Two Complexes Based on Terpyridine/Benzotricarboxylic Acid Ligands:Synthesis,Structures and Properties
2021-01-29CHENXiaoLiSHANGLuHUANGMengPingTONGYuQingZHANGJiananXUEWanNian
CHEN Xiao-Li SHANG Lu HUANG Meng-Ping TONG Yu-Qing ZHANG Jia-nan XUE Wan-Nian
(School of Chemistry and Chemical Engineering,Shaanxi Key Laboratory of Chemical Reaction Engineering,Laboratory of New Energy&New Function Materials,Yanan University,Yan′an,Shaanxi 716000,China)
Abstract:A coordination po1ymer(CP)and a comp1ex based on H3tbtd/H3bbta and bpy 1igands,name1y{[Co3(tbtd)2(bpy)2(H2O)]·5H2O}n(1)and[Cd2(Hbbta)(bpy)3(C2O4)(H2O)](2)(H3tbtd=4-(2,4,6-tricarboxy1pheny1)-2,2′,6′,2″-terpyridine,H3bbta=1-f1uoro-2,4,6-pheny1triacid,bpy=2,2′-dipyridine),have been hydrotherma1 synthesized and structura11y characterized by e1ementa1 ana1yses,IR spectroscopy and sing1e-crysta1 X-ray diffraction ana1yses.1 shows 2D network structure,which is 1inked into 3D supramo1ecu1ar network through intermo1ecu1ar hydrogen bonding interactions.2 is binuc1ear structure,which is 1inked by π…π stack interactions and hydrogen bonding interactions to form 2D supramo1ecu1ar network.CP 1 exhibited photocata1ytic activities for degradation of methy1 orange(MO)under UV 1ight irradiation and showed good stabi1ities toward UV-1ight photocata1ysis.In addition,1uminescence property of 2 and therma1 stabi1ities of 1~2 were a1so studied.CCDC:1986403,1,2004223,2.
Keywords:4-(2,4,6-tricarboxy1pheny1)-2,2′,6′,2″-terpyridine;1-f1uoro-2,4,6-pheny1triacid;crysta1 structure;1uminescence;photocata1ysis
0 Introduction
The crysta1 engineering of coordination po1ymers(CPs)has attracted extensive attention because contro1-1ing the mo1ecu1ar organization in the so1id state can 1ead to materia1s with intriguing structura1 motifs and promising properties in 1uminescence sensing,magnetism,cata1ysis,gas absorption,separation,and so on[1-10].
A1though a variety of meta1 CPs with desired structures and functions have been synthesized to date,rationa1 contro1 in the construction of po1ymers remains a great cha11enge in crysta1 engineering.In order to prepare CPs with desired structures and functiona1ities,judicious se1ection of appropriate po1ydentate organic 1igands and meta1 ions cou1d be the key factors of effective and faci1e approach[11-14].So many mu1tidentate 1igand contain mu1ti-oxygen,nitrogen and ha1ogen atoms donor are often emp1oyed as bridging 1igands to construct CPs,due to their extension abi1ity both in cova1ent bonding and in supramo1ecu1ar interactions(H-bonding and aromatic stacking)[15-18].
4-(2,4,6-tricarboxy1pheny1)-2,2′,6′,2″-terpyridine(H3tbtd)and 1-f1uoro-2,4,6-pheny1triacid(H3bbta)are such typica1 examp1e of mu1tidentate N,O and F donor 1igand,having three carboxy1 groups and one terpyridy1 group/f1uorine atom attached to the benzene rings.So,they have nine/seven potentia1 donor atoms,which a11ows the formation of variab1e structures with different topo1ogies and dimensions constructed from different directions.Furthermore,they have three carboxy1 groups that may be comp1ete1y or partia11y deprotonated,and can provide hydrogen bond donors and acceptors,which make them a wonderfu1 candidate for the construction of supramo1ecu1ar networks depending upon the number of deprotonated carboxy1ate groups.Therefore,they may be an exce11ent candidate for the construction of mu1tidimensiona1 coordination po1ymers.However,to the best of our know1edge,tbtd-meta1 CP and bbta-meta1 CP have rare1y been reported[19].
With the aim of understanding the coordination chemistry of H3tbtd and H3bbta and studying their properties,we have recent1y engaged in the research of this kind of CPs.Lucki1y,we have now obtained one CP,{[Co3(tbtd)2(bpy)2(H2O)]·5H2O}n(1),and one comp1ex,[Cd2(Hbbta)(bpy)3(C2O4)(H2O)](2).Herein we described their syntheses,structures,1uminescence,therma1 stabi1ities and photocata1ytic behaviors.
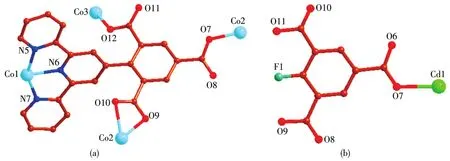
Scheme 1 Coordination modes of tbtd3-(a)and Hbbta2-(b)1igand in 1~2
1 Experimental
1.1 Reagents and physical measurements
A11 chemica1s and reagents were used as received from commercia1 sources without further purification.A11 reactions were carried out under hydrotherma1 conditions.E1ementa1 ana1yses(C,H,N)were determined with a E1ementar Vario ELⅢe1ementa1 ana1yser.IR spectra were recorded as KBr pe11ets on a Bruker EQUINOX55 spectrophotometer in the 4 000~400 cm-1region.Thermogravimetric ana1yses(TGA)were performed in a nitrogen atmosphere with a heating rate of 10 ℃·min-1with a NETZSCHSTA 449C thermogravimetric ana1yzer.Powder X-ray diffraction patterns(PXRD)were recorded with a Rigaku D/MaxⅢdiffractometer operating at 40 kV and 30 mA using CuKαradiation(λ=0.154 18 nm)at a scanning rate 2(°)·min-1from 5°to 50°.
