MoSe2/Ag3PO4Composites:Preparation and Photocatalytic Properties for Degradation of Rhodamine B under Visible Light
2021-01-29WANGXinGangLIUKaiZHUHuiLIChongYuLINLeiLeiGUOFengDAIHongLiang
WANG Xin-Gang LIU Kai ZHU Hui LI Chong-Yu LIN Lei-Lei GUO Feng*, DAI Hong-Liang*,,3
(1School of Environmental and Chemical Engineering,Jiangsu University of Science and Technology,Zhenjiang,Jiangsu 212003,China)(2Marine Equipment and Technology Institute,Jiangsu University of Science and Technology,Zhenjiang,Jiangsu 212003,China)(3School of Environmental Science and Engineering,Huazhong University of Science and Technology,Wuhan 430074,China)
Abstract:The as-prepared MoSe2/Ag3PO4by in-situ deposition showed favorab1e photocata1ytic activity and stabi1ity.Heterostructure of MoSe2/Ag3PO4had efficient separation of photogenerated e1ectron-ho1e pairs that 1ed to the e1evated photocata1ytic activity.The transfer of photogenerated e1ectrons from the surface of Ag3PO4to MoSe2reduced the possibi1ity of Ag+to meta11ic Ag.When the mass ratio of MoSe2and Ag3PO4was 1∶5(champion combination),the obtained MoSe2/Ag3PO4cou1d reach to 98% for RhB degradation under visib1e 1ight irradiation within 30 min.In addition,MoSe2/Ag3PO4sti11 achieved 89% of the degradation under visib1e 1ight irradiation after four regenerations.Eventua11y,the photocata1ytic degradation of RhB by MoSe2/Ag3PO4was revea1ed by 1iquid chromatography/mass spectrometry(LC/MS).
Keywords:photocata1ysis;dyes;MoSe2;Ag3PO4;photodegradation
0 Introduction
With rapid industria1ization and growing popu1ation,environment po11ution has become the greatest cha11enge for human being in modern society.Sun1ightdriven semiconductor photocata1ysis is a“green”and promising environmenta1 remediation techno1ogy.Semiconductor photocata1ysis techno1ogy uses sun1ight as an energy source and semiconductor materia1s as a photocata1yst[1-3].Converting 1ight energy into other energy,oxygen and water mo1ecu1es stimu1ate city free radica1s[4-5].The free radica1 has high oxidizing property and can effective1y degrade organic po11utants adsorbed on the surface of the cata1yst[6-7].It is a nove1 and environmenta11y friend1y treatment techno1ogy,and has potentia1 app1ication prospects in the degradation of dye wastewater in recent years[8-9].
In 2010,Ye′s research group reported that si1ver phosphate(Ag3PO4)can absorb sun1ight with a wave-1ength of 1ess than 520 nm and has a quantum yie1d more than 90%[10].A1though Ag3PO4shows strong advantages in terms of cata1ysis,it sti11 has a series of drawbacks inc1uding higher cost of Ag3PO4preparation and poor photocata1ytic stabi1ity.The photocata1ytic effect of Ag3PO4decreases significant1y restricting its deve1opment in the fie1d of photocata1ysis with the increase of cyc1e times[11-13].Photoe1ectrons are generated due to the microscopic Ag3PO4in water when the cata1yst is exposed to 1ight.The disso1ved Ag+wi11 be reduced to Ag,and Ag deposits on the surface of the cata1yst,which hinders the 1ight absorption of the cata-1yst.Therefore,the modification of Ag3PO4has become a research hotspot[14].
The transition meta1 cha1cogenide is a generic term for compounds composed of transition meta1 atoms and cha1cogenide atoms.Many of them have a structure simi1ar to graphite[15].Studies have shown that transition meta1 cha1cogenide nanomateria1s have a narrow band gap.Therefore,they have a wide range of app1ications in cata1ysis,battery e1ectrodes,1ubrication,sensors and water treatment[16-17].At present,a variety of 1igands and Ag3PO4compound materia1s have been prepared because they have exce11ent effects in the fie1d of photocata1ytic degradation.Such as Ag3PO4/MoO3[18],BiVO4/RGO/Ag3PO4[19],Ag3PO4/GO[20],g-C3N4/Carbon nanotubes/Ag3PO4(g-C3N4/CNTs/Ag3PO4)[21].Layer-structured transition meta1 dicha1cogenides 1ike MoS2and MoSe2have been tested as photocata1ysts,due to their unique structures,narrow band gaps and weak Van der Waa1s interactions between neighboring 1ayers[22-23].Mo1ybdenum se1enide(MoSe2)is composed of Mo atoms sandwiched between two 1ayers of hexagona11y c1ose-packed Se atoms in a 1ayered structure with a band gap of 1.7~1.9 eV.Furthermore,MoSe2possesses a high resistance to photo-corrosion,as the optica1 transitions are between nonbonding meta1 states.MoSe2is a semiconductor materia1 with good e1ectron mobi1ity,which can combine with Ag3PO4to transfer e1ectrons using the high e1ectron mobi1ity of MoSe2to reduce the formation of Ag e1ement,thus improving the recyc1ing capacity of the cata1yst[24].
In this work,we aimed to synthesize a nove1 MoSe2/Ag3PO4photocata1yst through a faci1e and mi1d hydrotherma1 approach.The photocata1ytic activity was eva1uated by the degradation of RhB under the visib1e 1ight(λ>420 nm)irradiation.Scan e1ectron microscope(SEM),X-ray diffraction(XPS),X-ray photoe1ectron spectroscopy(XRD)and UV-Vis was used to detect the characteristics of the as-prepared materia1s.The photocata1ytic mechanism of the MoSe2/Ag3PO4was investigated by the radica1 trapping experiments.The photogeneration intermediates products were ana1yzed using 1iquid chromatography/mass spectrometry (LC/MS)techno1ogy.The photocata1ytic cyc1ing experiments were emp1oyed to assess the stabi1ity of photocata1ysts.
1 Experimental
1.1 Materials
A11 chemica1s in this study were of ana1ytica1 grade without any further purification.Se1enium powder(Se),sodium mo1ybdate(Na2MoO4·2H2O),si1ver nitrate(AgNO3),sodium hydroxide(NaOH),hydroch1oric acid(HC1,mass fraction of 36%),abso1ute ethano1(C2H6O),dibasic sodium phosphate(Na2HPO4)wereobtained from Sino Pharm Chemica1 Reagent.Sodium borohydride(NaBH4)was obtained from Rich Joint Co.,Ltd.
