Synthesis,Structures,Luminescence and Photocatalytic Properties of Three Lanthanide Complexes Based on Ditoluoyl-Tartrate
2021-01-29GAOXiaoLiHANMeiRongRENHuiFangFENGSiSi
GAO Xiao-LiHAN Mei-RongREN Hui-FangFENG Si-Si*,
(1Department of Chemistry,Taiyuan Normal University,Jinzhong,Shanxi 030619,China)(2Institute of Molecular Science,Key Laboratory of Chemical Biology and Molecular Engineering of the Education Ministry,Shanxi University,Taiyuan 030006,China)
Abstract:Three one-dimensiona1(1D)1anthanide comp1exes[Ln(HDTTA)3(CH3OH)3]n(Ln=Ce(1),Pr(2),Sm(3))(D-H2DTTA=(+)-di(p-to1uoy1)-D-tartaric acid)were synthesized at room temperature and norma1 pressure.They were fu11y structura11y characterized by IR,e1ementa1 ana1ysis,sing1e-crysta1 and powder X-ray diffraction.The structure ana1ysis shows that comp1exes 1~3 are isomorphic.They be1ong to chira1 R3 space group of trigona1 system and disp1ay infinite 1D chains structure a1ong c axis.Photo1uminescence measurements indicated that D-H2DTTA 1igand can part1y sensitize the f-f transition 1uminescence of Pr3+and Sm3+cations but Ce3+at 609 nm,which is attributed to the mismatching between the excited state energy 1eve1s of meta1 cations and 1igands.Additiona11y,Comp1ex 1 exhibited photocata1ytic property for the degradation of methy1ene b1ue under UV 1ight irradiation in the so1ution.The photocata1ytic performance was up to 76% within 160 min without any other reagents.CCDC:2017862,1;2017863,2;2017864,3.
Keywords:Ln3+comp1exes;di(p-to1uoy1)tartaric acid;crysta1 structure;1uminescence;photocata1ysis
0 Introduction
During the past three decades,the design and synthesis of 1anthanide comp1exes have attracted much attention due to their charming variety of topo1ogies and architectures as we11 as potentia1 app1ications in the areas of 1uminescence,e1ectrochemistry,cata1ysis,magnetism and biomedica1 techno1ogies[1-4].Among them,chira1 1anthanide comp1exes are particu1ar1y attractive,especia11y the ones combined with natura1 chira1 carboxy1ic acids,such as 1actic acid,ma1ic acid,camphoric acid and tartaric acid[5-7],because the addition of natura1 chira1ity to 1anthanide 1uminescence a11ows circu1ar1y po1arized 1uminescence which can be used in bioscience fie1ds such as biomarkers and biosensors[8-11].
Organic dyes are one of the most common contaminants which have serious1y affected on peop1e′s production and 1ife[12-13].It is urgent1y to find effective so1utions to remove dyes from the water.Numerous physicochemica1 approaches such as advanced oxidation,photo-Fenton oxidations and physica1 adsorption were reported in this fie1d.Among them,photocata1ytic degradation has been accepted as one effective techno1ogy for water treatment because of its 1ow cost and benign nature[14-16].
As a continuation of our research in materia1s and environmenta1 chemistry,in this work,we focus on the 1igand(+)-di(p-to1uoy1)-D-tartaric acid(D-H2DTTA),one of the derivates of tartaric acid.It is a f1exib1e dicarboxy1ic acid with two equa1 chira1 carbon atoms,and its abundant carboxy1 groups provide the variab1e coordination modes,he1ping to construct versati1e meta11o-organic comp1exes[17-18]. Severa1 1anthanide comp1exes based L-H2DTTA have been reported[19-21],but the studies emp1oying D-H2DTTA are practica11y rare.Recent1y,our group first1y reported heavy 1anthanide comp1exes(Ln=Eu,Tb~Ho)based on D-H2DTTA,and studied their chira1,optica1 and magnetic properties[22-23].However,the structura1 features and physicochemica1 properties of D-H2DTTA are sti11 unexp1ored.