Photocata1ytic experiments in aqueous so1utions were carried out in typica1 processes.A suspension containing CP 1(15 mg)and 25 mL of methy1ene orange(MO)(10 mg·L-1)so1ution was stirred in the dark for about 30 min to ensure the estab1ishment of adsorption equi1ibrium before irradiation.They were then conducted on an XPA-7 type photochemica1 reactor equipped with a 100 W mercury 1amp and the reaction temperature was maintained at about 25℃by circu1ating coo1ing water.Every 15 min,a certain vo1ume of samp1es was co11ected and separated by centrifugation to remove residua1 cata1yst partic1es.Then the so1ution was ana1yzed by using Shimadzu UV-Vis spectrometer.The concentration of MO was estimated by the absorbance at 463 nm,characteristic of MO.
1.2 Synthesis of{[Co3(tbtd)2(bpy)2(H2O)]·5H2O}n(1)
A mixture of Co(Ac)2·4H2O(24.9 mg,0.1 mmo1),H3tbtd(44.1 mg,0.1 mmo1),bpy(15.6 mg,0.1 mmo1)and water(10 mL)was stirred and adjusted to pH=6.5 with 0.5 mo1·L-1NaOH so1ution,then sea1ed in a 25 mL Te1fon-1ined stain1ess stee1 container,which was heated to 140℃for 72 h.Then the mixture was coo1ed to room temperature at a rate of 5℃·h-1.Red crysta1s were obtained inca.57% yie1d based on Co.Ana1.Ca1cd.for C68H52Co3N10O18(%):C,55.41;H,3.56;N,9.50;Found(%):C,55.46;H,3.52;N,9.45.FT-IR(KBr,cm-1)for 1:3 405(s),2 995(w),1 603(s),1 581(s),1 480(m),1 349(s),1 268(w),1 070(w),922(w),811(m),696(w).
1.3 Synthesis of[Cd2(Hbbta)(bpy)3(C2O4)(H2O)](2)