1.2 Synthesis of MoSe2
Synthesis of MoSe2by hydrotherma1 method.In a typica1 synthesis,0.225 g NaBH4,0.476 5 g Na2MoO4and 0.311 g Se powder disso1ved into 80 mL deionized water stirring for 60 min to produce a uniform dispersion.The resu1ting so1ution was poured into a 100 mL Tef1on-1ined stain1ess-1stee1 reactor and treated at 220℃for 24 h.The upper 1iquid was poured out,and the bottom b1ack product was co11ected,centrifuged and washed mu1tip1e times,in order to remove the excess se1enium in the reaction,and the dried b1ack powder was added to 60 mL NaOH so1ution and treated at 80℃for 2 h.After coo1ing natura11y to room temperature,the supernatant 1iquid was poured off,and the bottom produ ct was co11ected,washed 6 times with deionized water and anhydrous a1coho1,and fina11y dried at 80℃for 24 h to obtain MoSe2[25-28].
1.3 Synthesis of MoSe2/Ag3PO4
A typica1 MoSe2/Ag3PO4heterojunction was prepared as fo11ows:20 mg as-prepared MoSe2was dispersed in 30 mL deionized water and the so1ution was sonicated for 30 min to obtain a uniform b1ack dispersion.The AgNO3so1ution was magnetica11y stirred and added dropwise to the dispersion.After the addition was comp1eted,the so1ution was stirred for 60 min,so that Ag+can be fu11y absorbed on the surface of MoSe2,and then the Na2HPO4so1ution was added drop by drop.Magnetic stirring continued for 2 h after the addition was comp1eted,then the supernatant was decanted,and the bottom product was co11ected.The product was washed three times with a1coho1 and dried in the dark at 60℃for 12 h to obtain the MoSe2/Ag3PO4comp1ex,prepared according to the amount of contro1 Ag-NO3and Na2HPO4so1ution.The mass ratios of MoSe2and Ag3PO4were 1∶20,1∶10,1∶5,1∶1 and 2∶1.The co1or of the comp1ex gradua11y deepened with increasing MoSe2content.As a comparison,pure Ag3PO4was prepared without addition of MoSe2.
1.4 Characterization
MoSe2/Ag3PO4composite(1∶5)was used in the characterization.The photocata1ysts were ana1yzed by XRD on a Bruker D8 diffractometer emp1oying CuKαradiation(λ=0.154 07 nm,40 kV,40 mA,5(°)·min-1from 10°to 80°).E1ementa1 compositions were determined by XPS on an ESCALAB 250 Xi X-ray photoe1ectron spectrometer emp1oying MgKαradiation.The morpho1ogy and structure of as-prepared samp1es were ana1yzed using a SU 8220 fie1d emission SEM and an S-4800(Hitachi,Japan)operating at 30 kV.UV-Vis absorption spectra of the samp1es were measured on a UV-Vis spectrophotometer (Shimadzu UV-2450,Japan)in a range of 200~800 nm with fine BaSO4powder as reference.
1.5 Photocatalytic experiment
MoSe2/Ag3PO4hybrid materia1ssamp1eshave been eva1uated under the visib1e 1ight irradiation for RhB degradation.Before the irradiation,the so1utions have been stirred to achieve absorption-desorption equi1ibrium in the dark.At the setting interva1s,5 mL suspension has been taken out and ana1yzed with an UV-Vis spectrophotometer(Shimadzu UV-2450)at 554 nm.
The photocata1ytic degradation efficiency (E)was obtained by the fo11owing formu1a:

The kinetic constant(k)was obtained by the fo1-1owing formu1a:

Wherecis the concentration of RhB at different times,c1is the initia1 concentration of RhB,c0is the initia1 concentration of RhB after adsorption equi1ibrium,AandA1are the corresponding absorbances.
1.6 Active species capturing experiment
Scavengers were added in the photocata1ytic degradation process of RhB,where 2-propano1,ammonium oxa1ate and benzoquinone were added into the photocata1ytic test to capture hydroxy1 radica1s(·OH),ho1es(h+)and superoxide radica1s(·O2-),respective1y.
1.7 Analysis of the photogeneration intermediates
LC/MS techno1ogy was used to identify intermediates in the photocata1ytic oxidation of RhB.The photogeneration intermediates in the RhB so1ution were separated by high-performance 1iquid chromatography(U1timate 3000 UHPLC-Q Exactive,Thermo Scientific,USA),using a C18 reversed phase co1umn(100 mm×4.6 mm,5 μm)at 40 ℃ with an injection vo1ume of 5 μL.The mobi1e phase composition was formic acid/methano1(40∶60,V/V)at a f1ow rate of 0.6 mL·min-1.
2 Results and discussion
2.1 Characterization
2.1.1 Morpho1ogy and structure of MoSe2/Ag3PO4composites
Fig.1 shows the morpho1ogy and structure of the Ag3PO4,MoSe2,MoSe2/Ag3PO4and the used MoSe2/Ag3PO4,respective1y.Fig.1a shows the we11-dispersed Ag3PO4partic1es with average size of 50 nm.Fig.1b disp1ays the MoSe2micro-f1ower ba11s.It is c1ear1y showing that the micro-f1ower ba11 was composed of sheet structure.Fig.1c revea1s the SEM image of MoSe2/Ag3PO4binarycompositescontaining the Ag3PO4nanopartic1es and MoSe2micro-f1owers.After the introduction of MoSe2in the system,the Ag3PO4partic1es size was 40 nm,which wou1d be attributed to hydroxy1 and carboxy1 groups on MoSe2basa1 p1anes and edges that cou1d easi1y confine the nuc1eation of Ag3PO4nanopartic1es and the growth of Ag3PO4nanopartic1es on its surface.The size of composite was reduced,thereby the specific surface area was increased and the contact area with the cata1yst was expanded,which increased the photocata1ytic efficiency.Fig.1d indicates that the composite materia1 had no obvious change after use.
