Hg isotopes
The samples were prepared by pre-concentration following a previous method(Sun et al.2013).Briefly,Hg was preconcentrated by double-stage tube furnace combustion,and the vaporized Hg0was subsequently trapped by a 5 mL freshly 40 % concentrated mixture of HNO3and HCl(HNO3:HCl=2:1,V/V).Before Hg isotope measurement,THg concentration of all trapping solutions was analyzed by cold vapor atomic fluorescence spectrometry(CVAFS,Tekran 2500,Canada)using the US-EPA method 1631(1631,2002).The recovery of Hg was 97.2±6.3 %(n=5,mean±1σ)during thermolysis the standard reference material BCR482(lichen,480±20 ng g-1),and the recoveries of Hg were 92.5±4.3%(n=9,mean±1σ)for foliage samples,90.6±3.2 %(n=6,mean±1 σ)for branch samples,93.7±2.3 %(n=4,mean±1σ)for bark samples,91.8±3.5 %(n=4,mean±1σ)for wood samples,and 90.7±3.2 %(n=4,mean±1σ)for rock samples respectively,according to THg measured by Lumex RA-915+.Hg isotopic compositions were determined by a Nu Plasma II Multiple-collector inductively coupled plasma mass spectrometry(Nu Instruments,UK),following a previous method(Yin et al.2010).

Fig.1 Site locations for biomasses collection at different altitudes from Mt.Ailao.S1,S2 and S3 represent three different elevations in forest respectively(S1:N24.5398°,E101.0278°,2450 m a.s.l;S2:N24.53817°,E101.02968°,2550 m a.s.l;S3:N24.53629°,E102.03129°,2650 m a.s.l)
Mass dependent fractionation(MDF)and mass independent fractionation(MIF)of Hg isotopes,in units of permil(‰),were expressed as the following equations(Bergquist and Blum 2007b):

xxx is the mass of each Hg isotope from 199 to 202 amu,the factorβxxxis 0.252 for199Hg,0.502 for200Hg and 0.752 for201Hg,respectively.
During the Hg isotopes analysis,in addition to repetitive measurement(typically n=2),we also used the UMAlmade´n as a second standard and measured once in every 10 samples.Results of UM-Almade´n(-0.52±0.06 ‰forδ202Hg,0.03±0.05 ‰ forΔ199Hg,-0.01±0.05 ‰forΔ200Hg and-0.02±0.10 ‰forΔ201Hg,2SD,n=5)and BCR-482 (-1.53±0.22 ‰ for δ202Hg,-0.66±0.08 forΔ199Hg,-0.06±0.03 ‰ forΔ200Hg and-0.67±0.06 ‰ forΔ201Hg,2SD,n=5)were consistent with the recommended values(Bergquist and Blum 2007a;Blum and Bergquist 2007;Estrade et al.2010).
3 Results and discussion
3.1 Distribution of THg in biomass

Fig.2 THg concentrations measured in the vegetative samples in this study
The measured THg concentrations(Fig.2)in matured foliage ranged from 29.6 to 105.9 ng g-1.THg concentrations in the fresh litter were 74±19 ng g-1for CW(mean±SD,n=9),56±13 ng g-1for LX(mean±SD,n=9),and 42±10 ng g-1for SN(mean±SD,n=9),respectively.The varied foliage THg was attributed to tree species that yield different uptake flux and reemission flux(Du et al.2018;Louis et al.2001;Luo et al.2016;Poissant et al.2008;Yuan et al.2019).The assimilated Hg in foliage is expected to be transferred to the trunk(Frescholtz et al.2003).HgIIin the soil can be utilized and moved through the root(Ericksen et al.2003;Rea et al.2002),which only play the secondary role in accumulating Hg in-ground biomass(Cui et al.2014).The observed THg concentrations were 5.4±2.5 ng g-1for branch(mean±SD,n=27),11±4.0 ng g1for bark(mean±SD,n=27)and 1.6±0.7 ng g-1for trunk(mean±SD,n=27).