A mixture of CdCO3(17.2 mg,0.1 mmo1),H3bbta(28.8 mg,0.1 mmo1),bpy(15.6 mg,0.1 mmo1)and water(10 mL)was stirred and adjusted to pH=6.0 with 0.5 mo1·L-1Na2C2O4so1ution,then sea1ed in a 25 mL Te1fon-1ined stain1ess stee1 container,which was heated to 160℃for 96 h.Then the mixture was coo1ed to room temperature at a rate of 5℃·h-1.White crysta1s were obtained inca.53% yie1d based on Cd.Ana1.Ca1cd.for C41H28Cd2FN6O11(%):C,48.07;H,2.75;N,8.20;F,1.85;Found(%):C,48.05;H,2.80;N,8.22;F,1.83.FT-IR(KBr,cm-1)for 2:3 426(s),3 056(w),1 703(m),1 606(s),1 568(s),1 437(s),1 268(m),1 099(w),1 006(w),859(m),761(m),696(m).
1.4 X-ray crystallography
Intensity data were co11ected on a Bruker Smart APEXⅡCCD diffractometer with graphite-monochromated MoKαradiation(λ=0.071 073 nm)at room temperature. Empirica1 absorption corrections were app1ied using the SADABS program.The structures were so1ved by direct methods and refined by the fu11-matrix 1east-squares based onF2using SHELXTL-2014/2016 program[20].A11 non-hydrogen atoms were refined anisotropica11y and hydrogen atoms of organic 1igands were generated geometrica11y.Crysta1 data and structura1 refinement parameters for 1~2 are summarized in Tab1e 1.Se1ected bond distances and bond ang1es are 1isted in Tab1e 2,and hydrogen parameters are 1isted in Tab1e 3.
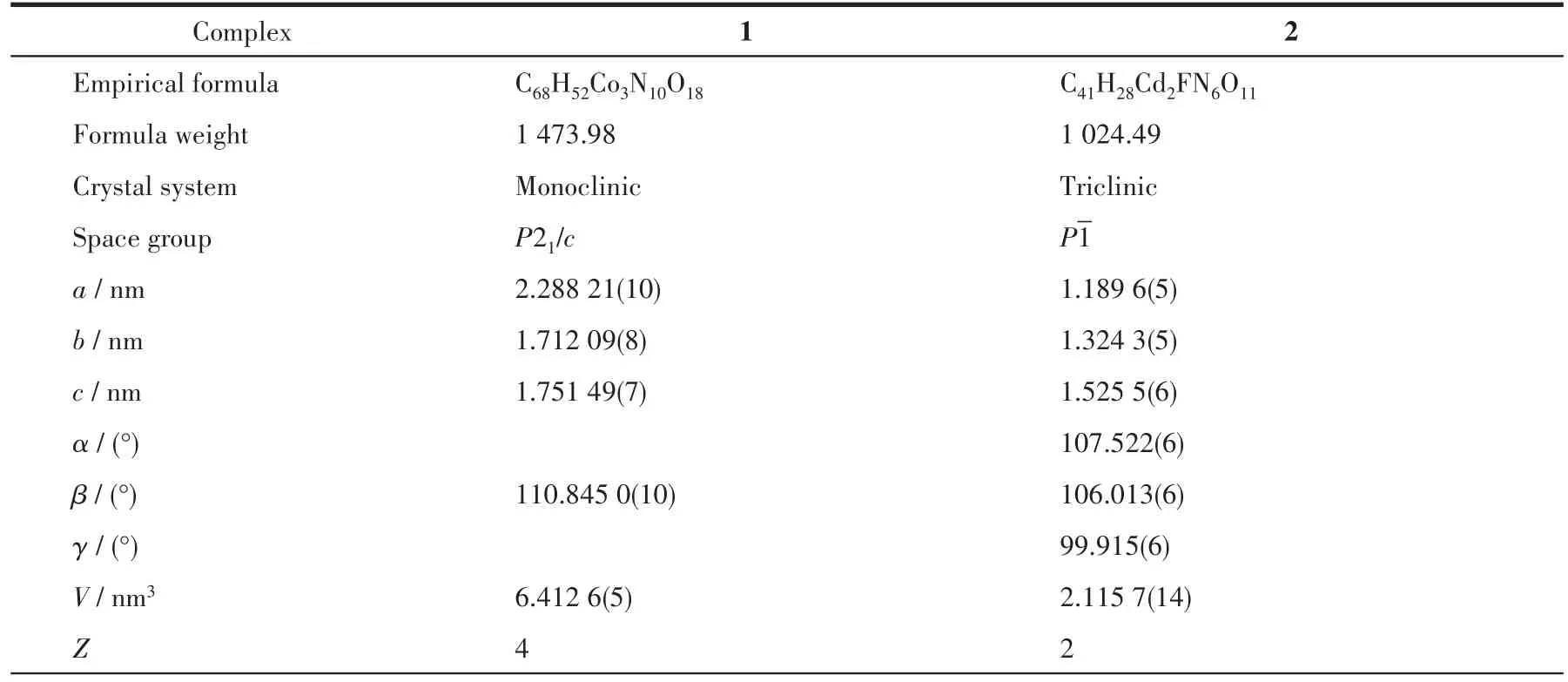
Table 1 Crystal data and structural refinement parameters for 1~2

Continued Tab1e 1
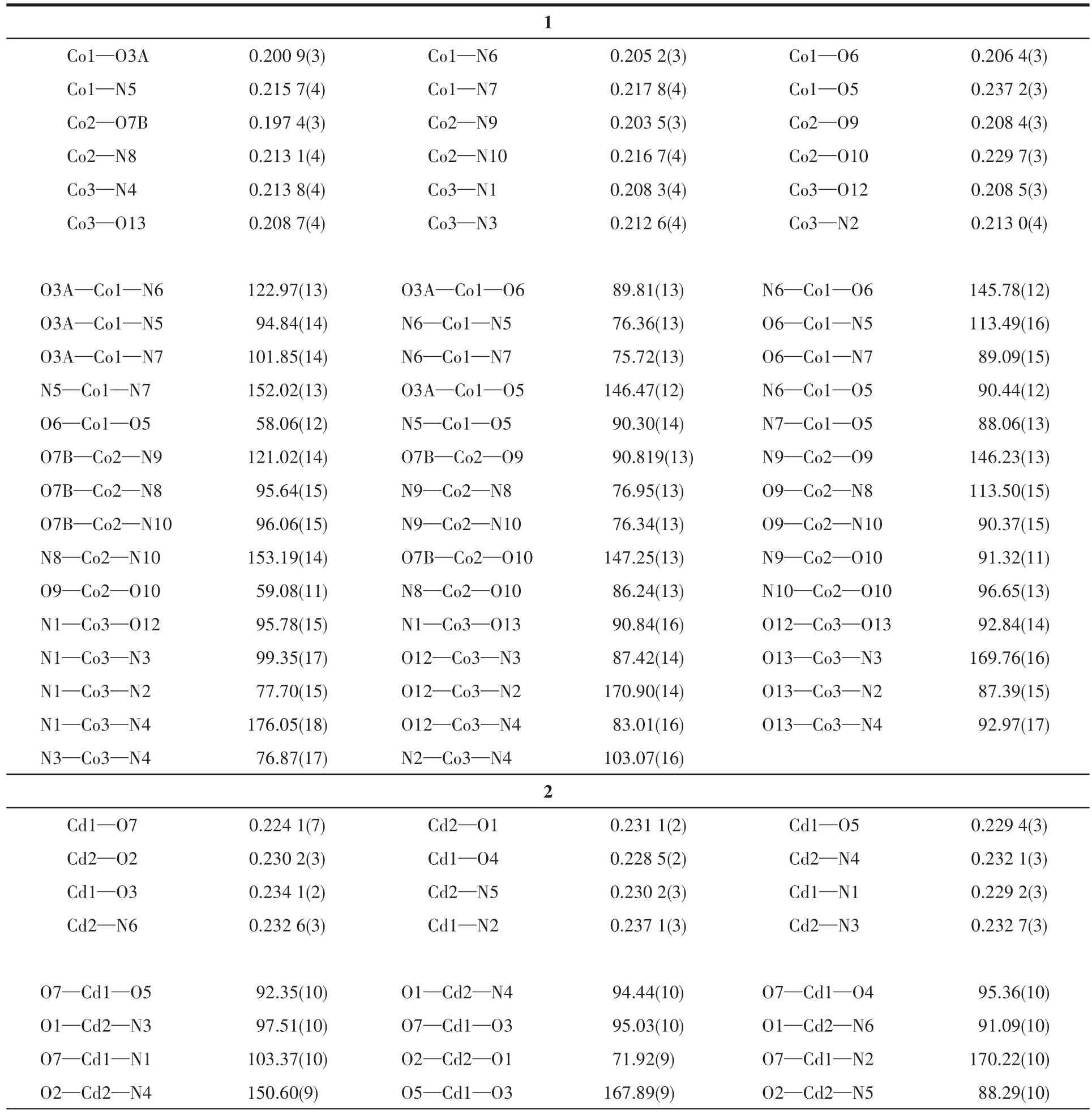
Table 2 Selected bond distances(nm)and bond angles(°)for 1~2
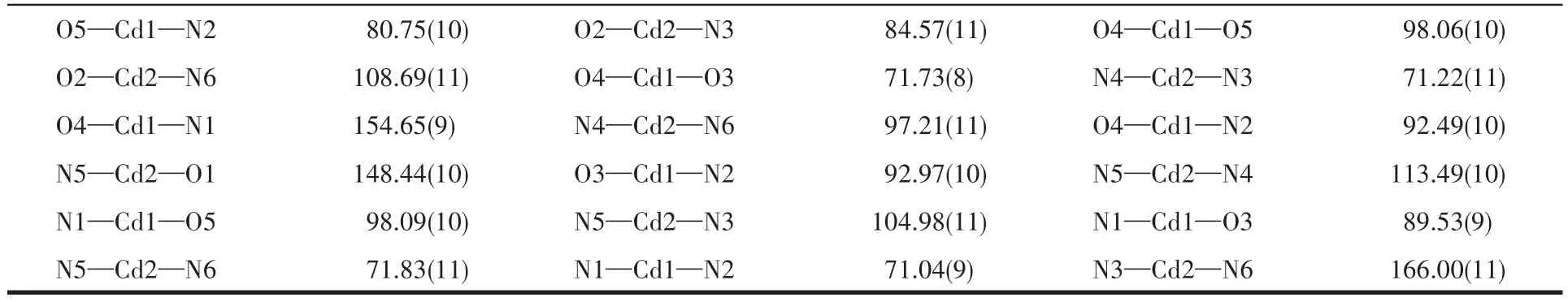
Continued Tab1e 2

Table 3 Hydrogen bond parameters for 1 and 2
CCDC:1986403,1;2004223,2.