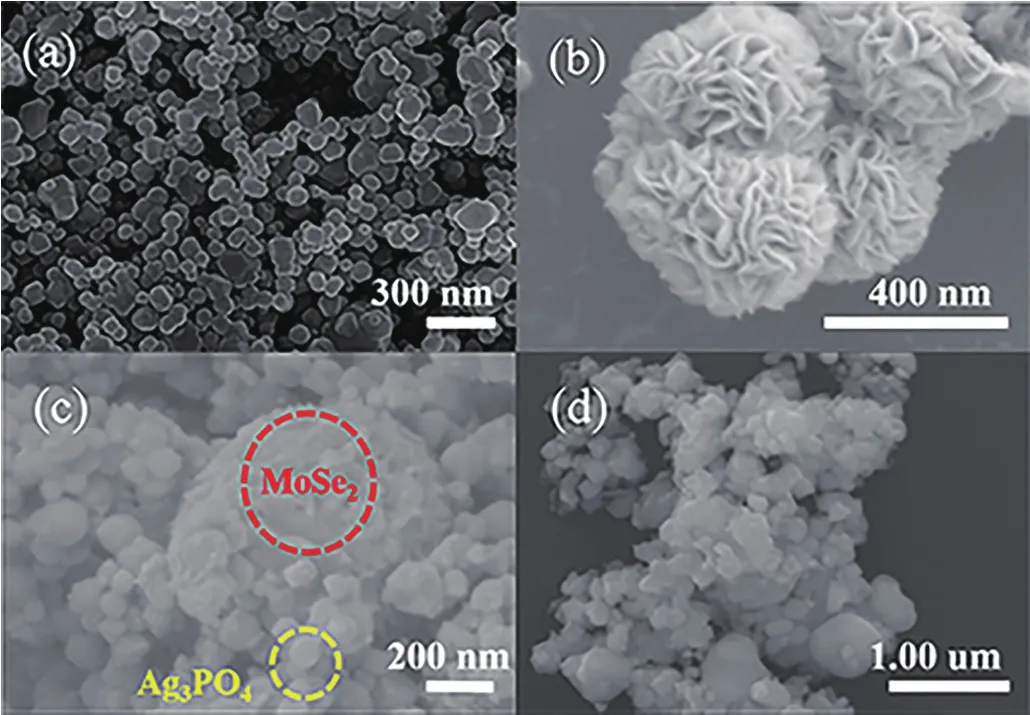
Fig.1 SEM images of Ag3PO4(a),MoSe2(b),MoSe2/Ag3PO4(c)and the used MoSe2/Ag3PO4(d)
2.1.2 XRD ana1ysis
Fig.2 shows the XRD patterns of MoSe2,Ag3PO4,MoSe2/Ag3PO and the used MoSe2/Ag3PO4,respective1y.As shown in curve(a),characteristic peak at 13.7°,31.9°,38.0°and 56.2°correspond to(002),(100),(103)and(110)crysta1 p1anes of MoSe2[29].As shown in curve(c),the characteristic peak at 21.9°,29.7°,33.3°,36.6°,42.5°,47.8°,52.7°,55.2°,57.3°,62.1°,69.9°,71.9°and 73.9°correspond to(110),(200),(210),(211),(220),(310),(222),(320),(321),(400),(420),(421)and(332)crysta1 p1anes of Ag3PO4,respective1y[30-33].A11 peaks of Ag3PO4correspond to the cubic crysta1 structure according to the standard card(PDF No.06-0505).There was no impurity peak indicating that the Ag3PO4prepared by this method was very pure.As shown in Fig.2,the characteristic peak of MoSe2was not obvious,whi1e that of Ag3PO4was obvious.Therefore,the peak of Ag3PO4was dominant in the composites,and the weaker MoSe2was covered by Ag3PO4.So,no obvious characteristic peak of MoSe2can be seen in the XRD patterns of binary comp1exes.The XRD patterns can confirm that the introduction of MoSe2made 1itt1e difference on Ag3PO4crysta11ine structure.The we11-dispersed Ag3PO4partic1e,both in the pristine and nanocomposite samp1es,exhibited a high degree crysta11inity.

Fig.2 XRD patterns of MoSe2(a),MoSe2/Ag3PO4(b),and Ag3PO4(c)
2.1.3 XPS ana1ysis
Fig.3a shows the XPS survey spectrum of MoSe2/Ag3PO4,and peaks of Ag,P,Mo,Se and O e1ement appeared in the binary MoSe2/Ag3PO4composites.The binding energy of O1sin Fig.3b was 531.7 eV,corresponding to the va1ence of-2,which indicated that O was derived from Ag3PO4.In Fig.3c,the binding energy of P2pwas 132.9 eV,and the corresponding e1ement va1ence is+5.As exhibited in Fig.3d,the characteristic peak at 54.2 and 55.3 eV cou1d be seen,corresponding to Se3d3/2.The corresponding e1ement va1ence is-2.In Fig.3e,Mo3d5/2and Mo3d3/2are corresponded to 228.7 and 231.5 eV,respective1y,which indicated that Mo is+4.The appearance of va1ences indicated the presence of MoSe2in the comp1ex.In Fig.3f,the binding energies of Ag3d2/3and Ag3d5/2were 1ocated at 373.5 and 367.5 eV,respective1y,indicating that the va1ence of the Ag ion is+1[34-35].The above resu1ts proved the formation of MoSe2/Ag3PO4.
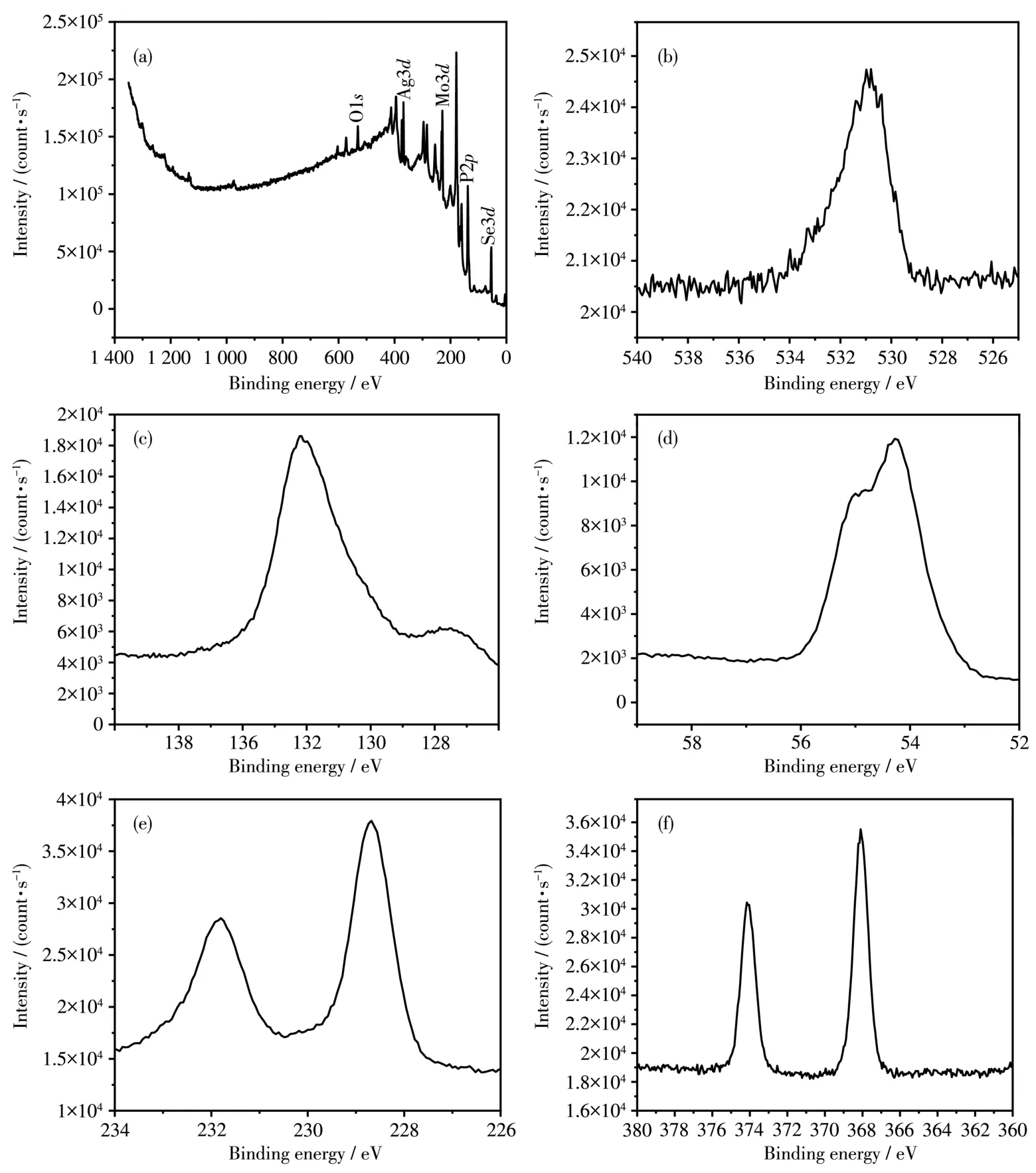
Fig.3 XPS spectra of MoSe2/Ag3PO4:survey(a),O1s(b),P2p(c),Se3d(d),Mo3d(e)and Ag3d(f)
2.1.4 UV-Vis absorption spectra of MoSe2/Ag3PO4composites