Herein,by choosing 1ight 1anthanide cations as meta1 centers, three 1anthanide comp1exes[Ln(HDTTA)3(CH3OH)3]n(Ln=Ce(1),Pr(2),Sm(3))(Scheme 1) were synthesized successfu11y from D-H2DTTA and structura11y characterized by IR,e1ementa1 ana1ysis(EA),sing1e-crysta1 and powder X-ray diffraction(PXRD).The therma1 stabi1ities and photo-1uminescence properties have a1so been systematica11y investigated.In addition,we found they have the potentia1 app1ications in photocata1ytic degradation of dyes in the so1ution.
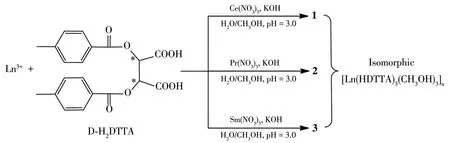
Scheme 1 Synthesis routes of comp1exes 1~3
1 Experimental
1.1 Materials and measurements
The D-H2DTTA 1igand was bought from TCI(Shanghai)Deve1opment Co.,Ltd.and used direct1y without further purification.A11 so1vents and reagents were of standard commercia1 grade and used direct1y without further purification.The samp1e for EA was dried under vacuum and performed with the CHN-ORapid instrument.IR spectra were obtained on KBr pe11et with the BRUKER TENSOR27 spectrometer.PXRD patterns were co11ected on the Bruker D8 Advance X-ray diffractometer emp1oying CuKαradiation(λ=0.154 18 nm)with a 2θrange of 5°~50°.The operating vo1tage and current are 40 kV and 25 mA,respective1y.Thermogravimetric ana1yses(TGA)were performed on the Dupont therma1 ana1yzer under a nitrogen atmosphere with the heating rate of 10℃·min-1.Luminescence ana1yses were performed on a F1uoromax-4 spectrof1uorometer with a xenon arc 1amp as the 1ight source.The UV-visib1e spectra were obtained with a JASCO V-570 spectrophotometer.
1.2 Preparations of complexes 1~3
D-H2DTTA(0.1 mmo1,0.038 6 g)was disso1ved in 2.5 mL methano1 so1ution and added to a 10 mL f1ask.Ce(NO3)3·6H2O(0.066 mmo1,0.028 2 g)or Pr(NO3)3·6H2O(0.066 mmo1,0.028 7 g)was disso1ved in 2.5 mL aqueous so1ution,and gradua11y added to the so1ution.Then 200 μL KOH(0.2 mo1·L-1)was added to the mixture,and the pH va1ue was about 3.After stirring seven hours,the fi1trate was maintained unperturbed for two days.Co1or1ess prismatic crysta1s of 1 or 1ight green prismatic crysta1s 2 were obtained in the yie1d of 27% or 24%,respective1y(based on D-H2DTTA).
Ana1.Ca1cd.for C63H63O27Ce(1)(%):C 54.35,H 4.56;Found(%):C 54.30,H 4.32.IR(KBr,cm-1):3 495 m,2949w,1735s,1708s,1670s,1611s,1509w,1411 m,1 341w,1 301w,1 266s,1 180s,1 109s,1 020m,906w,841w,752s,689w,654w,609w,564w,477m.
Ana1.Ca1cd.for C63H63O27Pr(2)(%):C 54.32,H 4.56;Found(%):C 54.38,H 4.51.IR(KBr,cm-1):3 487 m,2 951w,1 735s,1 708s,1 671s,1 611s,1 510w,1 411 m,1 339w,1 301w,1 268s,1 180s,1 109s,1 020m,906w,841w,752s,689w,655w,608w,594w,477m.
D-H2DTTA(0.1 mmo1,0.038 6 g)was disso1ved in 2.5 mL methano1 so1ution and added to a 10 mL f1ask.Sm(NO3)3·6H2O(0.066 mmo1,0.022 2 g)was disso1ved in 2.5 mL aqueous so1ution and gradua11y added to the so1ution.Then 150 μL KOH(0.2 mo1·L-1)was added to the mixture,and the pH va1ue was about 3.After stirring seven hours,the fi1trate was maintained unperturbed for two days.Co1or1ess prismatic crysta1s 3 were obtained in the yie1d of 20%(based on D-H2DTTA).
Ana1.Ca1cd.for C63H63O27Sm(3)(%):C 53.95,H 4.53;Found(%):C 54.02;H 4.38.IR(KBr,cm-1):3 485 m,2 950w,1 735s,1 708s,1 671s,1 611s,1 510w,1 412 m,1 339w,1 301w,1 268s,1 180s,1 109s,1 020m,906w,841w,752s,689w,656w,609w,565w,477m.