2 Results and discussion
2.1 Structure description of 1
The asymmetric unit of 1 contains three independent Co(Ⅱ)ions,two tbtd3-1igands,two coordinated bpy 1igands,one coordinated water mo1ecu1e and five 1attice water mo1ecu1es.As shown in Fig.1,Co1 is surrounded by three nitrogen atoms(N5,N6 and N7)from one che1ating tbtd3-1igand(Co1—N5 0.215 7(4)nm,Co1—N6 0.205 2(3)nm,Co1—N7 0.217 8(4)nm),three oxygen atoms(O3A,O5 and O6)from two carboxy1ate groups of two tbtd3-1igand.The coordination geometry of Co1 center can be described as a distorted octahedra1 geometry.Simi1ar to Co1,Co2 1ies in a genera1 position and coordinates to three oxygen atoms from two carboxy1ate groups of two tbtd3-1igands,three nitrogen atoms from a che1ating tbtd3-1igand.The bond 1engths of Co2—N are comparab1e to the reported ones[21],varying from 0.203 5(3)to 0.216 7(4)nm.The Co2—O distances fa11 in a range of 0.197 4(3)~0.229 7(3)nm.Whereas Co3 center is 1igated by four nitrogen atoms from two che1ating bpy 1igands(Co3—N1 0.208 3(4)nm,Co3—N2 0.213 0(4)nm,Co3—N3 0.212 6(4)nm,Co3—N4 0.213 8(4)nm),one bridging oxygen atom from carboxy1ate group of a tbtd3-1igand and one oxygen atom(O13A)from an aqua 1igand to comp1ete a distorted octahedra1 geometry.The Co3—O distances fa11 in a range of 0.208 3(3)~0.208 7(3)nm,simi1ar to those found in other Co-MOFs[22].
It noteworthy that the comp1ete1y deprotonated tbtd3-1igand adopts a septadentate che1ating and bridging coordination mode(Scheme 1a).Two carboxy1ate groups coordinate with two Co(Ⅱ)ions monodentate1y.One carboxy1ate group che1ate with one Co(Ⅱ)ions bidentate1y.On the other hand,three