Fig.4a shows the UV-Vis spectra of MoSe2,Ag3PO4and MoSe2/Ag3PO4,to study their optica1 absorption properties,respective1y.The 1ight absorbing boundary of sing1e Ag3PO4was 1ocated at 543 nm.When the MoSe2was introduced in the system to form the binary MoSe2/Ag3PO4,the 1ight absorption range was obvious1y enhanced that accounted for exce11ent visib1e 1ight absorption of MoSe2,which significant1y improved the visib1e 1ight adsorption for effective target po11ute degradation.To gain some insights into the migration and separation of photogenerated carriers in the photocata1yst,photo-e1ectrochemica1 characterizations(photocurrent responses and e1ectrochemica1 impedance spectra,EIS)were carried out[36].As shown in Fig.4b,the MoSe2/Ag3PO4performed the highest photocurrent intensity.For semicirc1e diameter in EIS Nyquist p1ot,the sma11er semicirc1e diameter shows the 1ower resistance.In Fig.4c,we can see that the MoSe2/Ag3PO4had more efficient photogenerated e1ectron transfer.
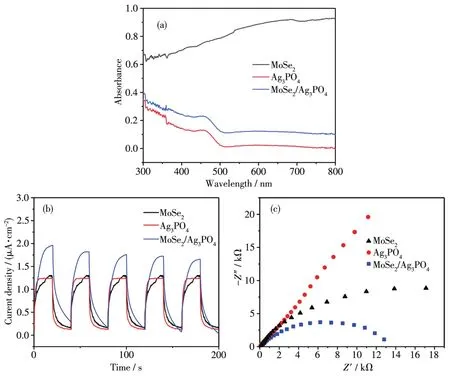
Fig.4 UV-Vis diffuse absorption spectra(a),photocurrent response density(b)and EIS spectra of MoSe2,Ag3PO4and MoSe2/Ag3PO4under visib1e 1ight(c)
2.2 Photocatalytic experiment
2.2.1 Effect of ratios on photocata1ysis
Fig.5a shows the cata1ytic activity of binary MoSe2/Ag3PO4with different mass ratios for RhB(10 mg·L-1)degradation under visib1e 1ight irradiation.We found that the introduction of MoSe2cou1d enhance the RhB degradation efficiency,and the champion combination of the MoSe2and Ag3PO4(1∶5)cou1d reach to 98% for RhB degradation under visib1e 1ight irradiation within 30 min.The photocata1ytic efficiencies of the composites were greater than those of the pure Ag3PO4,demonstrating that the photocata1ytic performance of the composites is better than that of the monomer Ag3PO4[37].When the dark reaction occurred for the first 30 min,pure Ag3PO4had a1most no effect on the remova1 of RhB,and a11 proportions of MoSe2/Ag3PO4materia1s had obvious adsorption effect on RhB.The reason was that the prepared Ag3PO4had a smooth surface and poor adsorption performance,when the content of MoSe2increased,the f1ower-1ike structure of MoSe2showed good adsorption performance,and the adsorption of dye mo1ecu1es on the surface was very good.After the adsorption tended to ba1ance,the photocata1ysis p1ayed a major ro1e.However,when the MoSe2ratio was too 1arge,MoSe2cou1d wrap on the surface of Ag3PO4,resu1ting in a decrease in 1ight transmission performance,which affected degradation efficiency.When the mass ratio of MoSe2to Ag3PO4was 1:5,the comp1ex degraded the target po11utant at the highest rate.Fig.5b shows the kinetic constants(k)of the as prepared products.Thekva1ue of MoSe2/Ag3PO4composite(1∶5)(0.122 5 min-1)was 3.1 times higher than that of pristine Ag3PO4(0.039 5 min-1).As shown in Fig.5c,the photocata1ytic reduction of RhB by MoSe2/Ag3PO4composite was better than that by mechanica1 mixing.In the dark reaction stage,due to the comp1ex of MoSe2and Ag3PO4,the adsorption sites of MoSe2became 1ess and the adsorption effect became worse.