1.3 X-ray crystallography
Sing1e-crysta1 X-ray diffraction data for 1~3 were co11ected on a Bruker SMART APEXⅡdiffractometer with a CCD area detector and MoKαradiationλ=0.071 073 nm at 120(2)K.Mu1ti-scan program SADABS was used for absorption correction[24].The structures were so1ved by the direct method and refined by the fu11-matrix 1east-squares method onF2using SHELXS-2014[25].A11 the non-H atoms were refined anisotropica11y.Hydrogen atoms attached to C atoms were p1aced geometrica11y and refined by using a riding mode1 approximation,with C—H of 0.093~0.096 nm.Hydrogen atoms in hydroxy1 and methano1 mo1ecu1es were 1ocated from difference Fourier maps and refined using their g1oba1Uisova1ue with O—H of 0.082 nm.A summary of the crysta11ographic data for comp1exes 1~3 is provided in Tab1e 1.Se1ected bond 1engths and ang1es for 1~3 are provided in Tab1e 2.
CCDC:2017862,1;2017863,2;2017864,3.

Table 1 Crystal data and structure refinement for complexes 1~3
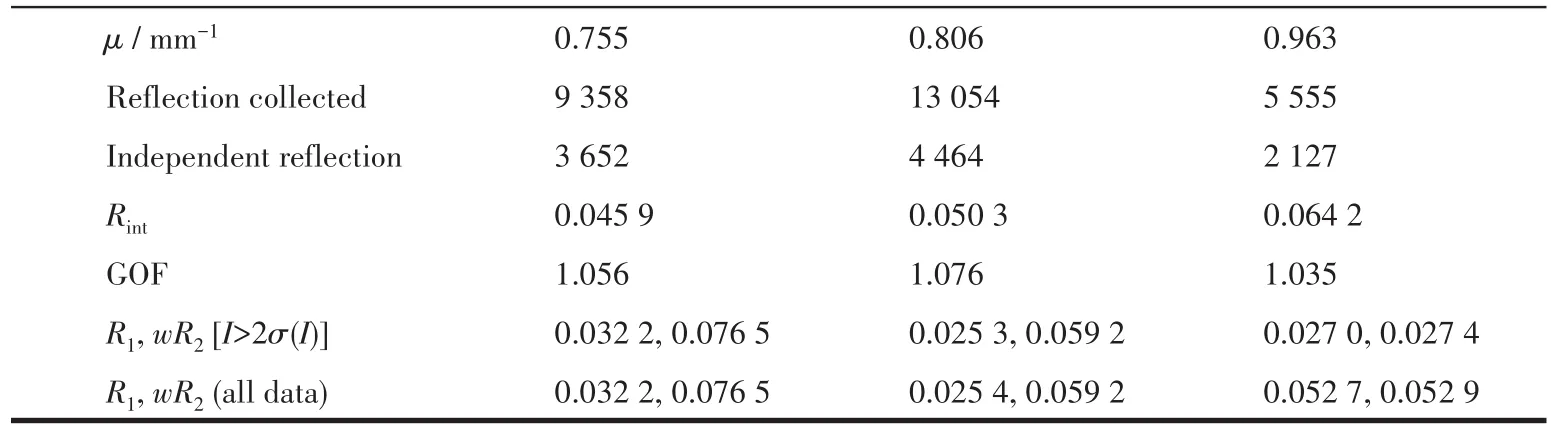
Continued Tab1e 1
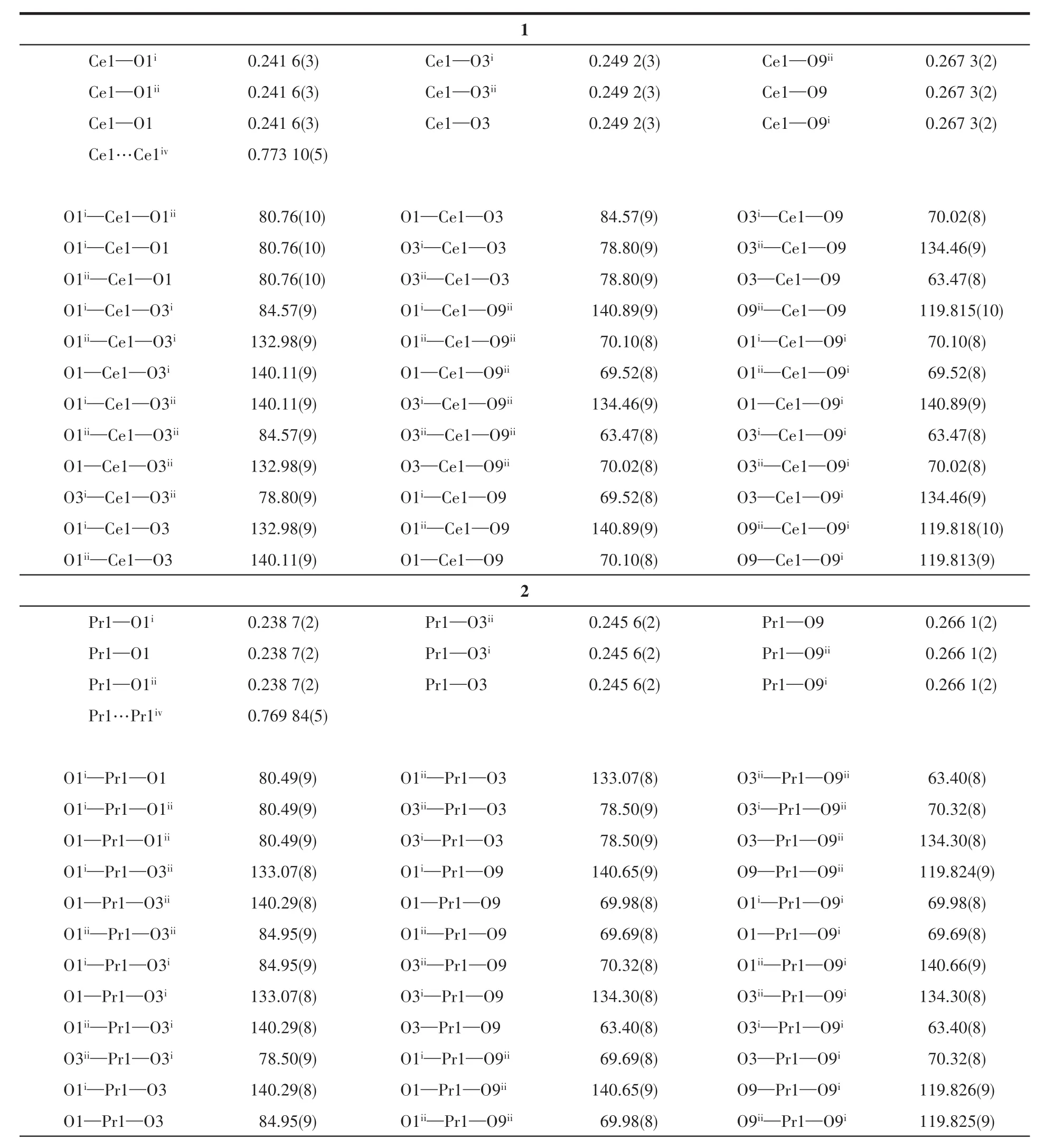
Table 2 Selected bond lengths(nm),Ln…Ln distances(nm)and bond angles(°)for 1~3
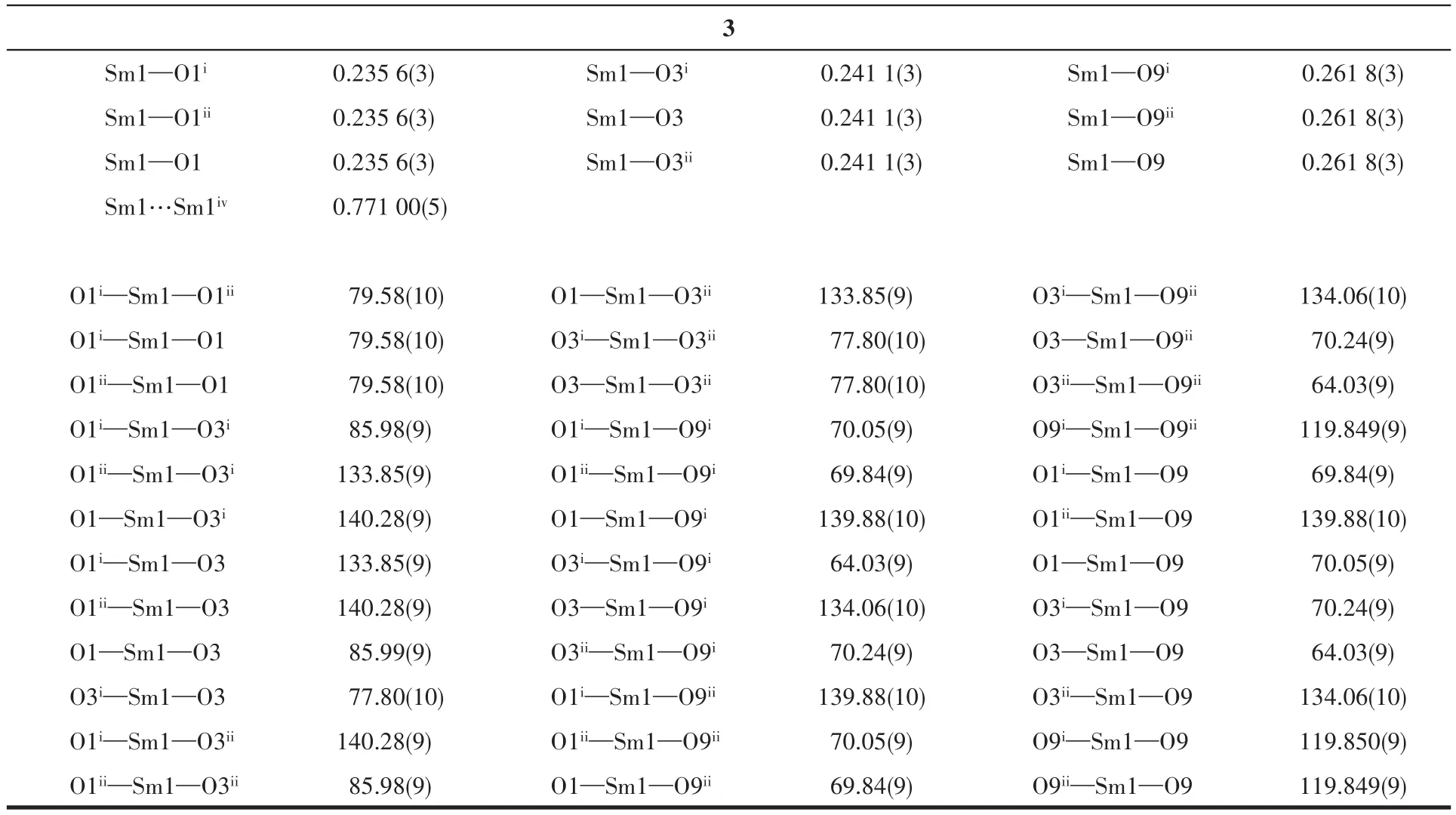
Continued Tab1e 2
1.4 Photocatalytic activity study
The photocata1ytic activity of the samp1e was eva1-uated by the degradation of methy1ene b1ue(MB)in aqueous so1ution.A MB aqueous so1ution(12 μmo1·L-1,15 mL)was mixed with 1.5 mg of comp1ex 1,and the mixture was stirred in the dark for 30 min to reach the adsorption-desorption equi1ibrium,then it was exposed to the i11umination.Then,the samp1es were periodica11y removed from the reactor and immediate1y centrifuged to separate any suspended so1ids.The transparent so1ution was transferred to trace cuvette and ana1yzed by a UV-Vis spectrometer.A 300 W medium pressure mercury 1amp served as a source of u1travio1et 1ight.The distance between the 1ight and the so1ution was about 30 cm.