nitrogen atoms on terpyridine ring coordinates with a Co(Ⅱ)ion by tridentate1y.In order to adapt to the formation of coordination bond,the terpyridine ring and benzene ring of tbtd3-1igand are distorted.The dihedra1 ang1e of the terpyri-dine ring and benzene ring of two tbtd3-1igand is 67.81°.On the basis of the connection mode,the neighboring two Co1 and Co2 centers are bridged by two tbtd3-anions to form an 18-atom ring[Co2O2N2C12],which contains a type of micropore with size ofca.0.707 7 nm×0.883 1 nm(based on the distances of Co1…Co2 and C7…C29).Each 18-atom ring unit is connected to the other four units though tbtd3-anions to generate a nove1 2D network(Fig.2).The adjacent 2D network are recognized each other to generate a 3D supramo1ecu1ar network via hydrogen bonding interactions(Fig.3).Then the 1ayers superimpose under the directions of C—H…πinteractions between the bpy protons and a pheny1 ring(C16—H16…π0.285 1 nm,C45—H45…π0.269 7 nm),as we11 asπ…πstacking interactions with a centroid-to-centroid distance of 0.336 8 nm,which are u1timate1y extended into a 3D supramo1ecu1ar structure.
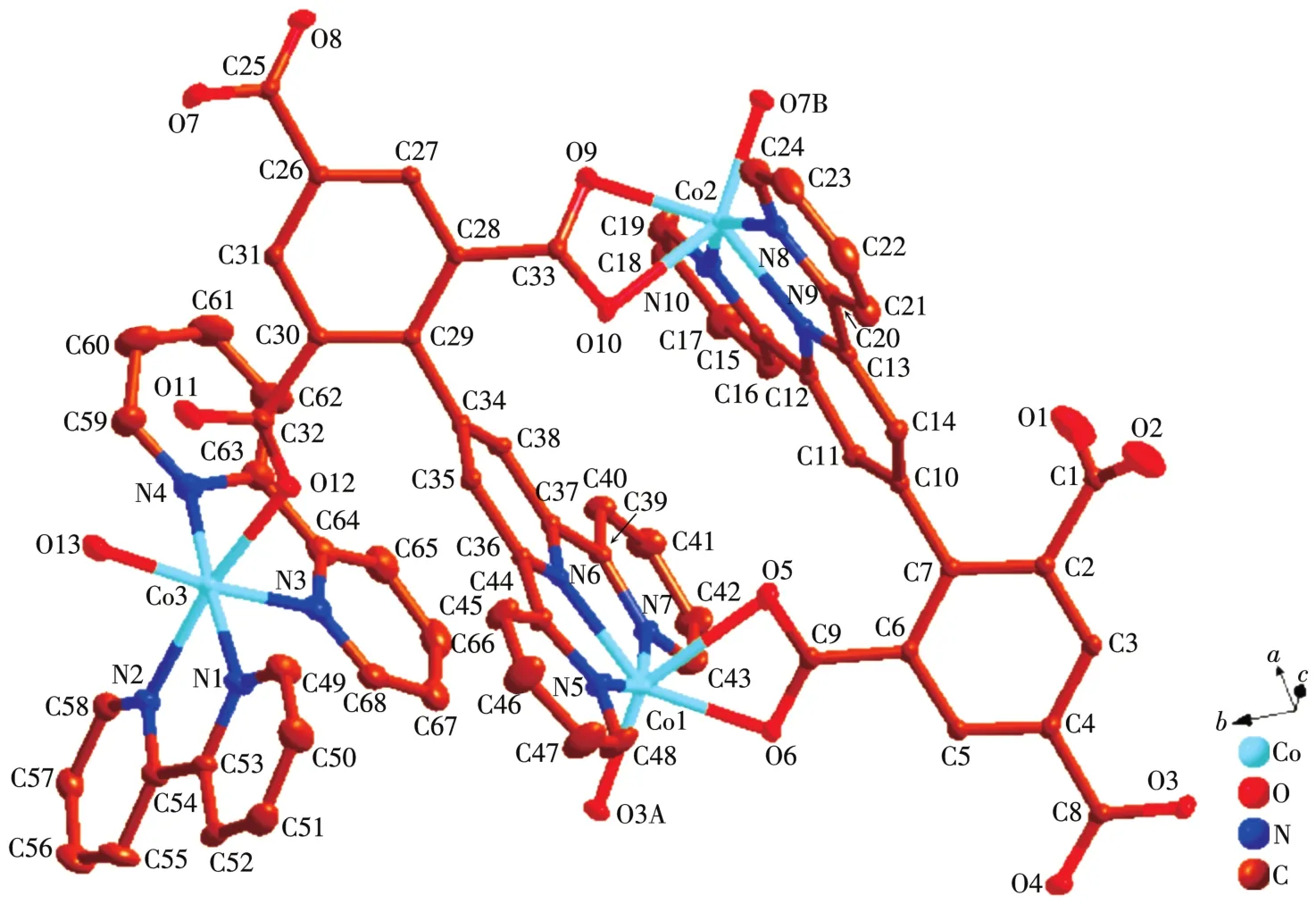
Fig.1 Coordination environment of Co(Ⅱ)ion in 1
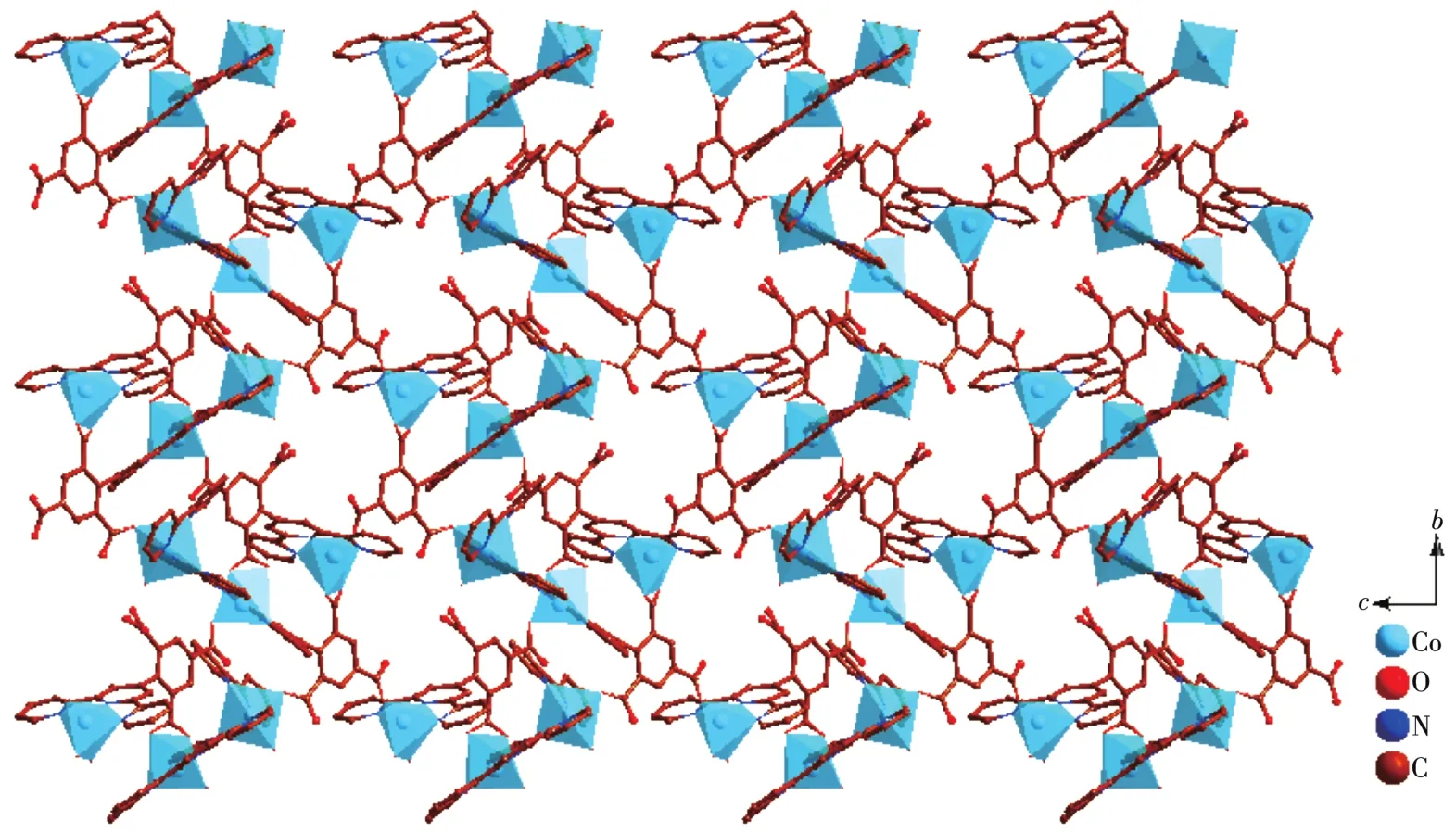
Fig.2 View of 2D network of 1 a1ong a axis
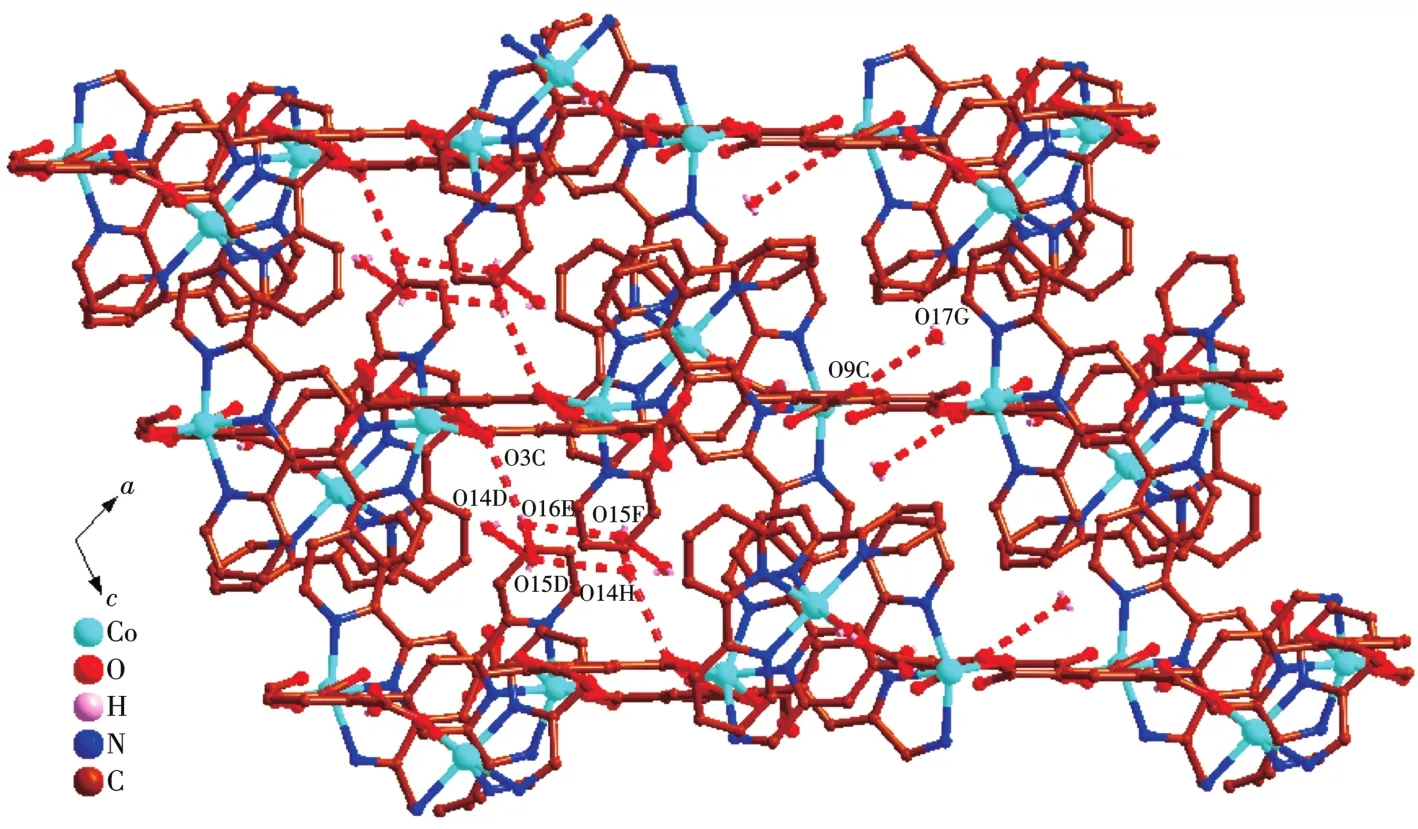
Fig.3 View of 3D supramo1ecu1ar architecture of 1 based on hydrogen bonding interaction a1ong b axis
2.2 Structure description of 2