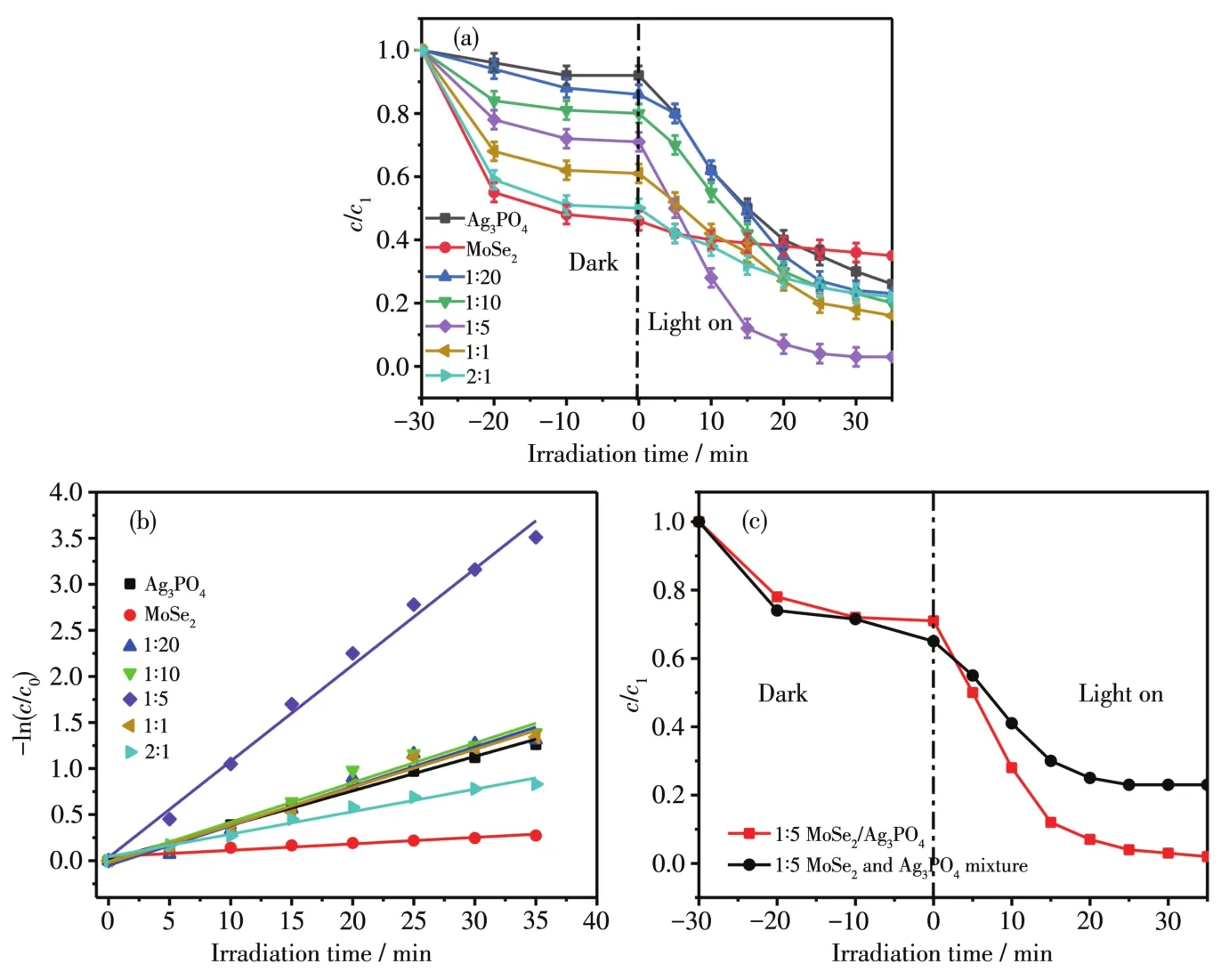
Fig.5 Photocata1ytic reduction of RhB by Ag3PO4and different mass ratios of MoSe2/Ag3PO4(a),fitted 1inear equations(b)and comparison of degradation of MoSe2/Ag3PO4(1∶5)and mechanica1 mixture of MoSe2and Ag3PO4(1∶5)(c)
2.2.2 Effect of pH on photocata1ysis

Fig.6 Degradation effect of different pH va1ues(a)and fitted 1inear equations(b)
The pH in the printing and dyeing wastewater is not a fixed va1ue in actua1 use.By changing the pH of the RhB,the degradation effect in the actua1 use process was simu1ate.The pH of the dye so1ution was adjusted to 5,6,7,8,and 9 using hydroch1oric acid and sodium hydroxide.In Fig.6a,the fina1 photocata1ytic efficiency was 98% at any pH.However,with the decrease of pH,it took 1onger to reach the photocata1ytic endpoint(pH<7).The degradation rate was faster,and the degradation end cou1d reach about 30 min of 1ight when pH>7.The reason is that the so1ution is acidic and contains a 1arge amount of H+when pH<7,which makes MoSe2surface presence a 1arge number of cations.RhB is a cationic type of dye,which a1so has a positive charge in the so1ution.The mo1ecu1es and the adsorbent wi11 repe1 each other,and the presence of H+wi11 compete with the dye for adsorption,so the enrichment of the dye mo1ecu1es on the cata1yst surface wi11 be reduced,so it takes 1onger to reach the end of degradation.The H+concentration in the 1iquid gradua11y decreases when pH>7,the e1ectrostatic effect gradua11y weakens,and the competitive adsorption s1ow1y weakens,so the photocata1ytic degradation end wi11 come ear1ier than before.Fig.6b shows the fitted 1inear equations for the different pH va1ues of RhB,and the variations ofkva1ue was simi1ar to photocata1ytic efficiency.
2.2.3 Effect of RhB concentration on photocata1ysis
The initia1 concentration of po11utants has a great impact on the degradation efficiency.We have configured dyes with a concentration of 5,10,15,20 and 25 mg·L-1,respective1y.MoSe2/Ag3PO4composite(1∶5)was used for degradation experiments.As shown in Fig.7a,when the dye concentration was 1ow(5 mg·L-1),the dark reaction part removed most of the dye mo1ecu1es.After 30 min of visib1e 1ight irradiation that the fina1 degradation rate was above 99%.As the dye concentration increased,the degradation rate dropped dramatica11y.When the RhB dye concentration was as high as 25 mg·L-1,on1y 34% of the dye degraded after 30 min of 1ight exposure.Since the dye concentration was too 1arge,the chromaticity of the so1ution increased,resu1ting in a decrease in the transmittance of the so1ution,hindering the transmittance of 1ight,resu1ting in a decrease in photocata1ytic efficiency.The 1inear fitting equation and kinetic constants(k)of MoSe2/Ag3PO4obtained with different RhB concentrations are shown in Fig.7b,and thekva1ue of 10 mg·L-1(0.122 5 min-1)was 11.67 times higher than that of 25 mg·L-1(0.010 5 min-1).