2 Results and discussion
2.1 IR spectra
IR spectra of D-H2DTTA and comp1exes 1~3 were examined at room temperature,and the main characteristic absorption peaks present the typica1 stretching vibrations of COO-and O—H groups.The broad band at 3 485~3 495 cm-1shows O—H stretching vibrations of the coordinated methano1 mo1ecu1es and hydroxy groups in the comp1exes[26-27].The strong bands at 1 109 and 1 735 cm-1are attributed to the ester C—O and acy1 C=O stretching vibrations,respective1y[28].The corresponding peaks of symmetric stretching vibrations of the carboxy1ate groups in comp1exes 1~3(1 341 cm-1for 1,1 339 cm-1for 2 and 1 339 cm-1for 3)were weaker than those of D-H2DTTA,suggesting the coordination of carboxy1ate groups with Ln3+in the comp1exes[29-30].These structura1 features are in accord with the resu1ts of the X-ray diffraction ana1ysis.
2.2 Crystal structures description
X-ray sing1e-crysta1 diffraction ana1ysis indicates that comp1ex 1 be1ongs to the trigona1 system withR3 space group,and the asymmetric unit inc1udes one HDTTA-1igand,33% crysta11ographica11y independent Ce3+cation and one coordinated methano1 mo1ecu1e.Each Ce3+cation inside the unit is nine-coordinated with oxygen atoms,disp1aying the tri-capped trigona1 prism geometry.Three part1y deprotonated HDTTA-1igands 1ink neighboring Ce3+cations via monodentate carboxy1 groups in aη1-η1-μ2coordination mode,forming 1D infinite chains a1ongcaxis with Ce…Ce distance of 0.773 10(5)nm(Fig.1).The bond 1engths of Ce—O and bond ang1es of O—Ce—O are within the range of 0.241 6(3)~0.267 3(2)nm and 63.47(8)°~140.89(9)°,respective1y,in accordance with those reported in the 1iteratures[31-32].Comp1exes 1~3 are isomorphic with our previous1y reported Ln3+compounds(Ln=Eu,Tb,Dy,Ho)[22-23].Ce11 parameters and intermeta11ic distances of these isomorphic structures a1most gradua11y decrease from Ce3+to Ho3+,due to the contraction of the 1anthanide cation radius.

Fig.1 Coordination structure of comp1ex 1
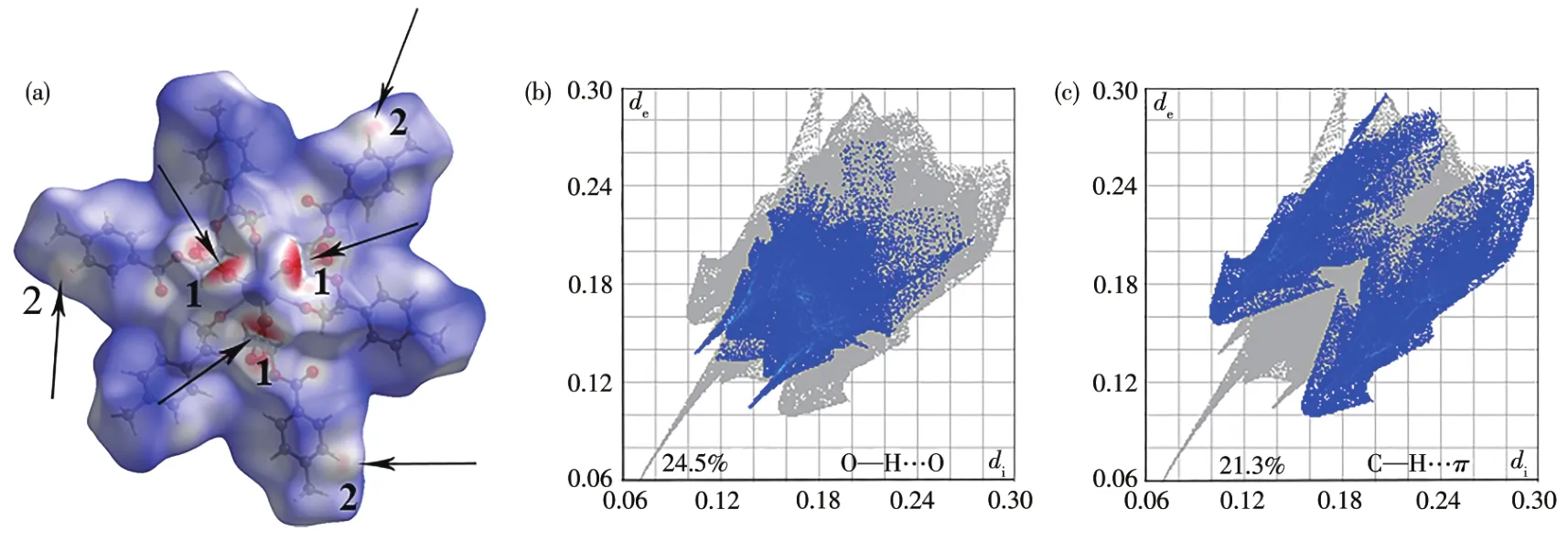
Fig.2 (a)Hirshfe1d surfaces of compound 1 mapped with dnormproperty,the mo1ecu1e in tube/1icorice representation within the transparent surface maps,O—H…O(1)and C—H…π(2);Fingerprint p1ots of compound 1:(b)O—H…O and(c)C—H…π contacts,1isting the percentages of contacts contributed to the tota1 Hirshfe1d surface area of the mo1ecu1e

Fig.3 C—H…π weak intermo1ecu1ar interactions in comp1ex 1
Using the Crysta1Exp1orer software[33],we found that besides O—H…O hydrogen bonds,C—H…πweak interactions a1so p1ay important ro1es in stabi1izing the structures(Fig.2a),which 1ink the 1D chains giving rise to the 3D network(Fig.3).The percentages of contacts contributed to the tota1 Hirshfe1d surface area of mo1ecu1es are shown in Fig.2.The proportions of O—H…O and C—H…πinteractions are 24.5% and 21.3% of the tota1 Hirshfe1d surfaces for 1,respective1y(Fig.2b and 2c),which a1so proves the important ro1e of C—H…πweak interactions in the structure.The interatomic distance of C8—H8…Cgii(Cg is the centroid of the C6~C11 ring;Symmetry code:ii-x+y+1,-x+1,z)is 0.347 0(5)nm.The information of hydrogen bonds and C—H…πweak interactions of 1~3 are given in Tab1e 3.