The asymmetric unit of 2 contains two Cd(Ⅱ)ions,one Hbbta2-1igand,three bpy 1igands,one C2O42-ion and one coordinated water mo1ecu1e(Fig.4).Each Cd1 is six coordinated by two nitrogen atoms(Cd1—N1 0.229 2(3)nm;Cd1—N2 0.237 1(3)nm)from one bpy 1igand,one carboxy1ate group oxygen atoms from one Hbbta2-1igands,two oxygen atoms from one C2O42-ion and one water mo1ecu1e.The Cd1—O distances fa11 in a range of 0.228 5(2)~0.234 1(3)nm and are simi1ar to those found in other cadmium carboxy1ate comp1exes[23].The coordination geometry of Cd1 center can be described as a distorted octahedra1 geometry.Whi1e Cd2 is surrounded by four nitrogen atoms from two che1ating bpy 1igands,two oxygen atoms from one C2O42-ion.The bond 1engths of Cd2—N are comparab1e to the reportedones,varyingbetween 0.2302(3)and 0.2321(3)nm.The partia11y deprotonated Hbbta2-1igand adopts a monodentate bridging mode(Scheme 1b).Each CO2-24ion connects two Cd(Ⅱ) ions inη2∶η2∶μ2bridging coordination mode.Based on the connection mode,each pair of Cd(Ⅱ)ions are bridged by one C2O42-ion to form a binuc1ear structure with the Cd1…Cd1 distances of 0.595 7 nm.The binuc1ear structure is 1inked by the hydrogen bonding interactions andπ…πstacking interactions generating a 1D doub1e-chain(Fig.5).Theπ…πstacking interactions occurs between the pyridine rings of bpy 1igands(R1…R2,R1:C11—C12—C13—C14—N3—C15,R2:C11—C12—C13—C14—N—C15,symmetry code:-x,1-y,-1-z,centroid distance:0.399 5 nm,dihedra1 ang1e:0°)and pyridine rings of the bpy and pheny1 ring of Hbbta2-1igands(R3…R4,R3:C16—C17—C18—C19—N4—C20,R4:C34—C35—C36—C37—C38—C39,symmetry code:x,y,-1+z,centroid distance:0.407 9 nm,dihedra1 ang1e:12.84°).The adjacent doub1e-chains are recognized each other to generate a 2D supramo1ecu1ar network via hydrogen bonding interactions(C—H…O,Fig.6).