Table 1 shows THg concentration of plant tissues in forest ecosystems observed at global sites.Previous studies(JA et al.2003;Kang et al.2019;Siwik et al.2009;Sun et al.2017;Yang et al.2018;Zhou et al.2016,2017)have focused on the distribution of Hg in foliage and trunk.The data show a wide range of THg concentrations in foliage from 3 to 160 ng g-1.The large variation may be the resulted from the tree species,which were verified in previous studies(Juillerat et al.2012;Richardson and Friedland 2015;Siwik et al.2009);foliage age,which resulted in long exposure and accumulation time in longevity foliage(Ericksen et al.2003;JA et al.2003;Millhollen et al.2006;Rea et al.2002;Selvendiran et al.2008;Siwik et al.2009);climate conditions and atmospheric THg concentrations,which affected the THg accumulation in foliage(Frescholtz et al.2003).These factors influence uptake and absorption by stoma(Demers et al.2013b;Laacouri et al.2013;Yuan et al.2019;Zheng et al.2016).The tree trunk has low levels of THg concentration(Table 1).In most areas,the THg levels in the trunk were below 5 ng g-1(Kang et al.2019;Siwik et al.2009;Yang et al.2017,2018).Our results indicate that THg content in the trunks of the subtropical evergreen broad-leaved forest from Ailao Mountain ranged from 0.8 to 2.4 ng g-1.Data for bark and branch,constituting 7 %–10 % of biomasses in forest ecosystems,are rarely reported.Available data suggested bark contained significantly higher THg than branch(Zhou et al.2016,2017).Our study shows a similar trend(Fig.2),suggesting that despite differences in sampling heights and tree species,the mechanism of Hg retention in tree branch and bark was similar.
Globally,forest biomass and soil constitute some of the largest reservoirs of actively cycling Hg(Juillerat et al.2012),especially in tropical and subtropical forest ecosystems(Wang et al.2019).Atmospheric Hg0could be taken up by foliage via stomata or absorbed on the surface(Laacouri et al.2013;Yuan et al.2019).Further,atmospheric Hg was assimilated by foliage and partly released(~30%)again to the atmosphere(Yuan et al.2019),resulting in a large proportion of Hg accumulation.The accumulated Hg in foliage could be transferred to the trunk based in a previous study(Frescholtz et al.2003),while another study showed the opposite results that THg concentrations in branch and trunk were influenced by THg level in soil(Ericksen et al.2003).Meanwhile,the HgIIin the soil can be utilized and moved through the root(Ericksen et al.2003).Such a process that occurred at root is expected to be a secondary process in accumulating Hg in-ground biomass such as branch and trunk.
