Ni(OH)2/Ni/g-C3N4 composite: An efficient electrocatalyst for hydrogen evolution
2021-02-24ZHANGJieZHAOYuWUAilianLIJiaWANGYuxue
ZHANG Jie ,ZHAO Yu ,WU Ai-lian ,LI Jia ,WANG Yu-xue
(Institute of Clean Chemical Engineering, College of Chemistry and Chemical Engineering, Taiyuan University of Technology,Taiyuan 030024, China)
Abstract: The preparation of efficient catalysts in hydrogen evolution reaction (HER) is an urgent task at present.In this work, Ni(OH)2/Ni/g-C3N4 composite catalyst was prepared through liquid phase impregnation with in-situ reduction, which was used to compose the cathode with carbon paper (CP) for the microbial electrolysis cell (MEC).With the help of SEM,TEM, XRD, XPS and electrochemical analysis techniques, the structure, properties and electrocatalytic performance in hydrogen evolution of the Ni(OH)2/Ni/g-C3N4 composite were investigated.The results indicate that the Ni(OH)2/Ni/g-C3N4 catalyst exhibits excellent electrochemical activity for hydrogen evolution in the MEC.Using the Ni(OH)2/Ni/g-C3N4 catalyst,the current density reaches 100 A/cm2 at a small overpotential of 1881 mV, with a low charge transfer resistance of 10.86 Ω and a low Tafel slope of 44.3 mV/dec, which is much superior to pure g-C3N4 catalyst and CP, and even comparable to the Pt catalyst, suggesting that the Ni(OH)2/Ni/g-C3N4 composite can be a potential candidate of HER catalyst in MEC.
Key words: Ni(OH)2/Ni/g-C3N4;hydrogen evolution reaction;microbial electrolysis cell
Up to date, almost four fifths of current energy supply comes from fossil fuel[1], which may be completely exhausted in hundred years[2].Hydrogen is a pollution-free and renewable energy source, which can be stored and transported easily[3,4].Nevertheless, most of the current hydrogen energy is produced from nonrenewable fossil fuels.Electrolysis system for water splitting is receiving high priority due to its low energy consumption, low cost and pollution-free hydrogen production[5].Microbial electrolytic cell (MEC) is a new electrolysis system to generate hydrogen from organic matter[6,7].It is generally believed that precious metals(such as platinum[8]and palladium[9]) present prominent properties for the hydrogen evolution reaction (HER);however, the expensiveness and scarceness of the precious metals limit their industrial application to a large extent.Consequently, it is extremely urgent to exploit highly efficient non-noble metal catalysts for HER.
Nickel-based materials (Ni-based alloys and Nibased composites) are one of the most promising nonnoble metal catalysts for HER.Nickel atom possesses an unfilled 3dorbit, which are paired with single electrons in 1S orbital of hydrogen atoms, and hydrogen adsorption bonds are easily formed between Ni atoms and hydrogen atoms[10].Nevertheless, the overall catalytic performance of nickel-based materials still needs to be improved due to the high hydrogen evolution overpotential of Ni.
Increasing specific surface area is an effective means to reduce hydrogen evolution overpotential.The graphitic carbon nitride (g-C3N4) possesses a twodimensional nano-sheet structure and a large specific surface area.In addition, g-C3N4has abundant N and a unique tri-s-triazine ring structure, which may enhance the electrocatalytic activity toward special electrochemical reactions[11].Bi et al[12]prepared a Ni@g-C3N4composite and its H2-production rate reached 8.41 μmol/h, indicating that the Ni@g-C3N4composite could enhance the H2production activity on the surface of g-C3N4.Wu et al[13]reported a facile co-electrophoretic/electrodeposition method to prepare the O@g-C3N4/Ni(OH)2/NF electrode; the electrochemical impedance measurement (EIS) data revealed that the O@g-C3N4/Ni(OH)2/NF hybrid electrode had low internal resistance and small charge transfer resistance.Cao et al[14]synthesized 2D/3D Ni(OH)2/g-C3N4(Ni/DOMCN) via the electrostatic method; the EIS Nyquist plots displayed the smallest semicircle of 25-Ni/DOMCN, indicating its small charge-migrating resistance.The 3D g-C3N4photocatalyst supported on the Ni(OH)2promoter (30%) exhibited the highest H2evolution rate (ca.87.2 μmol/h), about 76 times higher than that of pure 3D g-C3N4.
