层状钒青铜纳米片的制备及其锂离子电池阳极材料性能
2021-02-26马录芳谭超良
周 战,马录芳,谭超良
(1.洛阳师范学院化学化工学院,河南省功能多孔材料重点实验室,洛阳471934;2.香港城市大学电机工程系,香港九龙)
1 Introduction
Layered two-dimensional(2D)materials,such as graphene,transition metal dichalcogenides and layered metal oxides,have been proven to be promising in a wide range of applications,including electronics,optoelectronics,sensors,energy storage and conversion,biomedicine,etc.,owing to their unique physical,chemical and electronic properties[1—15].Among these 2D materials,layered 2D metal oxides have been widely explored as electrode materials for various rechargeable batteries,especially Li-ion batteries(LIBs)[16—20].Previous study[21]has demonstrated that layered 2D nanosheets normally have large surface area and short diffusion path as compared to other kinds of nanomaterials,making them promising electrode materials for high-performance LIBs.As one of the typical layered metal oxides,2D V2O5nanosheets have been extensively investigated as an electrode material due to its reasonable price and high theoretical specific capacity[22—26].For example,Xuet al.[24]reported the synthesis of 2D V2O5network by an one-step polymer-assisted chemical method and the synthesized V2O5delivered a high capacity(e.g.165 mA·h/g at 3 C)and excellent stability when used as a cathode for LIBs.Wanget al.[25]also reported the preparation of 2D V2O5@C nanosheets used as anode for LIBs,exhibiting a large discharge capacity(e.g.802 mA·h/g at 1 A/g),good cycling perfor⁃mance and high rate capability.In addition,Zhanget al.[26]reported that liquid-phase exfoliated 2D V2O5nanosheets exhibited a high discharge capacity of 370 mA·h/g at 0.05 C when used as the cathode for LIBs.However,previous reports still suffer from unsatisfied performance or relatively complicated synthetic processes.
It is known that adjacent layers of layered 2D materials are stacking together through weak van der Waals interactions.Therefore,it is feasible to exfoliate the bulk powders into nanosheets through the exfoliation techniques including mechanical exfoliation,liquid-phase exfoliation,intercalation-assisted liquid exfolia⁃tion,and so on[27—31].Importantly,the layered structure makes them ideal hosts to be intercalated with various intercalants,such as Li+,Na+,K+,Zn2+,Ca2+,Mg2+,Mn2+,polyaniline,polypyrrole and polythiophene[32—35].More importantly,the intercalation of layered materials by various intercalants can enlarge their interlayer spacing,making them promising in energy storage with enhanced capacity and long-term cycling stability,including LIBs,sodium-ion batteries(SIBs),Zn-ion batteries(ZIBs)and supercapacitors[35—44].For example,Xiaet al.[43]reported that the calcium vanadium oxide bronze can deliver a high capacity(340 mA·h/g at 0.2 C),good rate capability and very long cycling life when used as the cathode material for ZIBs.Genget al.[44]also reported that the interlayer Mn2+-doped layered vanadium oxide(Mn0.15V2O5·nH2O)exhibited enhanced electrochemical performance than that of the V2O5When used as the cathode for ZIBs.
In this paper,we report the preparation of layered 2D(NH4)2V6O16·H2O nanosheets by simply reacting commercial V2O5nanoparticles with ammonium persulfates in aqueous solution at room temperature.The com⁃mercial V2O5nanoparticles can be transformed into(NH4)2V6O16·H2O nanosheets with a size of 2—10µm and thickness of 50—250 nm due to the co-intercalation with ammonium ions and water molecules.Importantly,when used as an anode material for LIBs,the(NH4)2V6O16·H2O nanosheets exhibit much enhanced capacity,rate performance and cycling performance in comparison with commercial V2O5nanoparticles.Our study demonstrates that the(NH4)2V6O16·H2O nanosheets can be used as an excellent anode material for LIBs,which may be also promising for other rechargeable batteries,such as SIBs and ZIBs.
2 Experimental
2.1 Chemicals
Vanadium pentoxides(V2O5,99%)and ammonium persulfates(98%)were purchased from Aladdin.Polyvinylidenefluoride(PVDF,99.9%)andN-methyl-2-pyrrolidinone(NMP,A.R.)were obtained from Sigma-Aldrich.Acetylene black was purchased from Lion Corporation(Japan).The lithium ion battery elec⁃trolyte(LiPF6,1 mol/L),lithium foil,Separator(polypropylene film),and copper foil were obtained from Dongguan Shanshan Battery Materials Co.,Ltd.(China).