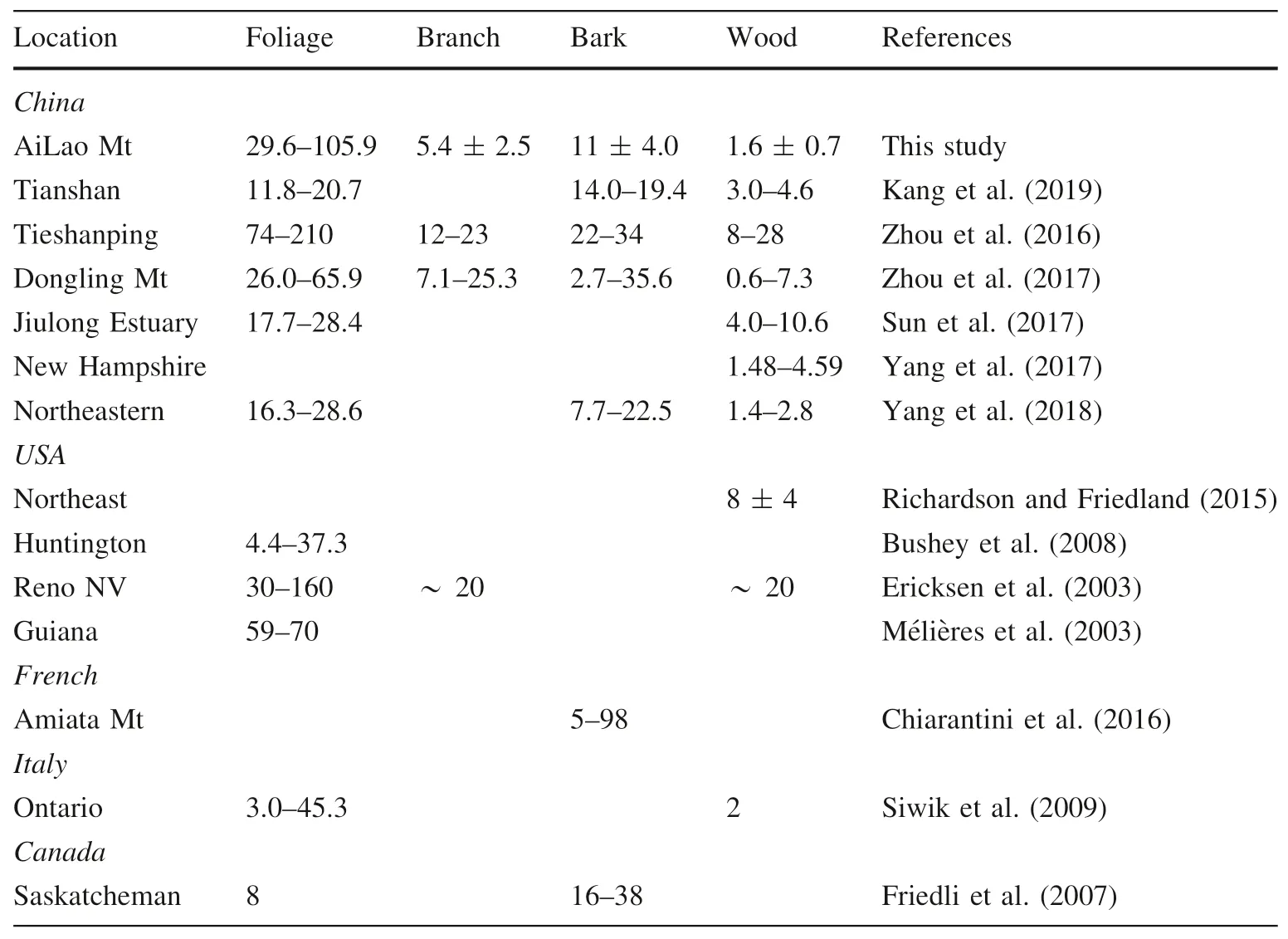
Table 1 THg concentration of biomass in related study sites

Fig.3 Isotopic signature of Hg for the vegetative and bed rocks in this study
3.2 Storage of THg in biomass
Given the large biomass mass in bark,branch,and trunk in a forest with a low turnover rate due to a relatively long life cycle,the storage and transformation of Hg in such biomass could play a significant role in Hg cycling and should not be ignored.At Mt.Ailao,the average ground biomass was 7.1 t ha-1,33 t ha-1,46 t ha-1,373 t ha-1for foliage,branch,bark,and trunk,respectively.The pools size of Hg was estimated using the following equation:
Poolbiomass=Cb×Mb. (3)where,Cb(ng g-1)and Mb(t ha-1)are the THg concentration and biomass of tree biomass samples,respectively(Zhou et al.2017).Table 2 shows the average pools of Hg in biomass followed in a descending order:wood(60μg m-2)>bark(51μg m-2)>foliage(41μg m-2)->branch(26μg m-2).The corresponding proportion was 33.7 %,28.7 %,23.0 %,and 14.6 % for the pool of biomass in wood,bark,foliage,and branch,separately.This distribution is different from the reported biomass pool(Zhou et al.2017)reported previously,which a pool size in the order of branch>foliage>bole>bark for Chinese pine,branch>bole>bark>foliage for a mixed broadleaf forest.For Larch and Oak,the order was similar:branch>bark>foliage>bole.These suggested that the tissue of the branch was the largest pool of Hg;and that the Hg reservoirs vary in different forest types due to the biomass affected by the canopy geometry,areal density of species,and individual differences of each plant(Bushey et al.2008;Richardson and Friedland 2015)and the THg concentration probably due to the physiological property of plants,such as migration rate,and retention in the xylem(Zhou et al.2017).Although wood has the lowest THg concentration at<2 ng g-1,it has the largest pool size of Hg due to its greatest biomass at 60μg m-2.The bark is the second large Hg reservoir due to the higher THg content and greater biomasses,up to 51μg m-2.The foliage has a slightly lower pool of Hg than bark at 41μg m-2,while the branch has the smallest pool of Hg at 26μg m-2.The ground biomass of the subtropical forest ecosystem store a large quantity of Hg mass at nearly 180μg m-2.