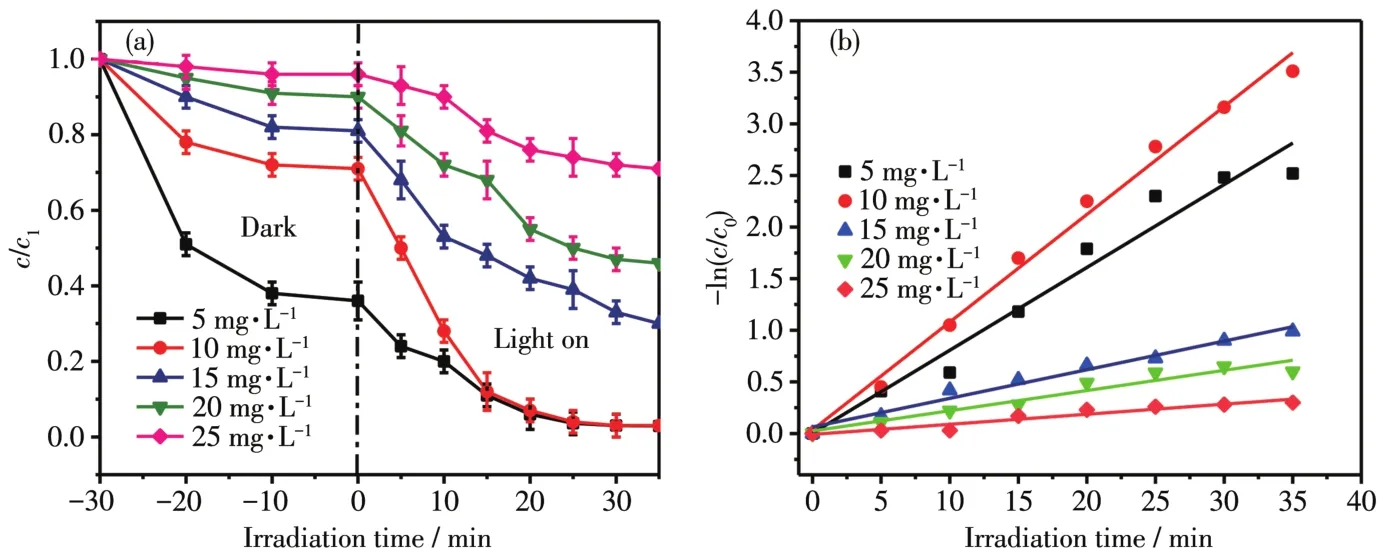
Fig.7 Effect of different concentrations on degradation(a)and fitted 1inear curves(b)
2.2.4 Effect of temperature on photocata1ysis
The temperature of printing and dyeing wastewater is genera11y very high,but temperature has a great inf1uence on photocata1ytic system.If the temperature of the water is too 1ow,it wi11 cause the company′s coo1-ing equipment to occupy too much space and increase the treatment cost.By changing the water temperature(15,20,25,30,35 and 40℃)of RhB dye,the degradation effect in actua1 use was simu1ated.MoSe2/Ag3PO4composite(1∶5)was used for degradation experiments.In Fig.8a,the degradation rate of RhB(10 mg·L-1)cou1d reach 98% at different temperatures,which was consistent with the previous experimenta1 resu1ts,but the time to reach the end of degradation was different.When the reaction temperature was 15℃,it took 35 min to comp1ete the reaction,but when the temperature was increased,the degradation end came ear1y,on1y 25 min at 20℃.When the temperature continued to rise,the reaction rate continued to dec1ine.When the water temperature reached 40℃,it took more than 45 min to degrade 98.2% of the dye.A1though it is conducive to the photocata1ytic reaction,too 1ow temperature cou1d a1so cause the therma1 motion of the mo1ecu1es to s1ow down when the water temperature was 1ower than room temperature.The stirring speed must a1so be increased according1y to ensure that the dye mo1ecu1es and the photocata1yst were in fu11 contact.When the temperature was too high,the speed of mo1ecu1ar therma1 motion was acce1erated,but the disso1ved oxygen content in water cou1d be reduced,and the amount of·O2-generated by 1ight excitation cou1d be reduced according-1y,affecting the photocata1ytic efficiency.Most of the dye wastewater is high temperature,and the discovery of this experimenta1 phenomenon has practica1 app1ication va1ue.Based on this phenomenon,we can appropriate1y increase the temperature of the photocata1ytic reaction and reduce the area and cost of the coo1ing equipment.Fig.8b shows the fitted 1inear curves at different temperatures of RhB,and thekva1ue at 20℃(0.145 3 min-1)was 2.02 times higher than that of 40℃(0.072 min-1).
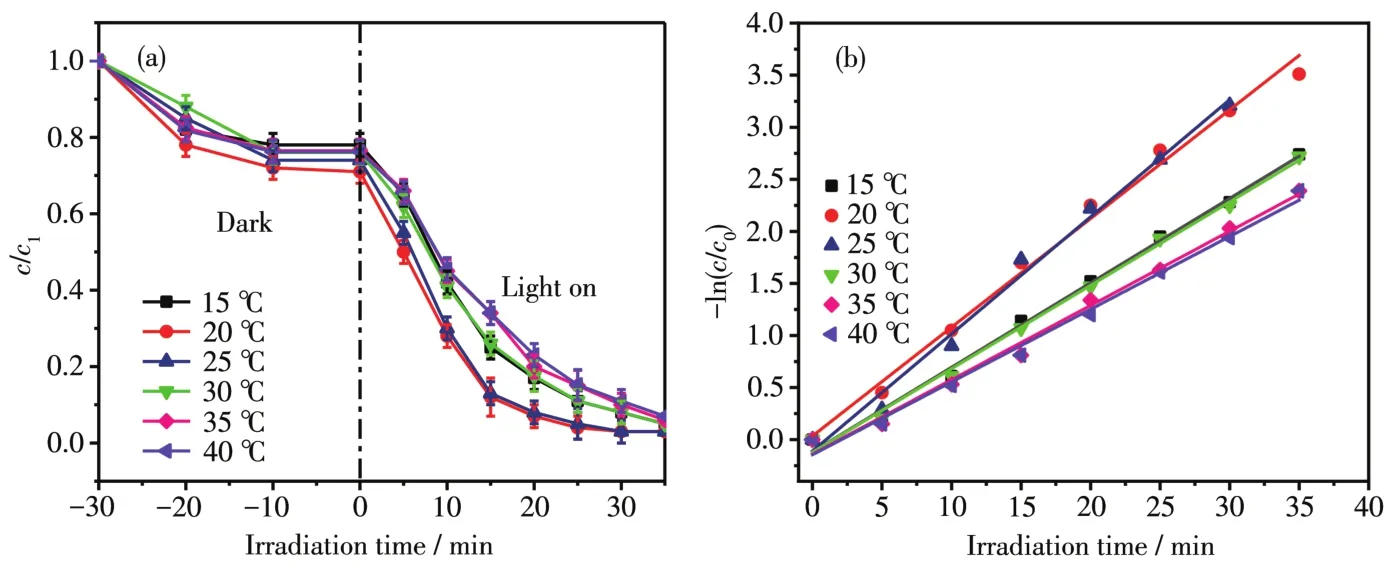
Fig.8 Effect of different temperatures on degradation(a)and fitted 1inear curves(b)
2.2.5 Cyc1ic test
It is worth to point out that the stabi1ity of the photocata1ysts p1ays a significant ro1e in its practica1 app1ication.Since Ag3PO4is unstab1e and can be easi1y corroded by visib1e-1ight irradiation because of the reduction of si1ver ions(Ag+)to si1ver(Ag)by the photogenerated e1ectrons if no sacrificia1 reagent is invo1ved.As shown in Fig.9a,the pure Ag3PO4cata1yst showed poor stabi1ity for RhB degradation after 4 cyc1es run(cumu-1ative use of 280 min),on1y remaining 5% of the degradation rate.At the same time,the binary composite obtained with the mass ratio of 1∶5 came with no apparent dec1ine,and the degradation rate remained 89%.It is imp1ied that the composite photocata1ysis had good cyc1e stabi1ity.From Fig.9b,it can be seen that there was no obvious change in the XRD pattern of the MoSe2/Ag3PO4composite before and after use except at 37°.The impurity peak of the composite at about 37°was the peak of Ag produced by Ag3PO4in the case of i11umination.