2.2 Synthesis of(NH4)2V6O16·H2O Nanosheets
The(NH4)2V6O16·H2O nanosheets were synthesized by a facile approach in aqueous solution at room temperature according to the method reported in literature[45].Typically,1.5 g of commercial V2O5powders and 18.3 g of ammonium persulfate[(NH4)2S2O6]were dissolved in 150 mL of DI water.After stirring the dark yellow solution at room temperature for 48 h,the golden-yellow product was collected by centrifuge,washed thoroughly with DI water,and drying at 80°C overnight to obtain the(NH4)2V6O16·H2O nanosheets.
2.3 Characterization
The morphology and structure characterization of the samples was performed by a scanning electron mi⁃croscopy(SEM,Sigma 500)and an H-8100 transmission electron microscopy(TEM).The crystal structure of the samples was analyzed by wide-angle powder X-ray diffraction(XRD,Bruker D8)with CuKαradiation.The valence state of the products was determined by X-ray photoelectron spectroscopy(XPS,EscaLab 250Xi).Thermogravimetric analysis(TGA)was collected on a DTG-60AH instrument from 30°C to 700°C at a heating rate of 5°C/min in the air flow.The Raman spectra were recorded on an Invia Raman spectrometer.
2.4 Electrochemical Measurements
The working electrodes are prepared by following procedure.70%(mass fraction)active materials,20%(mass fraction)acetylene black and 10%(mass fraction)PVDF binder were mixed in N-methyl-2-pyrrolidone(NMP)and ground in a mortar to prepare a homogeneous slurry.The resulting slurry was spread on a Cu foil current collector,which was then dried in a vacuum oven at 120 °C for 12 h.After that,the coin-type cells were assembled in an argon-filled glovebox.The Neware CT-3008W was carried out to record the chargedischarge profiles of the electrodes in the potential range of 0.01—3 V at different current rates(0.1,0.4 and 1 A/g).It is worth pointing out that the current rate of 0.1 A/g was used for the first 4 cycles to activate the materials before testing at 1 A/g.A Parstat 4000+workstation(Princeton Applied Research,USA)was used to measure the cyclic voltammetry(CV)curves and electrochemical impedance spectroscopy(EIS).CV curves in the potential range from 0.01 V to 3.0 VversusLi/Li+were measured at a scanning rate of 0.1 mV/s.EIS were measured from 0.01 Hz to 100 kHz with an AC amplitude of 5 mV.
3 Results and Discussion
The layered(NH4)2V6O16·H2O nanosheets were synthesized by reacting of commercial V2O5nanoparticles with(NH4)2S2O6in solution at room temperature for 48 h.Note that ammonium ions and water molecules are interacted into the layered V2O5to stable(NH4)2V6O16·H2O compound,thus the compound can be considered as an intercalated compound.The SEM image shows that the commercial V2O5samples are aggregated nanopar⁃ticles with a size of several hundred nanometers(Fig.S1,see the Supporting Information of this paper).After the reaction,the commercial V2O5nanoparticles are transformed into micro-sized(NH4)2V6O16·H2O nanosheets.As shown in Fig.1(A),the obtained(NH4)2V6O16·H2O nanosheets show a plate-like morpho-logy with a size of 2—10µm.The thickness of the(NH4)2V6O16·H2O nanosheets measured from its atomic force(AFM)height images is ranging from 50 nm to 250 nm[Fig.1(B)and Fig.S2,see the Supporting Information of this paper].The TEM image further confirms the sheet-like morphology of the(NH4)2V6O16·H2O sample with a micrometer lateral size[Fig.1(C)].Moreover,the associated selected area electron diffraction(SAED)pattern reveals the crystalline structure of the(NH4)2V6O16·H2O nanosheets[Fig.1(D)].Both of the samples are then characterized by powder X-ray diffraction(XRD).As shown in Fig.2(A),all the XRD peaks of the commercial V2O5nanoparticles match well with the standard PDF card of V2O5(JCPDS:41-1426),confirming its crystal phase.Note that the diffraction peak located at 15.349° corresponded to the(200)plane of V2O5,indicating the interlayer distance of layered V2O5.After intercalation,the XRD pattern of the obtained nanosheets is assignable to the standard PDF of(NH4)2V6O16·H2O(JCPDS:41-0492),confirming that the obtained nanosheets are(NH4)2V6O16·H2O.It is worth pointing out that the(200)peak of(NH4)2V6O16·H2O shifts to the lower degree(11.200°)as compared to that of the V2O5sample.Such shift can be considered asd-spacing expansion induced by the co-intercalation of ammonium ions and water molecules into layered V2O5.It is worth noting that the obtained(NH4)2V6O16·H2O nanosheets still keep the layered structure,similar to the layered V2O5[45].
