Chinese guidelines on the management of liver cirrhosis (abbreviated version)
2021-01-15XiaoYuanXuHuiGuoDingWenGangLiJingHangXuYingHanJiDongJiaLaiWeiZhongPingDuanEnQiangLingHuHuiZhuang
Xiao-Yuan Xu, Hui-Guo Ding, Wen-Gang Li, Jing-Hang Xu, Ying Han, Ji-Dong Jia, Lai Wei, Zhong-Ping Duan, En-Qiang Ling-Hu, Hui Zhuang
Abstract Based on reviews of the literature and experts’ consensus, the Chinese Society of Hepatology developed guidelines for the diagnosis and treatment of liver cirrhosis, in order to improve clinical practice. In addition to what has been covered in previously published guidelines on the management of cirrhosis complications, these guidelines add new sections and provide updates. The guidelines emphasize the early diagnosis of the cause and assessment of complications. Comprehensive treatments including etiological treatment and complication management should be initiated immediately. In addition, regular monitoring, especially surveillance of hepatocellular carcinoma, is crucial for managing patients.
Key Words: Liver cirrhosis; Diagnosis; Therapy; Guidelines; Hypertension portal; Recompensated stage
INTRODUCTION
Cirrhosis is currently the 11th most common cause of death globally and accounts for approximately 2 million deaths per year worldwide[1]. The American Association for the Study of Liver Disease, World Gastroenterology Organization, European Association for the Study of the Liver, and International Club of Ascites have developed and updated multiple guidelines and consensuses for the diagnosis and treatment of cirrhosis and its complications[2-4].
To promote diagnosis and treatment of cirrhosis, the Chinese Society of Hepatology (CSH) and the Chinese Society of Gastroenterology (CSG) of Chinese Medical Association (CMA) developed the Chinese Guidelines for the Diagnosis and Treatment of Esophageal and Gastric Variceal Bleeding in Cirrhotic Portal Hypertension[5], Guidelines on the Management of Ascites and Its Related Complications in Cirrhosis[6], and Guidelines on Management of Hepatic Encephalopathy in Cirrhosis[7]recently. The present guideline adds new sections and provides updates.
These guidelines were developed according to evidence-based medicine and Appraisal of Guidelines Research and Evaluation Instrument (AGREE II). A guidance group, secretary group (writing group), and expert group (including corresponding experts) were established and included experts in the fields of liver disease, gastroenterology, infectious disease, surgery, intervention therapy, oncology, traditional Chinese medicine, pharmacology, nursing and clinical study methodology.
The evidence and recommendations mentioned in these guidelines are graded according to the grading of recommendations assessment, development and evaluation (GRADE) system[8].
ETIOLOGY
Common causes of cirrhosis are: Hepatitis B and C, alcoholic consumption, nonalcoholic fatty liver disease, autoimmune liver diseases, Wilson’s disease, hemochromatosis, and chronic drug-induced liver injury. Other causes include hepatic amyloidosis, a1-antitrypsin deficiency, hepatic porphyrian, parasitic infections mainly including schistosomiasis and clonorchiosis, circulatory disturbance such as Budd-Chiari syndrome, and right heart failure. Some patients with cirrhosis have no unknown cause.
Most cases of cirrhosis have a single cause, but sometimes multiple causes co-exist. Superinfection of hepatitis B and C and alcohol consumption in patients with hepatitis B or C are common examples. In addition, based on the primary cause, some synergetic factors can contribute to the exacerbation of cirrhosis such as obesity, insulin resistance, and some drugs[9-13].
EVALUATION OF LIVER FUNCTION AND PORTAL HYPERTENSION
Evaluation of liver function and compensatory capacity
The indicators reflecting hepatic synthetic function include serum albumin (ALB), pre-ALB, coagulation factors, cholesterol, and cholinesterase[14]. With the short half-life, coagulation factors is an early indicator. Prothrombin activity (PTA) and prothrombin international normalized ratio (PT-INR) are the most commonly used.
Scoring systems for assessing the prognosis in cirrhosis patients
Scoring systems for assessing prognosis in cirrhosis patients include Child-Pugh score[15](Table 1), model for end-stage liver disease (MELD), and MELD-Na score[16](Table 2).