3.3 Sources and processes of Hg implicated from stable isotope data
Characteristics of Hg stable isotope in the forest ecosystem provided a useful fingerprint for tracing Hg translocation and transformation.As listed in Table 3,all the biomass samples exhibited distinctive negativeδ202Hg(-1.83 to-3.84 ‰)andΔ199Hg(-0.18 ‰ to-0.62 ‰),but near-zeroΔ200Hg(-0.08 ‰ to 0.04 ‰).For foliage,Hg isotopic composition was characterized with negative δ202Hg andΔ199Hg averaged with-2.95±0.96 ‰ and-0.35±0.13 ‰,respectively,while neglectableΔ200Hg averaged with-0.03±0.05 ‰(n=9,2σ).Such results were consistent with Hg isotopic compositions in leaves reported earlier(Enrico et al.2016;Fu et al.2016;Yu et al.2016;Yuan et al.2019),which was attributed to the uptake process that causes preferential absorption of lighter isotopes.Subsequently,the reemission process that occurred at leaves produces negative odd-MIF remained in the residue(Yuan et al.2019).
Hg isotopic compositions in-branch averaged-2.80±0.90 ‰ and-0.35±0.21 ‰ forδ202Hg andΔ199Hg respectively, while Δ200Hg averaged 0.01±0.09 ‰(n=6,2σ).Bark showed negativeδ202Hg andΔ199Hg as-2.46±1.13 ‰ and-0.44±0.19 ‰respectively,but negligibleΔ200Hg as-0.05±0.07 ‰(n=4,2σ).Wood also displayed negativeδ202Hg and Δ199Hg with-2.72±0.46 ‰ and-0.35±0.12 ‰independently, but insignificant Δ200Hg with-0.01±0.04 ‰(n=4,2σ).The similar MIF values among foliage,branch,bark,and trunk(Fig.3)revealed Hg in biomasses were mainly derived from foliage transport,and there was no MIF generated during transmission(Sun et al.2017;Yin et al.2013a).The slope ofΔ199Hg/Δ201Hg is a useful fingerprint to identify the geochemical processes since the transformation in the tree does not shift Hg odd-MIF(Estrade et al.2009;Ghosh et al.2013;Jiskra et al.2012;Koster van Groos et al.2014;Wiederhold et al.2010;Yin et al.2013b).The slope ofΔ199Hg/Δ201Hg in all the samples is found to be 1.05(R2=0.86),suggesting that mercury has undergone Hg(II)photoreduction before entering the tree biomass(Fig.4).

Table 2 THg concentration in vegetative biomasses and their associated storage
Given the Hg in-ground biomass were mainly contributed by atmospheric Hg0uptake through foliage and bedrock through the root(Sun et al.2017;Yin et al.2013a),the contribution of foliage and bedrock can be calculated.Different from the MDF of Hg isotopes,which can be caused in almost all processes,including chemical processes(Bergquist and Blum 2007a;Sun et al.2016;Yang and Sturgeon 2009;Zheng et al.2019;Zheng and Hintelmann 2009,2010b),physical process(Estrade et al.2009;Ghosh et al.2013;Jiskra et al.2012;Koster van Groos et al.2014;Wiederhold et al.2010;Yin et al.2013b),and biogeochemical process in which microorganisms were involved(Bergquist and Blum 2007a;Kritee et al.2008,2007,2013;Malinovsky and Vanhaecke 2011;Perrot et al.2015),the odd-MIF of Hg isotopes is considered to be caused only in some special geochemical processes,such as photochemical process,methylmercury photodegradation,equilibrium evaporation and organic matters dark reduction/oxidation(Bergquist and Blum 2007a;Blum et al.2013;Estrade et al.2009;Ghosh et al.2013;Jimenez-Moreno et al.2013;Jiskra et al.2012;Wiederhold et al.2010;Yang and Sturgeon 2009;Zheng et al.2019;Zheng and Hintelmann 2009,2010a,2010b).Therefore,odd-MIF is utilized to build a two end-member mixing model to quantify of the source of Hg in-ground biomass in the following equation based on a previous study(Yin et al.2013a).