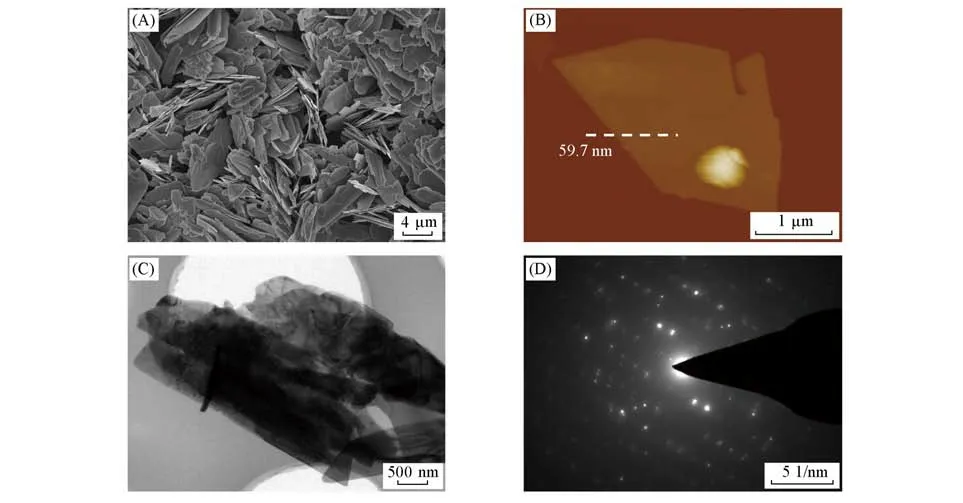
Fig.1 SEM image of the(NH4)2V6O16·H2O nanosheets(A),AFM height image of a typical(NH4)2V6O16·H2O nanosheet(B),TEM image(C)and its corresponding SAED pattern(D)of the(NH4)2V6O16·H2O nanosheets
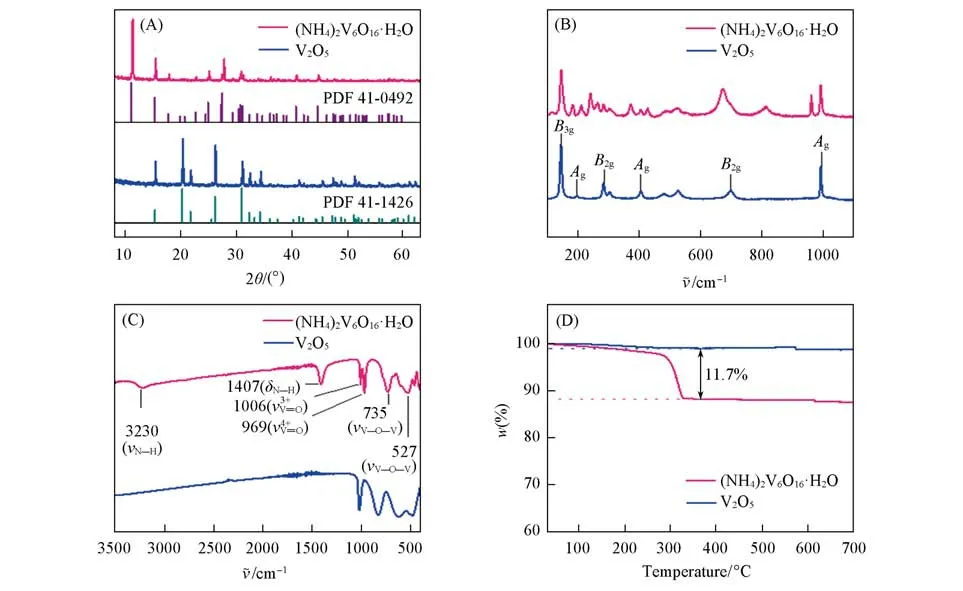
Fig.2 XRD patterns(A),Raman spectra(B),FTIR spectra(C)and TGA curves(D)of the commercial V2O5 nanoparticles and(NH4)2V6O16·H2O nanosheets
The commercial V2O5nanoparticles and(NH4)2V6O16·H2O nanosheets were further characterized by Raman spectroscopy[Fig.2(B)].The Raman spectrum of the commercial V2O5nanoparticles shows its charac⁃teristic peaks at 146,198,286,404,706 and 994 cm—1,which are corresponded to the OA—V—OBbond bending vibration modeB3g,the OA—V—OBbond bending vibration modeAg,V—OCbond bending vibration modeB2g,V—OB—V bond bending vibration modeAg,V—OCbond stretching vibration modeB2gand V—OAbond stretching vibration modeAg,respectively[46].The Raman spectrum of(NH4)2V6O16·H2O nanosheets shows similar peaks as the V2O5,but with more peaks at low frequency region,which might be originated from the vibration of NH4+in the(NH4)2V6O16·H2O nanosheets.