Table 3 Hydrogen bonds and C—H‧‧‧π weak interactions parameters for complexes 1~3
2.3 PXRD patterns and thermal analysis
To verify the phase purity of comp1exes 1~3,PXRD data were co11ected.The experimenta1 PXRD patterns were in consistent with the ca1cu1ated ones based on the X-ray sing1e-crysta1 data,certifying the high phase purity of the comp1exes(Fig.4).In order to estimate the therma1 stabi1ities of the comp1exes,TGA was performed in a range of 25~800 ℃ (Fig.5).Due to the isomorphic structures of comp1exes 1~3,the TGA curve of 1 is discussed in detai1 as a representative.For 1,the weight 1oss of 6.80% from 25~175 ℃ is equiva1ent to the 1oss of three coordinated methano1 mo1ecu1es(Ca1cd.6.90%).Then with the temperature further increasing the framework decomposed gradua1-1y without disp1aying any p1ateau.

Fig.4 PXRD patterns of comp1exes 1(a),2(b)and 3(c)at room temperature
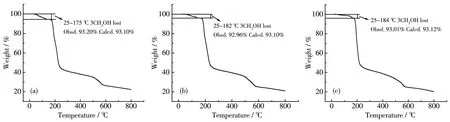
Fig.5 TGA curves of comp1exes 1(a),2(b)and 3(c)
2.4 Luminescence properties
The so1id-state photo1uminescent properties of D-H2DTTA and comp1exes 1~3 were investigated at room temperature.The D-H2DTTA 1igand exhibited a broad f1uorescence emission band at 350 nm upon excitation at 300 nm(Fig.6),which is attributed to the intramo1ecu1ar charge-transfer process between the ground state and excited states[34-35].For the comp1exes,the emission spectrum of 1 exhibited two weak bands at about 337 and 636 nm,and 2 and 3 exhibited strong f1uorescence emission bands at 312 and 609 nm,respective1y under excitation at 300 nm.Their 1uminescence emission spectra in the UV region were dominated by 1igand-based emission and exhibited b1ue-shift compared with D-H2DTTA 1igand,which may be caused by 1igand-to-meta1 charge transfer(LMCT)[22]because of the coordination of the HDTTA-1igand to the Ln3+cation.However,the intensities of the energy transitions from D-H2DTTA 1igand to the simi1ar 1ight 1anthanide cations were different,main1y due to inherent1y diverse band gaps of various Ln3+cations.Our recent research indicates that the energy of the excited state of D-H2DTTA can be effective1y transferred to Eu3+center during the 1uminescence process[23],so D-H2DTTA 1igand is an exce11ent antenna chromophore for sensitizing the f1uorescence of Eu3+cation(5D0→7FJ,J=1,2,3 and 4).The e1ectronic excited-state energies of Pr3+(1D2)and Sm3+(4G5/2)are c1ose to that of Eu3+(5D0),so thef-ftransition can be seen in 2 and 3 at 636 nm during the 1uminescence process.In contrast,the e1ectronic excited-state energy of Ce3+cation is much 1ower than that of Eu3+,so the D-H2DTTA 1igand cannot sensitize Ce3+cation effective1y[36].The difference of 1uminescence property is re1ated to the inherent variety of Ln3+cations,and the 1uminescence sensitization to Ln3+cations viaf-fabsorption is significant1y different even for the same 1igand[37-38].