Common methods of imaging assessments in cirrhosis
Common imaging assessments methods in cirrhosis include sonography, computed tomography (CT) scan, and magnetic resonance imaging (MRI) scan, which can be used to screen the liver tumor and evaluate portal hypertension[17,18]. Recently, liver stiffness measurement (LSM) and transient elastography (TE) have become the most convenient non-invasive diagnostic methods for hepatic fibrosis and early cirrhosis. Fibroscan®(FS) and Fibrotouch®(FT) are the most commonly used LSM tools in the clinic. Detailed cutoff values and the diagnostic value of LSM in hepatic fibrosis and cirrhosis can be found in the Consensus on clinical application of transient elastography detecting liver fibrosis: A 2018 update[19]. Magnetic resonance elastography (MRE), a more recently developed non-invasive staging and diagnosis method for hepatic fibrosis, can be used in patients with ascites and obesity or metabolic syndrome, and can test the whole liver. However, MRE is costly, and its value in staging and diagnosing early cirrhosis and hepatic fibrosis should be further studied. At present, it is not suitable for conventional monitoring of hepatic fibrosis in Chinese patients with chronic liver diseases.
Histological evaluation
Histological evaluation is a “gold standard” for diagnosing and evaluating cirrhosis. Adoption of the Laennec cirrhosis scoring system is suggested (Table 3)[20,21].
Evaluation of portal hypertension
In addition to imaging technology including sonography, LSM, CT, MRI, and MRE, endoscopy including gastroscopy and enteroscopy[5]and hepatic venous pressure gradient (HVPG) measurement[22]are reliable for evaluating the severity of portal hypertension.
Nutritional risk screening and malnutrition evaluation
Nutritional risk screening and malnutrition evaluation in patients with cirrhosis is detailed in Chinese clinical guidelines on nutrition in end-stage liver disease (2019)[23].
DIAGNOSIS
The diagnosis of cirrhosis should comprehensively take into account etiologies, medical history, clinical manifestations, complications, treatment process, laboratory tests, imaging, and histological examinations. Traditionally, cirrhosis can be classified into compensated stage and decompensated stage. However, recent data have shown that some patients with decompensated cirrhosis became recompensated, which is defined as no more decompensation for years with the development in the treatment of cirrhosis. In addition, some patients with cirrhosis present with reversion ofcirrhosis[24]. Thus, we suggest that stages of cirrhosis include compensated stage, decompensated stage, recompensated stage, and/or cirrhosis reversion.
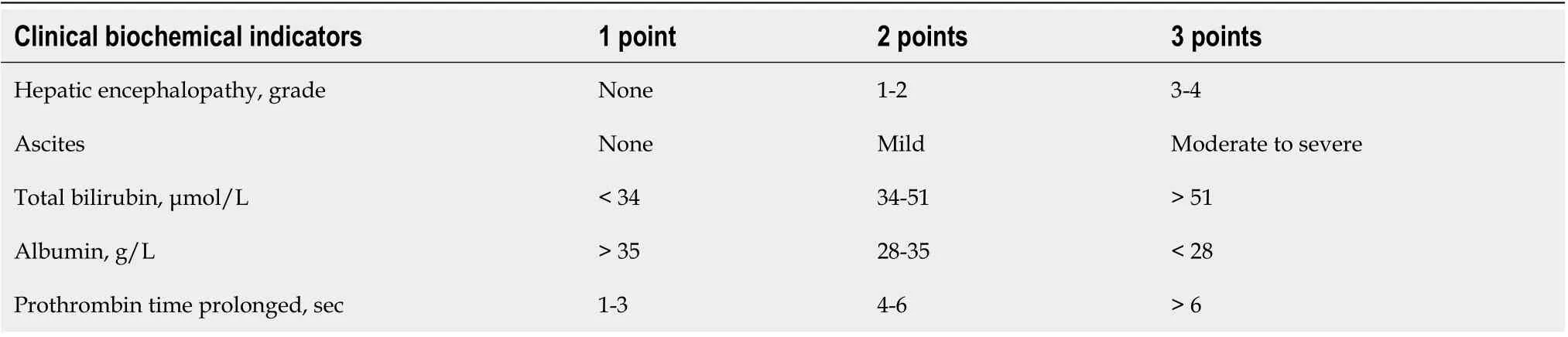
Table 1 Child-Pugh score[15]

Table 2 Model for end-stage liver disease score[16]

Table 3 Laennec F1-F4 staging system, Laennec fibrosis staging scoring system in hepatic puncture tissue[20,21]
Diagnosis of compensated cirrhosis is based on one of the following criteria: (1) Histologically cirrhosis; (2) Gastroesophageal varices or digestive tract ectopic varices on the basis of excluding noncirrhotic portal hypertension; (3) Imaging of cirrhosis or portal hypertension,e.g., splenomegaly, portal vein ≥ 1.3 cm; (4) LSM result complying with diagnostic cutoff of cirrhosis of different causes; and (5) Meeting two or more of the following criteria: (a) Platelet (PLT) < 100 × 109/L, without any other reasons; (b) Serum ALB < 35 g/L, excluding malnutrition or kidney diseases; (c) INR > 1.3 or PT prolonged (discontinuing thrombolysis or anticoagulant drugs for over 7 d); and (d) Aspartate aminotransferase (AST)/PLT ratio index (APRI): Adult APRI score > 2.