In this work, the Ni(OH)2/Ni/g-C3N4composite catalyst was prepared through liquid phase impregnation within-situreduction[15]and used to compose the cathode of microbial electrolysis cell(MEC) with the carbon paper (CP).With the help of SEM, TEM, XRD, XPS and electrochemical analysis techniques, the structure, properties and electrocatalytic performance in the hydrogen evolution reaction (HER)of the Ni(OH)2/Ni/g-C3N4composite were then investigated.
1 Experimental
1.1 Synthesis of g-C3N4
The bulk g-C3N4was prepared based on previous report[11].Briefly, 15 g urea was added into an alumina crucible with a cover and heated at 520 °C in a muffle furnace for 2.5 h with a heating rate of 10 °C/min.The solid product was ground into yellow powder and dispersed into deionized water with vigorous ultrasound for 3.0 h, followed by drying at 60 °C for 12 h, to get the g-C3N4nanosheets.
1.2 Synthesis of Ni(OH)2/Ni/g-C3N4 composite
Ni(OH)2/Ni/g-C3N4composite was prepared by using the similar liquid phase impregnation combined within-situreduction, as reported in the literature[15].Briefly, 49.53 mg of Ni(NO3)2·6H2O was dissolved in 20 mL of deionized water and 22.58 mg of polyvinyl alcohol (PVA) was then added; the mixture was stirred at room temperature for 1 h.After that, 200 mg of g-C3N4was added to the above solution and stirred for 2 h.In an ice water bath, 4.8 mL of NaBH4(30 mg) was added dropwise and stirred vigorously for 5 h.The precipitate was centrifuged and washed for 3 times with 20 mL of deionized water, followed by drying at 60 °C for 12 h.
1.3 Microbial electrolysis cell (MEC) construction
The same single-chamber MECs were used as reported in the reference[16].Plexiglas with a total volume of 80 mL was used in the single-chamber MECs, in which 20 mL of activated sludge from a local coking wastewater treatment plant (Yangjia Fortress,Taiyuan, China) and 60 mL of nutrient solution were injected at the same time.The anode was made of carbon felt (2 × 4 × 1 cm3) that had been running for 3 months in a dual chamber MFCs.In this work, three cathodes were compared, including the bare carbon paper electrode, the Pt electrode and the prepared Ni(OH)2/Ni/g-C3N4composite coating on CP.The distance between the anode and the cathode was 1.5 cm.The anode, cathode, and external power source (HB 17301 SL; HOSSONI, Inc., China) are connected in a loop by using a sheathed copper wire.
The reactor was operated in batch mode with a voltage of 0.7 V and placed in a constant temperature incubator at 35 °C.When the hydrogen production current of MEC was lower than 0.5 mA, 60 mL fresh nutrient solution was replaced and N2was injected for 15 min, while the electrode was exposed to air to prevent the growth of methanogens[17].After one cycle, all the catholyte were replaced, leaving the microorganisms at the bottom of the chamber.
1.4 Characterization
The crystalline phase of all samples was determined by X-ray diffraction (XRD) on a diffractometer (XRD-6000).The morphology, particle size and particle agglomeration were characterized by scanning electron microscopy (SEM) (JSM-7001F) and transmission electron microscopy (TEM) (JEM 2010).The chemical valence of various ions was analyzed by X-ray photoelectron spectroscopy (XPS) (ESCALAB 250Xi).
An electrochemical workstation (CHI660D,Chenhua, China) was used to perform all electrochemical tests.A single electrode was electrochemically measured in a three-electrode system with 100 mmol/L phosphate buffer solution (PBS) (pH = 7) as electrolyte,in which the as-prepared cathode electrodes were used as the working electrode.The Ag/AgCl electrode and Pt electrode were used as reference electrodes and counting electrodes, respectively.Linear sweep voltammetry (LSV) tests were conducted by sweeping the potential from -2.0 to 1.0 V with a scan rate of 10 mV/s.The potential sweep of the Tafel curve is-1.0-0.4 V with a scan rate of 10 mV/s.The amplitude of electrochemical impedance measurement (EIS) was 10 mV, and the frequency was 100 kHz to 10 MHz.The current was recorded with a multimeter (UNI-T 803, Uni-Trend Electronics Co., Ltd., Shanghai, China)every half an hour.The gas composition was analyzed by a gas chromatograph (Thermo Fisher Scientific, Wal tham, MA, USA), with argon as the carrier gas and packed TDX-01 column; TCD was used to detect H2and N2, whereas FID was used to measure CH4and CO2.