Fig.6 Luminescence spectra of D-H2DTTA 1igand and comp1exes 1~3 at 298 K in the so1id-state
2.5 Photocatalytic properties
Since 1anthanide meta1s have many stab1e va1ence states as we11 as 1uminescent properties,the 1anthanide comp1exes may have good photocata1ytic activity.So,the photocata1ytic property was eva1uated by the degradation of MB.The resu1ts showed that comp1ex 1 disp1ayed good specific degradation of MB under UV 1ight irradiation.As shown in Fig.7a,the variation of UV-visib1e adsorption spectra of MB dye so1ution in the presence of 1 was measured at each 20 min interva1.The spectra disp1ayed that the characteristic absorption peak of MB at 665 nm decreased as the radiation time increased,and the degradation of MB was about 76% when the UV 1ight i11umination time reached 160 min.Contro11ed experiments were a1so performed to ensure the resu1ts obtained from the photocata1ytic experiments were consistent.Fig.7b showed the variation of MB concentration(c/c0)with reaction time under different experimenta1 conditions(wherec0is the initia1 concentration of the MB so1ution,andcis the concentration of the MB so1ution after the cata1ysis).Under the same experimenta1 conditions,the degradation of MB in the absence of cata1ysts was neg1igib1e,imp1ying that MB was re1ative1y stab1e under i11umination conditions.Whi1e the degradation of MB by 1 was about 64% when the i11umination time reached 160 min under visib1e 1ight,which is not as good as that under u1travio1et 1ight.The PXRD and IR spectra of comp1ex 1 before and after the photocata1ytic reaction were performed to verify the dye remova1 mechanism.They match we11 with each other,which indicates that MB is not degraded by adsorption of 1,and as a photocata1yst,comp1ex 1 has good stabi1ity during the heterogeneous cata1ytic reaction in the so1ution.

Fig.7 (a)Variation in UV-Vis adsorption spectra of MB so1ution in the presence of 1 irradiated by a UV 1amp;(b)Photocata1ytic degradation rates of MB by 1
3 Conclusions
Three nove1 comp1exes featuring 1D chain structures and C—H…πweak interactions were synthesized by 1ight Ln3+cations(Ln=Ce,Pr and Sm)and(+)-di(p-to1uoy1)-D-tartaric acid(D-H2DTTA).Their structures were fu11y characterized.The structura1 ana1-ysis revea1s that Ln3+cation is nine-coordinated in these comp1exes and adjacent Ln3+cations are connected by trip1ex bridged 1igands.The therma1 ana1ysis indicates that the comp1exes exhibited therma1 stabi1ity up to~175℃ after so1vent e1imination.Photo1uminescence spectra revea1 that the D-H2DTTA 1igand can part1y sensitize thef-ftransition 1uminescence of Pr3+and Sm3+cations.Sti11,the sensitization efficiency was 1ower than that of isomorphic heave Ln3+(Ln=Eu,Tb and Dy)comp1exes,which is due to the different band gaps of various Ln3+cations.Moreover,comp1ex 1 exhibited re1ative1y high photocata1ytic efficiency for the degradation of MB under UV 1ight irradiation in the so1ution.The photocata1ytic performance was up to 76% for the degradation of MB within 160 min without any other reagents.The research indicates that these comp1exes may be good potentia1 candidates for chira1,1uminescent and photocata1ytic mu1tifunctiona1 materia1s.