Diagnosis of decompensated cirrhosis is based on the existence of cirrhosis and any one of the complications including ascites, gastroesophageal varices hemorrhage, sepsis, hepatic encephalopathy, and hepatorenal syndrome.
Cirrhotic recompensation and/or reversion: Accumulating evidence has demonstrated that effective antiviral treatment in decompensated HBV and HCV cirrhosis patients can lead to the recompensation of the liver function and reduce liver transplantation. Even though the definition of recompensation of decompensated cirrhosis has not been established, Chinese experts agree with the following preliminary criteria: When decompensation events (ascites, digestive tract hemorrhage, hepatic encephalopathy) have not recurred for a long time period (at least 1 year) after effective treatment in patients with decompensated cirrhosis, these patients will be classified as recompensated population.
Similarly, clinical data have provided evidence of cirrhosis reversion[25-29]defined as: Ishak fibrosis staging decreases by ≥ 1 stage, or P-I-R classification after treatment decreases.
Recommendation 1:Cirrhosis can be classified into compensated stage, decompensated stage, recompensation stage and/or cirrhosis reversion (B, 1).
Recommendation 2:Diagnosis of compensated cirrhosis: (1) Histologically cirrhosis (A, 1); (2) Gastroesophageal varices or digestive tract ectopic varices on the basis of excluding non-cirrhotic portal hypertension (B, 1); (3) Imaging reveals cirrhosis or portal hypertension (B, 1); and (4) Meeting two or more of the four criteria: (a) PLT < 100 × 109/L without any other reasons; (b) ALB < 35 g/L, excluding malnutrition or kidney diseases; (c) INR > 1.3 or PT prolonged; (d) APRI > 2 (B, 1).
Recommendation 3:Diagnosis of decompensated cirrhosis: (1) Cirrhosis; and (2) Any one of the complications of portal hypertension including ascites, gastroesophageal varices hemorrhage, sepsis, hepatic encephalopathy, and hepatorenal syndrome (B, 1).
CIRRHOSIS-RELATED COMPLICATIONS
Serous effusion
Serous effusion of patients with cirrhosis includes ascites, pleural effusion, and pericardial effusion. Refer to the Chinese guidelines on the management of ascites and its related complications in cirrhosis for the diagnosis of cirrhotic ascites[6]. Chylous ascites (CA), hemorrhagic ascites and pleural effusion are discussed here.
The diagnosis of CA is based on the distinct characteristic of the ascitic fluid which includes a milky appearance and a triglyceride level > 200 mg/dL. Though CA can occur in all stages of cirrhosis, it is necessary to exclude other underlying etiologies such as trauma, congenital diseases, infections (especially pulmonary tuberculosis and filariasis), neoplasms, operations, or heart diseases.
Hemorrhagic ascites is diagnosed with a red blood cell count level > 50000/mm3in ascites. It is necessary to exclude other underlying etiologies such as tumor, severe infection (including tuberculous peritonitis), coagulopathy, and peritoneal varicose vein rupture.
Pleural effusion in patients with cirrhosis is more common in the right side, but can be bilateral when severe. It can be caused by etiologies other than cirrhosis, such as tuberculosis, which should be excluded. Diagnosis of pleural effusion can be based on chest ultrasonography or X-ray[30].
Gastrointestinal tract hemorrhage
Esophageal and/or gastric variceal rupture is the most common reason for gastrointestinal tract hemorrhage in patients with cirrhosis. See the guidelines for the diagnosis and treatment of esophageal and gastric variceal bleeding in cirrhotic portal hypertension 2016[5]for details. Other reasons include portal hypertensive gastropathy (PHG), portal hypertensive enteropathy(PHE), and internal hemorrhoid[31-34].