To further confirm the intercalation of ammonium ions and water molecules,the commercial V2O5nanoparticles and(NH4)2V6O16·H2O nanosheets were characterized by Fourier transform infrared(FTIR)spectroscopy.As displayed in Fig.2(C),the FTIR spectrum of(NH4)2V6O16·H2O nanosheets exhibits a few additional peaks as compared to that of the commercial V2O5nanoparticles.The two bands at 735 and 527 cm—1are assignable to the asymmetric and symmetric stretching vibrations of V—O—V bonds[47].The peaks at 1006 and 969 cm—1are attributed to the stretching vibration of V4+=O and V5+=O groups,corresponding to the distorted VO6octahedra and VO5square pyramids,respectively[47].The peaks at around 3230 and 1407 cm—1are assigned to the asymmetric stretching vibration and symmetric bending of N—H bonds,indicating the presence of NH4+ions.FTIR results demonstrate the existence of NH4+in the framework of the as-synthesized(NH4)2V6O16·H2O nanosheets.X-Ray photoelectron spectroscopy(XPS)measurements were performed to characterize the electronic state of commercial V2O5nanoparticles and(NH4)2V6O16·H2O nanosheets.As shown in Fig.S3(A)(see the Supporting Information of this paper),the XPS survey spectrum of the(NH4)2V6O16·H2O nanosheets shows same peaks except an additional N1speak,which is further evident by the high-resolution XPS N1sspectra[Fig.S3(B),see the Supporting Information of this paper].The additional N1ssignal in the(NH4)2V6O16·H2O nanosheets can be attributed to NH4+.Both the XPS V2pand O1sspectra of the(NH4)2V6O16·H2O nanosheets are almost the same as compared to that of commercial V2O5nanoparticles,suggesting that the intercalation of ammonium ions and water molecules does not change the electronic structure of the oxide.In addition,thermogravimetric analysis(TGA)was performed in air atmosphere to investigate the two samples.As the temperature increases from 30 to 700 °C,the commercial V2O5powder remains stable over the entire temperature range[Fig.2(D)].While the(NH4)2V6O16·H2O nanosheets were found to lose weight suddenly byca.11.7% around 270—330 °C[Fig.2(D)].The weight change could be attributed to the thermal decomposition of NH4+and lose of water molecules,which is close to calculated weigh percentage of interacted NH4+and H2O(11.4%).All the aforementioned analysis suggests the preparation of(NH4)2V6O16·H2O nanosheets by co-intercalation of commercial V2O5nanoparticles with NH4+and H2O.