The catalytic performance in MEC was evaluated based on hydrogen recovery rate (RH2, %), cathode hydrogen recovery rate (Rcat, %), H2production rate (QH2,m3-H2/(m3·d)), energy recovery rate (ηw) and overall energy recovery rate (ηw+s), as reported in the reference[18].
2 Results and discussion
2.1 Physical characteristics of Ni(OH)2/Ni/g-C3N4
The SEM images shown in Figure 1 indicate that pure g-C3N4presents a flaky shape (Figure 1(a)) and the Ni(OH)2and Ni particles are embedded into the g-C3N4flakes (Figure 1(b)).The Ni(OH)2/Ni/g-C3N4electrode shows the shape of flower-like nanoclusters,with a rougher surface than pure g-C3N4.It is well known that the g-C3N4plane possesses many desirable coordination sites, including nitrogen lone-pair of electrons within its framework and a large number of oxygen-containing groups at the edges[19].Current results suggest that through mixing with g-C3N4nanosheets, the majority of metal ions are bonded to the inner surface of g-C3N4via strong chemical bonds,whereas fewer metal-ions are bound to the edge of g-C3N4by weak electrostatic force[20].

Figure 1 SEM images of g-C3N4 (a) and Ni(OH)2/Ni/g-C3N4 catalysts (b); TEM image of Ni(OH)2/Ni/g-C3N4 catalyst (c); XRD patterns of g-C3N4 and Ni(OH)2/Ni/g-C3N4 catalysts (d); survey spectrum and XPS spectrum (inset) of O 1s for the Ni(OH)2/Ni/g-C3N4 catalyst (e); XPS spectra of Ni 2p (f), N 1s (g), and C 1s (h) for the Ni(OH)2/Ni/g-C3N4 catalyst; N2 sorption isotherms of g-C3N4(a) and Ni(OH)2/Ni/g-C3N4 catalysts (i)
The TEM image of Ni(OH)2/Ni/g-C3N4(Figure 1(c))shows that Ni(OH)2and Ni particles grow uniformly and are staggeredly distributed in the interior of g-C3N4with two-dimensional lamellar structure; this feature can be reserved for subsequent hydrogen evolution reaction, to improve the efficiency of electrochemical reaction of electron transfer and promote the diffusion of hydrogen.
The XRD pattern of g-C3N4displays two diffraction peaks at 27.4° and 13.1° (Figure 1(d)), which are indexed as (002) and (100) crystal planes of g-C3N4,respectively.The (002) peak represents the characteristic interlayer stacking of conjugated aromatic system,whereas the (100) peak represents the in-plane structural packing motif of tri-s-triazine units[11].The(002) and (100) diffraction peaks can also be clearly observed on Ni(OH)2/Ni/g-C3N4.Besides, Ni(OH)2/Ni/g-C3N4shows three new peaks at 9.5°, 33.4° and 59.3°,which can be assigned to the (003), (101) and (110)crystalline facets of hexagonal α-Ni(OH)2, respectively(JCPDS 22-0444)[21].In contrast, the diffraction peaks at 44.76°, 52.24° and 76.83° are attributed to the (111),(200) and (220) lattice planes of standard Ni (JCPDS 03-1051), respectively[10].
The surface chemical state and electron transfer of Ni(OH)2/Ni/g-C3N4material were further characterized by XPS, as shown in Figure 1(e)-(h).The survey spectrum (Figure 1(e)) verifies the coexistence of Ni,N, C and O on the electrode surface.The XPS spectrum in Ni region of Ni(OH)2/Ni/g-C3N4shows signals at 855.5 and 861.2 eV, corresponding to the level of Ni 2p3/2, and peaks at 873.0 and 879.6 eV,belonging to the level of Ni 2p1/2.The peak intensity of Ni2+is higher than that of Ni0(ca.852.0 eV); in fact, the calculated ratio of Ni2+to Ni0is 48.5, suggesting that Ni2+probably plays the main catalytic role in hydrogen evolution.The N element exists in three forms of bonds(Figure 1(g)), including C-N=C of 398.4 eV, N-3C of 400.4 eV, and C-N-C of 399.3 eV, similar to those in g-C3N4.The C 1sspectrum (Figure 1(h)) can be deconvolved into two combined species; the peak at 284.8 eV comes from the C-N coordination in g-C3N4,whereas another peak at 287.9 eV belongs to the N-C=N bond.The peak in the O 1sXPS spectrum(Figure 1(e)) at 530.8 eV corresponds to the Ni-oxygen bond of Ni(OH)2/Ni/g-C3N4catalyst[10].All these results strongly suggest that Ni(OH)2and Ni particles have been embedded in g-C3N4.