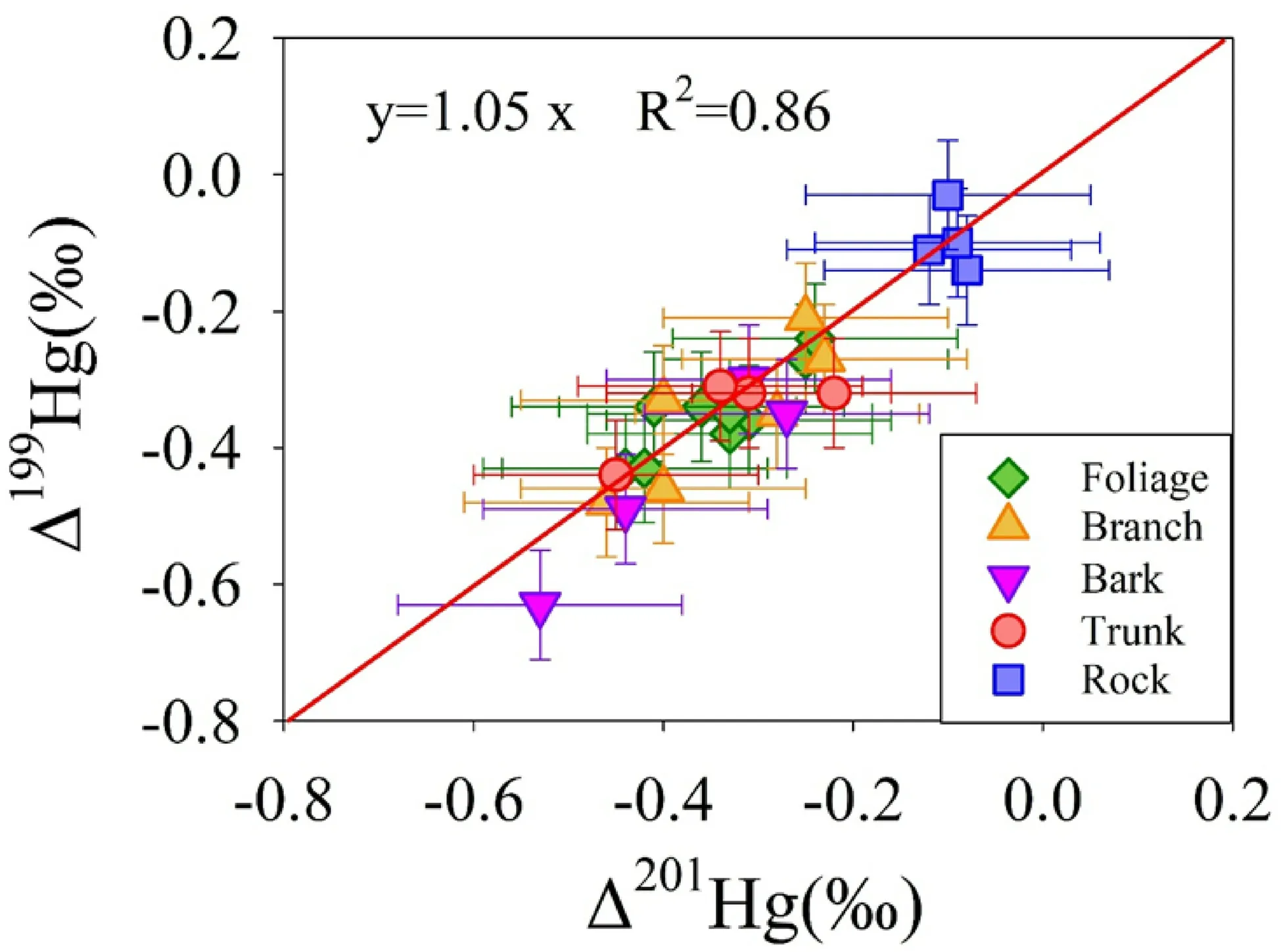
Fig.4△199Hg versus△201Hg for the vegetative samples in this study

whereΔ199Hgbiomassis theΔ199Hg values of biomass including samples of the branch,bark,and wood.Δ199Hg foliageandΔ199Hgrockare theΔ199Hg values of foliage Hg and rock Hg,respectively.Ffoliage(%)is the fraction of foliage Hg source.Considering the uncertainties that occurred at isotopic measurement,to further assess the uncertainty in calculation results,a Monte Carlo simulation was applied in the modeling calculation.More specifically,the every Hg isotopic signature,which was used in solving the above equations,was produced randomly around the mean with 1 standard error followed a normal distribution.Subsequently,the equation would be solved to get the fraction of different end-members.Such operations were applied to one million simulations to accomplish the simulation of a fraction of foliage or bedrock and its uncertainties.Results from the end-member mixing model(Fig.5)showed that atmospheric Hg followed by foliage transport was the dominant source for the ground woody biomass.Approximately 66.7 %of the Hg in the wood was derived from Hg transport through the foliage,while Hg in bark and branch were derived mainly from the foliage transport,up to 77 % and 80 % respectively.The much higher atmospheric Hg fractions in the woody biomass suggested that soil contributes little to the ground biomass Hg(Table 3).