2D V2O5nanosheets have been widely used as electrodes in various rechargeable batteries,specially LIBs[48,49].Therefore,the electrochemical lithium-ion storage properties of the commercial V2O5nanoparticles and(NH4)2V6O16·H2O nanosheets as anode materials for LIBs are evaluated in detail.Fig.3(A)presents the CV curves of the two electrodes at a scan rate of 0.1 mV/s in a voltage range from 0.01 V to 3.0 V.Two pairs of redox peaks of(NH4)2V6O16·H2O nanosheets located at 2.40/1.98 V and 1.23/0.72 V can be identified,which indicate the reversible intercalation process of Li+and the phase transformation during cycling.In con⁃trast,the potential gap of redox peaks of the commercial V2O5electrode is worse than(NH4)2V6O16·H2O nanosheets in the first CV since the larger activation polarization for(NH4)2V6O16·H2O nanosheets.In addi⁃tion,the first five charge and discharge voltage curves of the commercial V2O5nanoparticles and(NH4)2V6O16·H2O nanosheets at a current density of 0.1,0.4,and 1 A/g are shown in Fig.3(B,C)and Fig.S4(see the Supporting Information of this paper)respectively.It can be observed that both of them have the multiple dis⁃charge/charge voltage plateaus,corresponding to different redox reactions related to Li+insertion/extraction.The(NH4)2V6O16·H2O nanosheets exhibit higher capacity in comparison with that of the bulk commercial V2O5nanoparticles.Thereafter,the rate performance of the 2D(NH4)2V6O16·H2O nanosheets was investigated.Fig.3(D)clearly shows that the(NH4)2V6O16·H2O nanosheets has an excellent rate capability.The(NH4)2V6O16·H2O nanosheets delivers the discharge capacities of 1070 mA·h/g when the current density is 0.1 A/g.Even at the high current densities of 1.0 A/g,the discharge capacity remains approximately 355 mA·h/g.By comparison,the capacities of commercial V2O5is much less than that of(NH4)2V6O16·H2O nanosheets,especially at low current densities[Fig.3(D)].For example,the commercial V2O5delivers charge/discharge capacities of 584 and 316 mA·h/g at the current densities of 0.1 and 1 A/g,respectively.
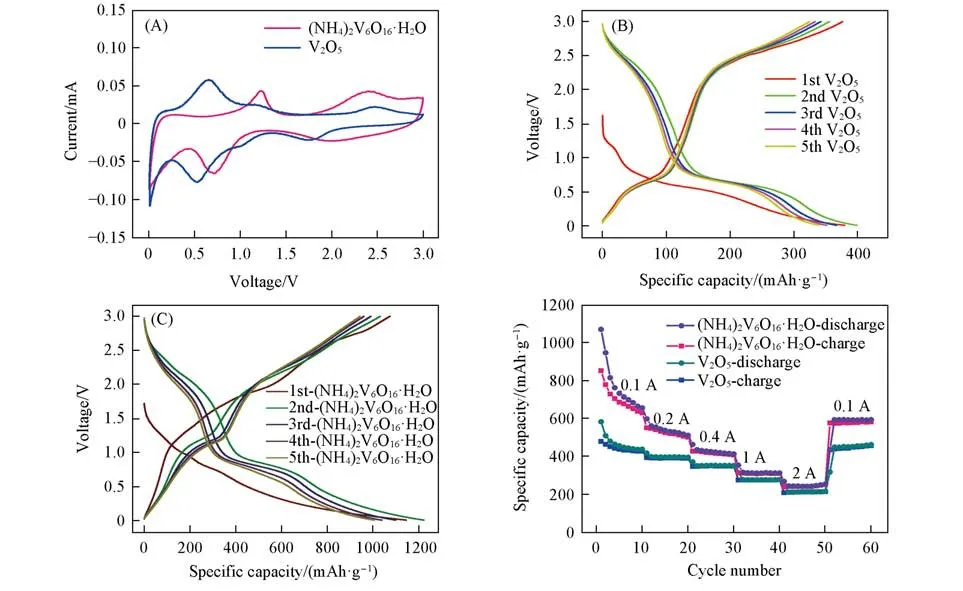
Fig.3 CV curves of commercial V2O5 nanoparticles and(NH4)2V6O16·H2O nanosheets(A),Galvanostatic charge⁃discharge profiles of commercial V2O5 nanoparticles(B)and(NH4)2V6O16·H2O nanosheets(C)for the first five cycles at 0.1 A/g,rate capabilities of commercial V2O5 nanoparticles and(NH4)2V6O16·H2O nanosheets at varying current rates(D)
The cycling performance of commercial V2O5and(NH4)2V6O16·H2O nanosheets were also investigated at different current densities of 0.1,0.4 and 1 A/g in a voltage range of 0.01—3 V.The capacity was calculated based on the mass of electrode materials.As shown in Fig.4(A)—(C),the(NH4)2V6O16·H2O nanosheets exhibited excellent cycle capacity retention.At a current density of 0.1 Ah/g,(NH4)2V6O16·H2O nanosheets delivers an average capacity of 1002 mA·h/g at the end of 70 cycles[Fig.4(A)],while the commercial V2O5nanoparticles gave an inferior capacity only around 349 mA·h/g at the same cycles.Although the discharge capacity of(NH4)2V6O16·H2O nanosheets decreased from 522 mA·h/g for the first cycle to 334 mA·h/g for the 50th cycle at 0.4 A/g due to the slow lithium ion diffusion and high charge-discharge resistance,its performance was surprisingly increased to 742 mA·h/g for the 450th cycle because the lithium intercalation and deintercalation during the cycling process could activate the materials to provide more active sties for lithi⁃um storage[Fig.4(B)].For the comparison,the commercial V2O5nanoparticles also presented good cycling performance at 0.4 A/g during the whole 450 cycles but with a much lower capacity.At the high current densi⁃ties of 1.0 A/g,the discharge capacity of(NH4)2V6O16·H2O nanosheets remains approximately 390 mA·h/g at the 450th cycle,while the commercial V2O5nanoparticles displayed lower discharge capacity(221 mA·h/g at the 450th cycle)than that of(NH4)2V6O16·H2O nanosheets[Fig.4(C)].All the aforementioned results suggest that the(NH4)2V6O16·H2O nanosheets have excellent cycling performance when used as a LIB electrode.