The N2adsorption-desorption isotherms (Figure 1(i)) were employed to determine the textural properties.Ni(OH)2/Ni/g-C3N4has a surface area of 72.38 m2/g,much higher than g-C3N4(39.53 m2/g), implying that Ni(OH)2/Ni/g-C3N4could have a better catalytic and adsorption activity than g-C3N4.
2.2 Electrochemical performance
The LSV curves of the CP, g-C3N4, Ni(OH)2/Ni/g-C3N4(with a loading ratio of 1/5, 2/5 and 3/5) and Pt catalysts are compared in Figure 2(a).Obviously, both g-C3N4and CP electrodes present lower electrocatalytic activity in HER, with high overpotentials.In contrast,Ni(OH)2/Ni/g-C3N4-1/5, Ni(OH)2/Ni/g-C3N4-2/5 and Ni(OH)2/Ni/g-C3N4-3/5 catalysts demand the overpotentials of 1950, 1881 and 1901 mV, respectively, to obtain the current densities of 100 A/cm2, whereas the Pt cathode needs an overpotential of 1498 mV.In particular,the Ni(OH)2/Ni/g-C3N4-2/5 catalyst exhibits higher electrocatalytic activity than other two Ni(OH)2/Ni/g-C3N4catalysts.g-C3N4presents a flake-like morphology with plenty of irregular interstitial pores[22], in which nickel particles are embedded.With an over high nickel loading, the pores within g-C3N4layers may be blocked,leading to the decline of hydrogen evolution performance.

Figure 2 LSV curves of Ni(OH)2/Ni/g-C3N4 composite catalysts with different Ni loading ratios (a) and coating amounts (b);Tafel plot (points represent raw data, lines represent fitted data) (c); EIS spectra of bare CP, Ni(OH)2/Ni/g-C3N4 and Pt catalysts (d)(the illustration in Graph (d) is the equivalent circuit used to simulate the HER kinetics process); CP curves (e)
For the Ni(OH)2/Ni/g-C3N4-2/5 catalyst with the optimum Ni loading, the effect of coating amount on the carbon paper on the electrochemical activity was then investigated (Figure 2(b)).For a coating amount of 2,4 and 6 mg, the overpotentials demanded are 1913, 1814 and 1881 mV, respectively, to obtain a current density of 100 A/cm2.In contrast, the Pt cathode demands an overpotential of 1498 mV.To sum up, the Ni(OH)2/Ni/g-C3N4-2/5 catalyst with a Ni loading ratio of 2/5 and coating amount of 4 mg on CP demonstrates the excellent electrocatalytic activity, comparable to that of the Pt electrode.
The Tafel slope can be used to explore the kinetics of hydrogen evolution through the electrode process[23];low Tafel slopes are indicative of high hydrogen generation rate.As shown in Figure 2(c), the Tafel slopes of CP, g-C3N4, Ni(OH)2/Ni/g-C3N4, and Pt catalysts are 95.27, 85.11, 44.3 and 35.81 mV/dec,respectively.It is widely believed that the mechanism of HER consists of three primary reactions named the Volmer, Heyrovsky and Tafel reactions[24].Volmer reaction is an electrochemical reaction that generates hydrogen atoms adsorbed to the surface of the electrode,Heyrovsky reaction is electrochemical desorption,whereas Tafel reaction undergoes another recombination step.
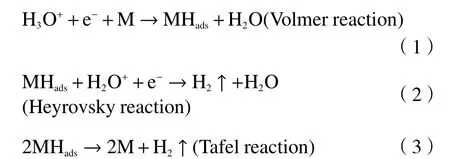
Above three electrodes (CP, Ni(OH)2/Ni/g-C3N4,and Pt) should abide by the Volmer-Heyrovsky reaction path, with Volmer reaction as the rate determining step[25].
To investigate the electrode kinetics, EIS measurements were performed with a cathode overpotential of 1500 mV.The Armstrong’s equivalent circuit on account of the slow discharge mechanism(Figure 2(d)) was obtained by fitting via Zsimpwin software.Charge transfer resistance (Rct) was obtained from the semi-circular diameter of the EIS Nyquist diagram, to evaluate the charge transfer process; small charge transfer resistances are indicative of excellent charge transfer property[26].Obviously, the charge transfer resistance of Ni(OH)2/Ni/g-C3N4(10.86 Ω) is far less than that of CP (30.2 Ω) and g-C3N4(12.25 Ω),and a little more than that of Pt (7.473 Ω), indicating the greatly promoted electron transfer ability and enhanced activity of the Ni(OH)2/Ni/g-C3N4catalyst, in accordance with the LSV and Tafel test results; that is,the Ni(OH)2/Ni/g-C3N4catalyst exhibits high electrocatalytic activity owing to its large surface area and excellent electron transfer ability.