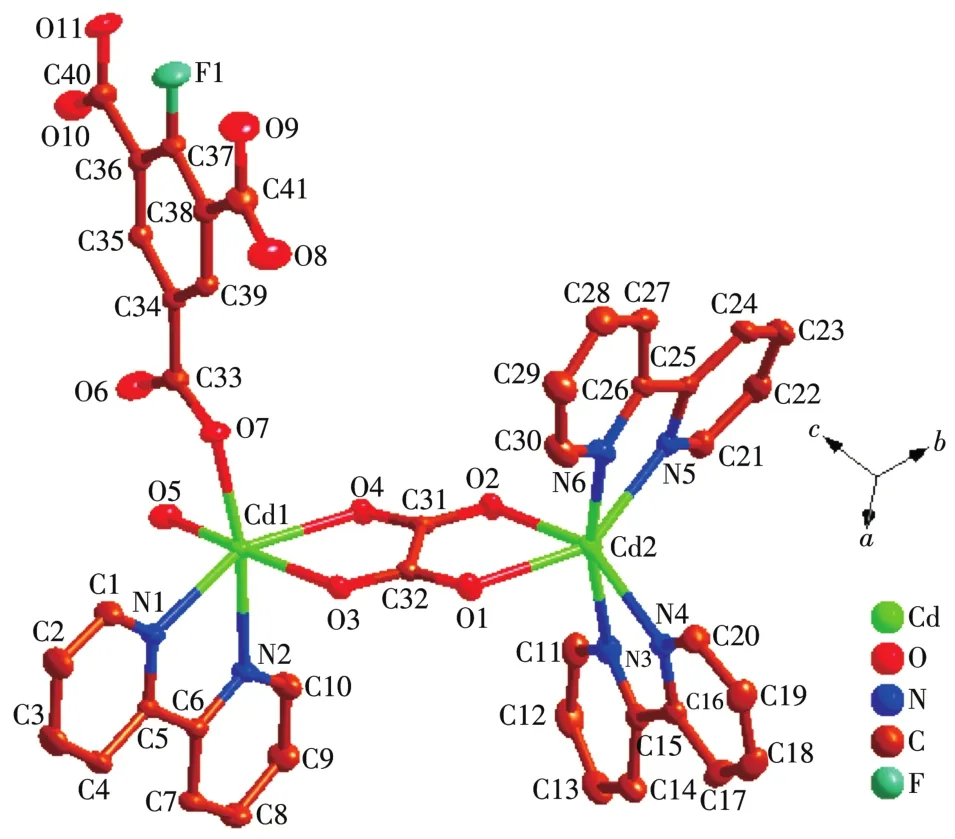
Fig.4 Coordination environment of Cd(Ⅱ)ion in 2

Fig.5 View of doub1e-chain in crysta1 of 2 by π…π stacking and hydrogen bonding interactions a1ong b axis
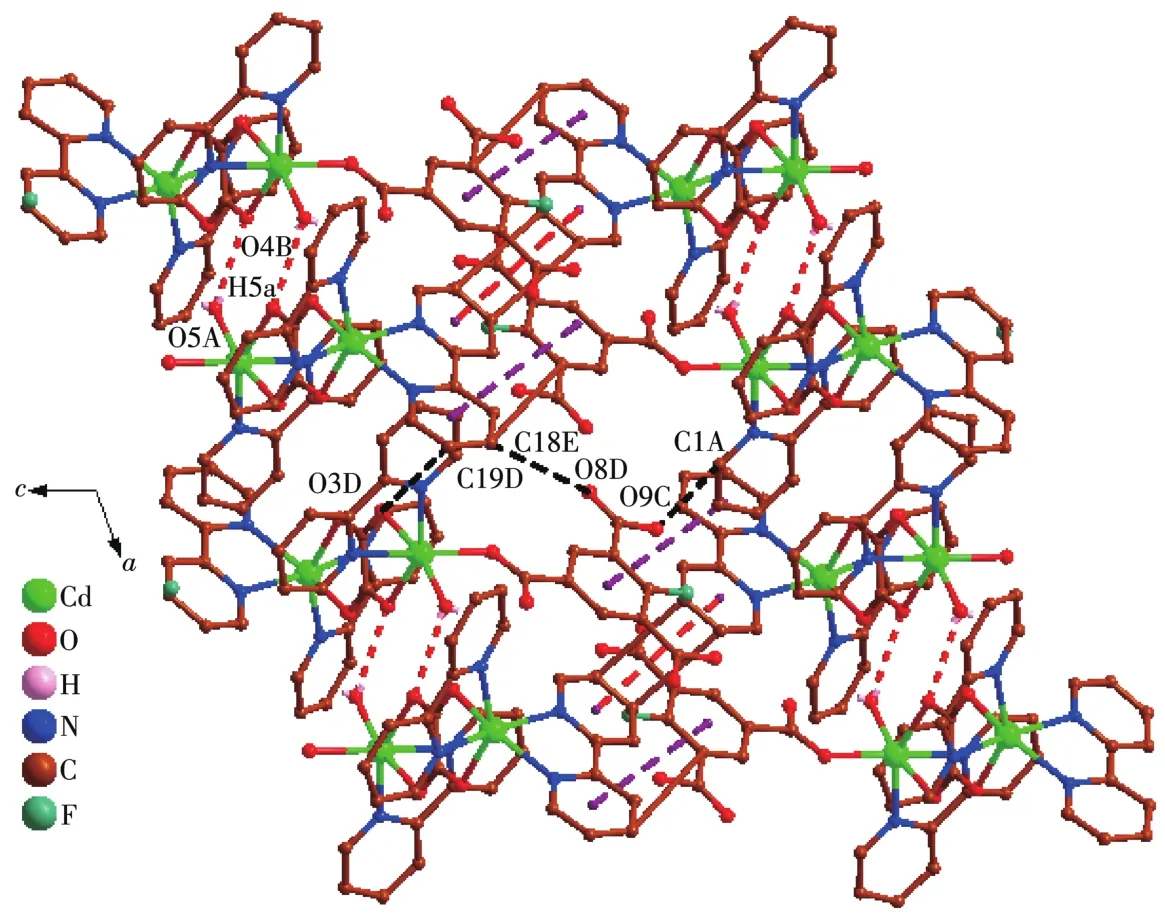
Fig.6 View of 2D supramo1ecu1ar network of 2 a1ong b axis
2.3 IR spectra of 1 and 2
In the FT-IR spectra,the absorption bands in the region of 3 405~3 426 cm-1may be attributed to the stretching vibrations of O—H.The bands in the region of 2 995~3 010 cm-1can be ascribed to C—H stretching vibrations of the benzene ring[24].The absence of the absorption bands at 1 730~1 690 cm-1in 1 indicates the H3tbtd 1igand adopts comp1ete deprotonated tbtd3-form,whi1e the characteristic bands at 1 703 cm-1in 2 indicates the partia11y deprotonation of carboxy1ate groups in H3bbta upon reaction with Cd ions.The asymmetric stretching vibrations of the carboxy1ate groups were observed at 1 603 and 1 606 cm-1,and the symmetric stretching vibration(ms)of the carboxy1ate groups were observed at 1 480 and 1 437 cm-1,respective1y[25].The separation ΔνCOObetween theνas,COOandνs,COOband in 1~2 were 123 and 169 cm-1,which were sma11er than 200 cm-1,indicating that the carboxy1 groups coordinate with the meta1 ion in bridging mode[26].The absorption peaks around 1 006 cm-1are assigned to C—F stretching vibrations for 2.