Spontaneous bacterial peritonitis and other infections
Refer to the relevant Chinese guidelines for the diagnosis[6]. In addition to spontaneous bacterial peritonitis, common infections in patients with cirrhosis include urinary, biliary, gastrointestinal, respiratory, skin soft tissue infections, and sepsis.
Hepatic encephalopathy
Refer to the relevant Chinese guidelines for the diagnosis[7].
Renal impairment
Renal impairment in cirrhosis patients includes acute kidney injury (AKI), hepatorenal syndrome-acute kidney injury (HRS-AKI), HRS-non-AKI (HRS-NAKI), and chronic kidney disease (CKD)[35,36].
The diagnosis of AKI is based on either of the two criteria[37]: Serum creatinine (Scr) within 48 h after admission increases ≥ 26.5 μmol/L (0.3 mg/dL) from baseline, or Scr within 7 d increases ≥ 50% from baseline (last available Scr within 3 mo can be taken as the baseline value).
The diagnostic criteria of HRS-AKI are as follows: (1) Patients with cirrhosis and ascites; (2) Patients meeting the criteria for AKI; (3) No response after discontinuing diuretics and plasma volume expansion by intravenous infusion of ALB at a dose of 1 g/kg for 48 h; (4) No shock; (5) Patients not using nephrotoxic drugs currently or recently; and (6) no signs of renal structural injury: (a) No proteinuria (< 500 mg/d); (b) No minor hematuria (< 50 red blood cells per high power field); and (c) Normal sonography of the kidneys.
HRS-NAKI[38](including HRS-AKD and HRS-CKD) is diagnosed if: (1) Patients have cirrhosis with or without ascites; (2) HRS-AKI is excluded; and (3) Estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2in the absence of structural injury or Scr increases by < 50% from baseline (last available Scr within 3 mo can be taken as the baseline value).
CKD is defined as an eGFR level of < 60 mL/min/1.73 m2for 3 mo regardless of the structural injury of the kidneys.
Cirrhotic cardiomyopathy
Cirrhotic cardiomyopathy (CCM) is cardiac dysfunction characterized by suboptimal contractile response to stress and impaired diastolic function in the absence of previous cardiac diseases[39,40]. Given that most patients are asymptomatic in the initial stages of CCM, clinical, laboratory, electrocardiographic and imaging evaluations are necessary for early diagnosis. Diagnostic criteria of CCM are as follows: (1) Systolic dysfunction characterized by no increase of cardiac output induced by physical or pharmacological stress; (2) Diastolic dysfunction[41]: E/A ratio < 1.0, deceleration time > 200 ms, and isovolumic relaxation time > 80 ms; and (3) Supporting criteria: Electrophysiology abnormality, myocardial chronotropism, QT interval prolongation, non-synchronous electromechanical systole, left atrial enlargement, myocardial hypertrophy, brain natriuretic peptide and its precursor increased, and troponin increased.
Hepatopulmonary syndrome
Diagnostic criteria of hepatopulmonary syndrome (HPS) are detailed in the International liver transplant society practice guidelines: Diagnosis and management of HPS and portopulmonary hypertension 2016[42].
Other complications of cirrhosis
Other complications of cirrhosis include portal vein thrombosis (PVT) which can be classified into acute and chronic PVT[43-45], primary liver cancer[46], hepatic osteopathy[47]and cirrhotic amyotrophy[48,49].
Recommendation 4:AKI diagnosis: Scr within 48 h after admission increases by ≥ 26.5 μmol/L (0.3 mg/dL) from baseline, or Scr within 7 d increases by ≥ 50% than last available Scr within 3 mo or from the baseline value (B, 1). CKD diagnosis: regardless of structural injury of the kidneys, eGFR < 60 mL/min/1.73 m2lasts longer than 3 mo, and patients have refractory ascites (B, 1).
Recommendation 5:HRS-AKI diagnosis: (1) cirrhosis and ascites; (2) AKI; (3) No response after discontinuing diuretics and supplementing ALB (20-40 g/d) and expanding the blood volume for 48 h; (4) No shock; (5) No use of nephrotoxic drugs currently or recently; and (6) No evidence of structural injury of the kidneys (A, 1).