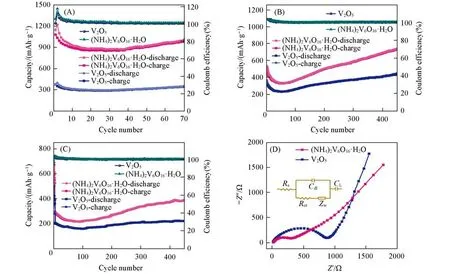
Fig.4 Cycling performance of commercial V2O5 nanoparticles and(NH4)2V6O16·H2O nanosheets at 0.1 A/g(A),0.4 A/g(B)and 1 A/g(C),nyquist-diagram of commercial V2O5 nanoparticles and(NH4)2V6O16·H2O nanosheets(Inset is the equivalent circuit diagram)(D)
EIS measurements were performed to reveal different electrochemical behaviors between the commercial V2O5and the(NH4)2V6O16·H2O nanosheets[Fig.4(D)].The Nyquist plots of the commercial V2O5and(NH4)2V6O16·H2O nanosheets are composed of the intercept at Z′-axis at the high frequency region,a semicir⁃cle in high to medium frequency regions and an inclined line in low frequency regions,corresponding to the re⁃sistance of electrolyte and cell components(Rs)and the charge transfer resistance(Rct).As listed in Table S1(see the Supporting Information of this paper),the value ofRctfor the(NH4)2V6O16·H2O nanosheets was 299.95Ω,which was significantly lower than that of the commercial V2O5counterpart(878.6Ω).This reduc⁃tion in charge transfer resistance results from the unique mesoporous nanosheet structure with larger surface area,which can shorten the pathways for Li+ion diffusion,thus leads to a higher rate capability.Based on the aforementioned results,we believed that the enhanced LIB performance of the(NH4)2V6O16·H2O nanosheets could be attributed to the following two reasons:(1)The 2D nanosheet structure endows the(NH4)2V6O16·H2O with faster transfer path for both lithium ions and electrons as compared to the commercial V2O5;(2)The expanded interlayer distance of the(NH4)2V6O16·H2O induced by co-intercalation of NH4+and H2O makes Li ions easier diffusion during the charge and discharge processes and more space for Li ion storages.
4 Conclusions
We have reported the preparation of layered 2D layered(NH4)2V6O16·H2O nanosheets by co-intercalation of NH4+and H2O into commercial V2O5nanoparticles.The ultrathin layered nanosheet structure provides short Li+diffusion pathways,large exposed surface and high electronic/ionic conductivity.Therefore,when used as anode material for LIBs,the as-synthesized(NH4)2V6O16·H2O nanosheets exhibited excellent electrochemical performances.Importantly,the discharge capacity is 390 mA·h/g under a current density as high as 1 A/g af⁃ter 450 cycles.We have demonstrated that the(NH4)2V6O16·H2O nanosheets can be a promising anode for LIBs.It is believed that this intercalated(NH4)2V6O16·H2O nanosheets could be also a promising electrode ma⁃terial in other rechargeable batteries,such as SIBs and ZIBs.
The supporting information of this paper see http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20200609.
This work is supported by the Project of Central Plains Science and Technology Innovation Leading Talents of Henan Province,China(No.204200510001),and the Funding Support from the Start-Up Grant from City University of Hong Kong,China(No.9610495).