In addition, the electrochemical stability the Ni(OH)2/Ni/g-C3N4catalyst was also investigated via chronoamperometry (CP) at constant current density of 2 mA/cm2for 1000 s.As shown in Figure 2(e), after experiencing a short voltage decrease, the potential keeps stable within 1000 s, demonstrating the excellent stability of the Ni(OH)2/Ni/g-C3N4catalyst.
2.3 MEC tests
Bare CP, Ni(OH)2/Ni/g-C3N4and Pt electrode were applied to MEC which had been running steadily for two months, to record the change of the current,collect the effluent gas, and measure the amount and composition of the effluent gas.As shown in Figure 3(a),for each cycle of the reaction, the current density of all electrodes first increases rapidly, then tends to be stable and finally decreases as nutrients have been depleted.High current densities indicate that additional electrons can be transferred to the cathode to generate hydrogen gas.During the five cycles of operation, the average current density of Ni(OH)2/Ni/g-C3N4catalyst is 16.75 A/m2, 184.4% larger than that of CP (5.89 A/m2)and 40.8% larger than that of Pt (11.89 A/m2), suggesting that the Ni(OH)2/Ni/g-C3N4catalyst possesses high and steady electrocatalytic activity and can be used for practical operation.
The gas produced via the cathode in each cycle of MEC operation was collected through the drainage method and its composition was determined by gas chromatography.As shown in Figure 3(b), the Ni(OH)2/Ni/g-C3N4catalyst exhibits the highest gas production(16.5 mL) and the highest hydrogen production rate;the gas consists of H2(90.32%), CH4(7.5%) and CO2(2.18%).In contrast, the gas production by the bare CP is the lowest (only 7.9 mL; 60.13% H2, 10.82% CH4and 29.05% CO2), whereas the Pt cathode produces 10.2 mL of gas (78.80% H2, 7.01% CH4and 16.78%CO2).Consistent with the LSV and Tafel test results,the Ni(OH)2/Ni/g-C3N4catalyst in MEC has a higher hydrogen production rate than CP and Pt, demonstrating the excellent electrocatalytic activity of the Ni(OH)2/Ni/g-C3N4catalyst.Nevertheless, the Ni(OH)2/Ni/g-C3N4catalyst cathode produces 7.5% methane, slightly higher than the Pt cathode (7.01%), which might be ascribed to the enrichment of methanogens resulted from long time oxygen-free operation, leading to a decrease of the hydrogen purity.
Table 1 gives the hydrogen recovery rate (RH2, %),cathode hydrogen recovery rate (Rcat, %), H2production rateQH2, m3-H2/(m3·d)), energy recovery rate (ηw) and overall energy recovery rate (ηw+s) of different cathodes in MEC.Obviously, the Ni(OH)2/Ni/g-C3N4catalyst exhibits prominent hydrogen evolution activity and can even be used as an alternative of Pt as a highly efficient hydrogen evolution catalyst.

Figure 3 Current generation for the cathode electrodes in the MEC (a) and the composition of MEC effluent gas per cycle (b)

Table 1 Energy efficiencies and hydrogen production in the MEC with different cathodes
3 Conclusions
The Ni(OH)2/Ni/g-C3N4composite catalyst was prepared through liquid phase impregnation withinsitureduction, which was used to compose the cathode with carbon paper (CP) for the microbial electrolysis cell (MEC).With the help of SEM, TEM, XRD, XPS and electrochemical analysis techniques, the structure,properties and electrocatalytic performance in hydrogen evolution of the Ni(OH)2/Ni/g-C3N4composite were investigated.
The results indicate that the Ni(OH)2/Ni/g-C3N4catalyst exhibits excellent electrochemical activity for hydrogen evolution in the MEC, owning to the synergy between flake-like C3N4and Ni.In particular, with the optimal Ni/g-C3N4mass ratio of 2/5 and carbon coating amount of 4 mg/cm2, the Ni(OH)2/Ni/g-C3N4-2/5 catalyst displays lower resistance and high H2production rate,even comparable to the Pt catalyst, suggesting that the Ni(OH)2/Ni/g-C3N4catalyst with excellent stability and electrocatalytic activity could be a potential candidate of HER catalyst in MEC.