Fig.5 Estimated fraction of atmospheric Hg in the vegetative biomass samples

Table3 Isotopic composition of mercury measured in ground tree biomasses and rocks Average THg ng g-1 2sd ‰Δ201Hg‰2sd ‰Δ200Hg‰2sd ‰Δ199Hg‰2sd ‰δ202Hg‰2sd ‰δ201Hg‰2sd ‰δ200Hg‰2sd ‰δ199Hg‰n Samples 485.4 111.6 5.2 1.0 467 0.14 0.19 0.24 0.19 0.03 0.10 0.06-0.34-0.34-0.39-0.33-0.10-0.02-0.67 0.05 0.09 0.07 0.04 0.04 0.05 0.03-0.03 0.01-0.05-0.01 0.01-0.01-0.06 0.13 0.21 0.29 0.12 0.09 0.05 0.08-0.35-0.35-0.44-0.35-0.10-0.03-0.66 0.96 0.90 1.13 0.46 0.24 0.06 0.22-2.95-2.80-2.46-2.72-0.29-0.52-1.53 0.79 0.56 0.91 0.28 0.17 0.18 0.20-2.56-2.44-2.24-2.37-0.32-0.41-1.81 0.46 0.39 0.47 0.26 0.09 0.08 0.12-1.51-1.40-1.29-1.38-0.14-0.25-0.72 0.33 0.23 0.39 0.17 0.07 0.06 0.11-1.09-1.06-1.06-1.03-0.17-0.16-1.04 9 6 4 4 4 5 5 Foliage Branch Bark Wood Rock UM-Almade´n BCR-482
4 Conclusion
This paper explores the THg distribution,storage,and Hg isotope composition in each part of over-ground tree biomass in the subtropical evergreen broad-leave forest of Ailao Mountain National Nature Reserve.We concluded that the THg in the biomass samples is derived mainly from the foliage transmission to ground biomass reaching 80 %,77 %,66.7%in branch,bark,and trunk,respectively.The net storage reservoir of Hg approximately is nearly 180μg m-2in-ground biomasses,suggesting that tree biomass acts as a sink in the Hg cycling in forest ecosystems.Approximately 100μg m-2was derived from foliage transport to branch,bark,and trunk,making up 80 %of the Hg reservoir in-ground biomasses,while bedrock only contributed 20 % Hg to the ground biomass.These indicate that the transmission of foliage Hg to branch,bark,and trunk is the main route of Hg biogeochemical cycling in forest ecosystems.
AcknowledgementThis work was funded by the National Natural Science Foundation of China(No.41430754).We thank Jin-Hua Qi for the assistance in sample collection.The Ailao Mountain Ecosystem Research Station was thanked for its fieldwork support.
杂志排行
Acta Geochimica的其它文章
- Early Silurian Wuchuan-Sihui-Shaoguan exhalative sedimentary pyrite belt,South China:constraints from zircon dating for K-bentonite of the giant Dajiangping deposit
- Niobium-tantalum oxide minerals in alluvial placer deposits from the Ngoura area,East-Cameroon
- Development of a 100 MPa water-gas two-phase fluid pressurization device
- Helium and argon isotope geochemistry of the Tibetan Qulong porphyry Cu-Mo deposit,China
- Neoproterozoic highly fractionated I-type granitoids of Shillong Plateau,Meghalaya,Northeast India:geochemical constraints on their petrogenesis
- Coprecipitation of metal ions into calcite:an estimation of partition coefficients based on field investigation