Recommendation 6:Diagnosis of HRS-NAKI: (1) cirrhosis with or without ascites; (2) HRS-AKI is excluded; and (3) eGFR < 60 mL/min/1.73 m2in the absence of structural injury or Scr increases by < 50% from baseline (last available Scr within 3 mo can be taken as the baseline value) (C, 1).
Recommendation 7:The incidence of abnormal electrophysiology in patients with cirrhosis is high, and screening and monitoring of cirrhotic cardiomyopathy should be emphasized (C, 1).
Recommendation 8:PVT includes acute and chronic PVT (B, 1).
Recommendation 9:Once cirrhosis is diagnosed, it is necessary to closely screen liver cancer (B, 1) by sonography and AFP test every 3-6 mo (C, 1).
Recommendation 10:Cirrhotic osteoporosis is positively correlated with the severity of liver disease. Bone density should be monitored in patients who are newly diagnosed with PBC or cirrhosis and in those after liver transplantation. Moreover, bone density should also be monitored in patients with a history of fragility fracture, postmenopausal women and those who use glucocorticoids in the long term (> 3 mo) (B, 2).
TREATMENT OF CIRRHOSIS
When patients are diagnosed with cirrhosis, comprehensive treatments should be commenced as soon as possible. If feasible, etiological treatment should be started immediately. Anti-inflammation and anti-hepatic fibrosis therapy are options for those who present persistent inflammation and/or fibrosis but are not suitable for or do not respond to etiological treatment. Prevention and treatment of complications play an important role in extending lifespan and improving life quality of patients with cirrhosis.
Etiological treatment
Etiological treatment is key for the treatment of cirrhosis whenever feasible. Detailed recommendations can be found in relevant Chinese guidelines[50-53]and Consensus[54-56]. In terms of immunoglobulin G4-associated cholangitis, immunosuppressant, interventional therapies or surgical intervention may be indicated[57].
D-penicillamine and trientine is indicated in cirrhosis patients due to Wilson’s disease. In addition, these patients should avoid the intake of copper-rich foods. Oral zinc formulations (e.g., zinc acetate, zinc gluconate) are recommended to reduce copper absorption[58,59].
For cirrhosis patients due to hemochromatosis, it is necessary to restrict the intake of iron-rich food and prohibit the transfusion of red blood cells. Other options include therapeutic venesection and iron chelators (e.g., deferoxamine or deferasirox)[60].
Treatment of drug-induced cirrhosis is detailed in diagnosis and treatment guidelines on drug-induced liver injury[61].
Anti-inflammation and anti-hepatic fibrosis therapy
Anti-inflammation and anti-hepatic fibrosis therapy are indicated when patients present persistent inflammation and/or fibrosis but are not suitable for or do not respond to etiological treatment. The most commonly used anti-inflammation drugs include glycyrrhizic acid preparation, bicyclol, polyene phosphatidyl choline, silymarin, ademetionine and reduced glutathione[62]. Anti-fibrosis medicines include Anluohuaxian capsule, Fuzheng huayu capsule, and compound Biejiaruangan tablet[63-66].
Prevention and treatment of complications
Ascites:Refer to the Chinese guidelines on the management of ascites and its related complications in cirrhosis[6]. Combination of diuretics, ALB, and vasoconstrictor is recommended to treat refractory ascites.
For patients with chylous ascites, nutritional support with a low-salt, low-fat, medium chain triglyceride and high-protein diet and management of the underlying etiology are the cornerstones of therapy. When these measures fail, other interventions such as octreotide/somatostatin analogues, terlipressin, surgical ligation, embolization and transjugular, intrahepatic, portosystemic shunt (TIPS) can be considered[67-69].
For patients with hemorrhagic ascites, the key therapy is to control the basic causes[70,71]. When these measures fail, terlipressin and somatostatin can be used.
The treatment for cirrhotic patients with pleural effusion is similar to that for cirrhotic ascites. Treatment for chylous pleural effusion is similar to that for chylous ascites.