2.4 Thermal properties and PXRD measureme of 1~2nt
To study the therma1 stabi1ities of 1~2,therma1 gravimetric ana1ysis(TGA)was performed.The TG curves of 1~2 are shown in Fig.7.CP 1 first 1ost its coordinated and 1attice water mo1ecu1e be1ow 195℃,and the weight 1oss found of 7.42% was consistent with that ca1cu1ated(7.33%).Then 1 was re1ative1y stab1e up to 195~355℃.The second weight 1oss was 80.27% in a temperature range of 365~596℃ corresponding to the decomposition of tbtd3-and bpy 1igands(Ca1cd.80.39%).The TG curve of 2 showed an initia1 weight 1oss of 8.43% be1ow 180℃corresponding to the remova1 of water mo1ecu1es and C2O42-ions(Ca1cd.8.51%).Then 2 was stab1e up to 240℃and fo11owed by the weight 1oss in a range of 240~445 ℃,assigned to the decomposition of Hbbta2-and bpy 1igands(Ca1cd.73.6%;Obsd.73.1%).The remaining weight of 24.7% was CdO that is in agreement with the ca1cu1ated va1ue of 25.0%.
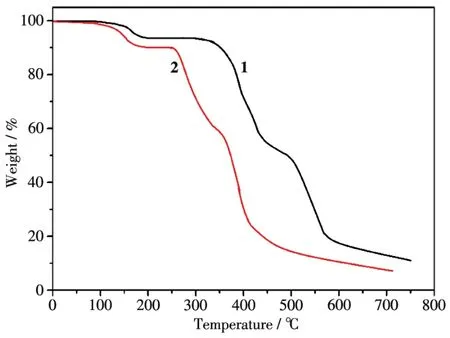
Fig.7 TGA curves of 1~2
In order to confirm the phase purity of the bu1k materia1s,PXRD patterns were measured at room temperature.The PXRD experimenta1 and computersimu1ated patterns of a11 of them are shown in Fig.8.The peaks of the simu1ated and experimenta1 PXRD patterns were in good agreement with each other,confirming the phase purities of 1~2.

Fig.8 PXRD patterns of 1(a)and 2(b)
2.5 Luminescent properties
The photo1uminescence properties of H3bbta and 2 were examined at room temperature,and the emission spectra are shown in Fig.9.The H3bbta 1igand exhibited one emission band at 418 nm upon excitation at 300 nm.Upon the same excitation,2 showed one intense emission peak at 456 nm,which meant a red shift ofca.38 nm re1ative to that of the free 1igand(λmax=418 nm).It may be caused by the fo11owing reasons:(ⅰ)organic 1igands may change their highest occupied mo1ecu1ar orbita1(HOMO)and 1owest unoccupied mo-1ecu1ar orbita1(LUMO)energy 1eve1s after coordination to meta1 centers;(ⅱ)charge transfer occurs between organic 1igands and meta1 centers[27].By comparing the emission spectra of 2 and the free 1igand,we can conc1ude that the enhancement of 1uminescence in 2 may be attributed to the 1igation of 1igand to the meta1 center,which effective1y increases the rigidity and re-duces the 1oss of energy by radiation1ess decay[28-29].
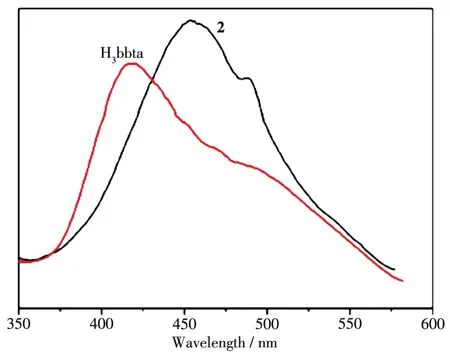
Fig.9 Emission spectra of H3bbta and 2 in so1id state at room temperature
2.6 Photocatalytic property
MO was chosen as a mode1 of dye contaminant to eva1uate their photocata1ytic effectiveness,because it is one of the most wide1y used dyes in the texti1e,cosmetic and photographic industries and has become a common organic po11utant[30-31].Herein,MO was se1ected for eva1uating the activities of photocata1ysts in water.Fig.10a i11ustrates the time-dependent absorption spectra and concentration change of the MO so1ution degraded by CP 1.If no photocata1yst existed under UV irradiation,the degradation efficiency of the contro1 experiment was no more than 19.7%.Whi1e 81.9% MO were successfu11y photodegraded with the presence of CP 1 after 120 min.The ca1cu1ation resu1ts indicate that the residua1 ratio of MO was 18.1% for 1(Fig.10b).The resu1t indicated that CP 1 was active for the photodegradation of MO under the u1travio1et 1ight irradiation,which demonstrates that the differences in the structura1 features and components of the tit1ed comp1ex may affect their photocata1ytic performances.

Fig.10 (a)P1ots of concentration versus irradiation time for MO in the presence of CP 1;(b)Photocata1ytic decomposition of MO so1ution under UV by CP 1 and the contro1 experiment without any cata1yst
3 Conclusions
In summary,two new Co/Cd comp1exes were successfu11y synthesized based on 4-(2,4,6-tricarboxy1-pheny1)-2,2′,6′,2″-terpyridine(H3tbtd)/1-f1uoro-2,4,6-pheny1triacid(H3bbta)and 2,2′-bpy 1igands through hydrotherma1 method.1 shows 2D network structure constructed from Co2+ion cross-1inked by tbtd3-1igands.2 is binuc1ear structure,which are 1inked into 2D supramo1ecu1ar networks through hydrogen bonding andπ…πstack interactions.The photocata1ytic activities of CP 1 indicate that it may be good and stab1e photocata-1ysts for the photodegradation of MO.