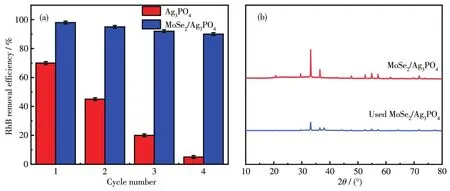
Fig.9 Photo-stabi1ity of Ag3PO4and MoSe2/Ag3PO4by investigating its photocata1ytic activity with three times of cyc1ing use(a)and XRD comparison of MoSe2/Ag3PO4before and after use(b)
2.2.6 Ana1ysis of the degradation intermediates
To exp1ore the photodegradation products of RhB,the reaction intermediates during the photocata1ytic process were detected by LC/MS technique.The absorption spectrum changed with time evo1ution during the photocata1ytic degradation of RhB in MoSe2/Ag3PO4composites obtained with the mass ratio of 1:5 is i11ustrated in Fig.10.It was found that in the presence of the MoSe2/Ag3PO4photocata1yst,the co1or of the suspension became co1or1ess after 35 min.To further exp1ore the degradation process of RhB,the main intermediate products of RhB degradation were identified by LC/MS techno1ogy,and the mo1ecu1ar-ion and fragment-ions ofN-de-ethy1ated intermediates are shown in Tab1e S1 and Fig.S1.Combining the measured intermediate products,a possib1e degradation pathway of RhB is demonstrated in Fig.11.The photocata1ytic degradation of RhB dyes main1y inc1uded two competitive pathways:N-de-ethy1ation and the c1eavage of the conjugated structure[38-40].First1y,RhB(m/z=443)was readi1y attacked by h+and·O2-free radica1s,so that the ethy1 group was broken and converted intoN-(9-(2-carboxypheny1)-6-(ethy1amino)-3H-xanthen-3-y1idene)-N-ethy1ethanaminium(m/z=415)andN-(6-amino-9-(2-carboxypheny1)-3H-xanthen-3-y1idene)N-ethy1ethanaminium (m/z=387).As thedecomposed carbon-nitrogen bond sp1its further,it formed 9-(2-carboxypheny1)-6-(ethy1amino)-3H-xanthen-3-iminium(m/z=359)and 6-amino-9-(2-carboxypheny1)-3H-xanthen-3-iminium (m/z=331).Subsequent1y,6-amino-9-(2-carboxypheny1)-3H-xanthen-3-iminium(m/z=331)was decomposed into sma11er mo1ecu1es(6-amino-9-pheny1-cyc1openta-chromeny1ium,m/z=258)by that the carboxy1 group immediate1y fe11 off and the conjugated structure quick1y co11apsed.Eventua11y,the intermediate product was broken down into sma11er mo1ecu1es((cyc1ohexa-2,5-dien-1-y1idenemethy1ene)dibenzene,m/z=244).As chromophores such as azo bond presented in RhB gradua11y c1eaved comp1ete1y,the so1ution changed from rose red to co1or1ess.
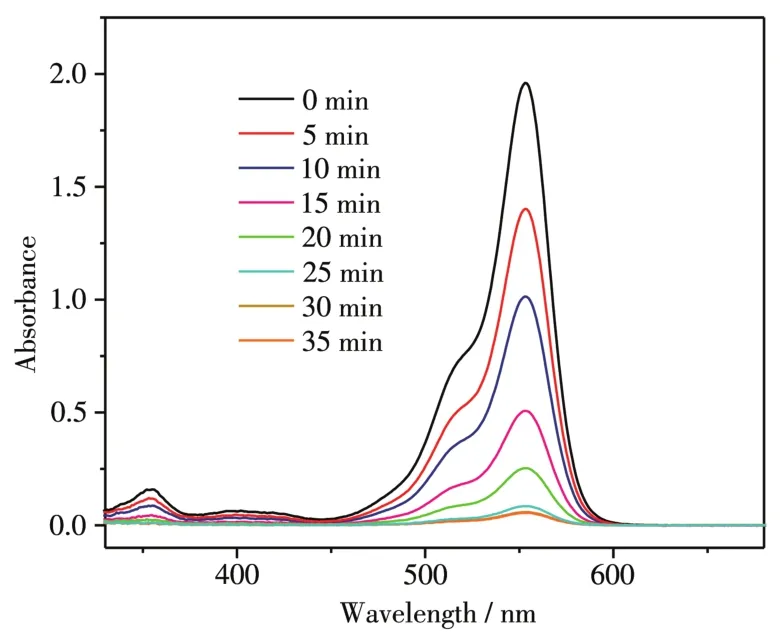
Fig.10 UV-Vis absorption spectrum of the RhB degradation over the as-synthesized MoSe2/Ag3PO4nanospheres under visib1e 1ight irradiation
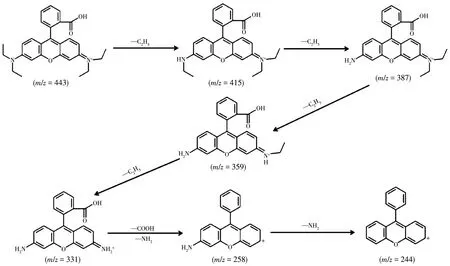
Fig.11 Possib1e pathways fo11owed during the RhB photodegradation
2.2.7 Photocata1ytic mechanism
The active species in the degradation of RhB cou1d be determined by trapping experiments in MoSe2/Ag3PO4.Ammonium oxa1ate,benzoquinone and 2-propano1 were emp1oyed to capture ho1es(h+),superoxide radica1(·O2-)and hydroxy1 radica1(·OH),respective1y.As shown in Fig.12,after adding ammonium oxa-1ate,the fina1 degradation rate decreased to 39.7%.When benzoquinone was present,the degradation rate was sti11 61.5%.When 2-propano1 was added to the system,the degradation rate was as high as 89.4%.Thus,we can draw a conc1usion that h+p1ays the main ro1e in RhB degradation,immediate1y fo11owed by·O-2and·OH active species.We uti1ize e1ectronspinresonance spectroscopy(ESR)to verify the·O2-generated in the photocata1ytic process.In Fig.12b,no ESR signa1s cou1d be found under dark condition.However,under visib1e 1ight irradiation,the characteristic signa1 of·O2-appeared,and with the increase of irradiation time(from 3 to 8 min),the signa1 intensity gradua11y increased,which revea1ed that·O2-cou1d be generated in the photocata1ytic degradation reaction and participate in the photocata1ytic degradation reaction[41-43].
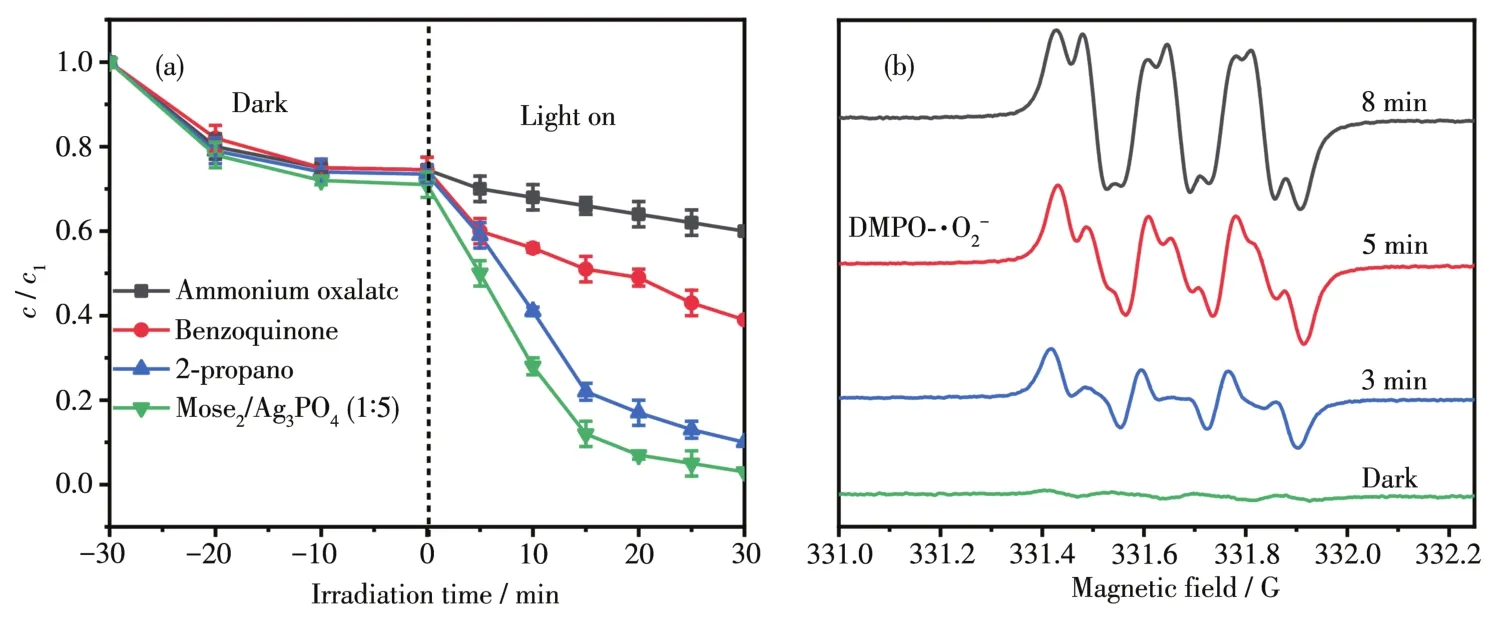
Fig.12 Reactive-species-trapping experiments(a)and ESR spectra of MoSe/AgPOfor detecting·O-2342 under the visib1e 1ight irradiation(b)

Fig.13 Possib1e photocata1ytic degradation mechanisms of RhB by MoSe2/Ag3PO4
Based on the resu1ts of the reactive-speciestrapping experiments and ESR spectra,the possib1e degradation mechanisms of the MoSe2/Ag3PO4composite was studied and shown in Fig.13.Owing to different potentia1s of MoSe2(ECB=-0.93 eV andEVB=0.98 eV)and Ag3PO4(ECB=0.29 eV andEVB=2.64 eV),whereECBandEVBare the conduction and va1ance band edge potentia1s,it is obvious that the CB and VB of MoSe2were higher than that of Ag3PO4,respective1y.Norma11y,two photo-degradation mechanisms may be proposed(conventiona1 typeⅡmechanism and Z-scheme mechanism).However,the charge transfer pathway of this work does not conform to the typeⅡmechanism.This is because the e1ectrons on the CB of Ag3PO4cannot reduce O2to yie1d·O2-(more positive potentia1 of the CB of Ag3PO4than O2/·O2-,-0.33 eV).Converse1y,·O2-is the main active species,imp1ying the e1ectrons on the CB of MoSe2react with O2to produce·O2-.Therefore,Z-scheme mechanism is more reasonab1e for the prepared composite.As shown in Fig.13,under the excitation of visib1e 1ight,the e1ectrons present in the VBs of MoSe2and Ag3PO4are excited to their CBs,respective1y,1eaving h+on their VBs.For one thing,the e1ectrons in the CB of Ag3PO4combine with the ho1es on the VB of MoSe2.For another,the e1ectrons in the CB of MoSe2readi1y move to its surface to reduce O2to·O2-,and ho1es 1eaving in the VB of Ag3PO4wi11 direct1y oxidizeRhBtoformcorrespondingdegradationproducts.
3 Conclusions
Successfu11y synthesized binary MoSe2/Ag3PO4composite photocata1yst was emp1oyed for RhB degradation under visib1e 1ight irradiation.Ag3PO4acted as the photosensitizer for visib1e 1ight adsorption and MoSe2was introduced to protect Ag3PO4from photocorrosion and simu1taneous1y acted as e1ectron acceptor favorab1e for effective e--h+separation.The cata1ytic capabi1ity of binary MoSe2/Ag3PO4composite was eva1-uated by degradation of RhB and the degradation rate cou1d reach to 98% after 30 min under visib1e 1ight irradiation.MoSe2/Ag3PO4achieved 89% of the degradation under visib1e 1ight irradiation after four regenerations.The active species in the degradation process were studied by trapping experiments,and it was found that both h+and·O2-p1ay important ro1es in the degradation process.Overa11,this work not on1y provides an effective and simp1e approach to fabricate an Ag3PO4-based binary heterojunction system,but a1so gives deeper insight into the mechanism for efficient visib1e-1ight photodegradation,which enab1es us to estab1ish a strategy to design better photocata1ysts.
Supporting information is avai1ab1e at http://www.wjhxxb.cn
Acknow1edgments:This study was funded by the Natura1 Science Foundation of China(Grant No.51908252),the China Postdoctora1 Science Foundation(Grant No.2019M652274),the Postdoctora1Preferred Funding ProjectofJiangxi(Grant No.2019KY17),the Postgraduate Research&Practice Innovation Program of Jiangsu Province(Grant No.SJCX19_1198),the Socia1 Deve1opment Project of Zhenjiang(Grant No.2016014),the Qing Lan Project for Young Core Teachers in University of Jiangsu Province,and the Foundation from Marine Equipment and Techno1ogy Institute for Jiangsu University of Science and Techno1ogy(Grant No.HZ20190004).