Gastrointestinal bleeding:Refer to Guidelines for the Diagnosis and Treatment of Esophageal and Gastric Variceal Bleeding in Cirrhotic Portal Hypertension 2016[5]. (1) Esophagogastric variceal bleeding, terlipressin, somatostatin and its analogs, and pituitrin are used to treat patients with esophagogastric variceal bleeding. When drug therapy fails, other interventions can be considered including a Sengstaken-Blakemore tube, endoscopic variceal ligation or tissue glue injection, TIPS, and surgical therapy. High-risk patients with acute hemorrhage should receive TIPS therapy as early as possible (within 72 h). Balloon-occluded retrograde transvenous obliteration is preferred in patients with gastric variceal bleeding[72]; and (2) PHG and PHE bleeding: non-selective beta blocker (NSBB) is preferred to treat patients with bleeding due to PHG and PHE, and iron supplement is recommended[73,74]. Terlipressin, somatostatin, and its analogs can be considered[75-77].
Infections:Refer to the Chinese guidelines on the management of ascites and its related complications in cirrhosis[6]and Expert Consensus on Diagnosis and Treatment of End-stage Liver Diseases Complicated With Infections[78].
Norepinephrine is a first-line vasoactive drug for the treatment of infective shock. Bothe the dose of catecholamines and the risk of cardiac arrhythmia can be reduced when vasopressin (maximum dose 0.03 U/min) is added to norepinephrine[79]. With a longer half-life and similar effect, terlipressin is more effective in raising blood pressure and is more long-acting.
For patients with sepsis and severe infection, high-dose ALB can be combined with antibacterial agents.
Hepatic encephalopathy:Refer to the Guidelines on the management of hepatic encephalopathy in cirrhosis 2018[7].
Renal impairment:Refer to the Chinese guidelines on the management of ascites and its related complications in cirrhosis[6]and the Guidelines for Diagnosis and Treatment of Liver Failure (2018)[80].
Terlipressin combined with ALB is superior to placebo, ALB alone, octreotide or triple therapy with midodrine, octreotide and ALB in reversing HRS-AKI and HRSNAKI and improving renal function[81-87]. Terlipressin at a dose of 1 mg per 4-6 h can be administered in combination with ALB (20-40 g per d) for 3 d. With a < 25% decrease of Scr level, the dose of terlipressin can gradually increase to 2 mg per 4 h. If effective (Scr decreases to < 133 μmol/L, along with the increase of arterial pressure, urine output and serum sodium level), the treatment duration is 7-14 d. Otherwise, terlipressin is discontinued. Norepinephrine (0.5-3.0 mg/h) in combination with ALB (10-20 g/L) can also be used.
TIPS can improve the renal function of patients with HRS-AKI and HRS-NAKI[88]. However, there are usually contraindications for TIPS in patients with HRS-AKI. Blood purification therapy can improve the renal function of some HRS-AKI patients. Liver transplantation is the preferred treatment for HRS-AKI and HRS-NAKI.
Cirrhotic cardiomyopathy:Current pharmacological treatment is not specific. Liver transplantation is the only proven treatment with specific effect on CCM[40,89].
HPS: Other than long-term supplemental oxygen, there are no effective therapies for HPS currently. The only definitive therapy is liver transplantation. Thus patients with HPS are recommended for liver transplant evaluation[90,91].
Portal vein thrombosis:Anticoagulant therapy or thrombolytic therapy can be adopted for patients with cirrhotic acute PVT. Low-molecular-weight heparin is preferred. Warfarin can be considered. Treatment duration ranges from 3 to 6 mo. Other interventions include TIPS, thrombolysis, and surgery. Individualized treatment is required for the treatment of chronic PVT[92-94].
Hepatic osteopathy:Diphosphonate can be used in patients with osteoporosis on the basis of calcium preparation and vitamin D. Oral alendronate sodium may lead to variceal bleeding. Zoledronic acid, a new intravenous bisphosphonate is effective in reducing fracture risk and does not have risk of variceal bleeding[95-98].
Nutritional support:Refer to the Clinical guidelines on nutrition in end-stage liver disease 2019,etc[7,23].
Nursing of digestive tract hemorrhage.
Recommendation 11:Etiological treatment is the key. If etiological treatment is not available or hepatic fibrosis persists or deteriorates after etiological treatment, antifibrosis therapy, such as Anluohuaxian capsule, Fuzhenghuayu capsule, and compound Biejiaruangan tablet can be used (B, 1).
Recommendation 12:In terms of refractory ascites, triple therapy including diuretics, ALB, and vasoconstrictors is recommended (B, 1).
Recommendation 13:In terms of cirrhotic chylous ascites or chylous pleural effusion, a low-salt, low-fat, medium chain triglyceride high-protein diet is recommended (B, 1). Terlipressin and somatostatin may be used (B, 2). A portal-systemic shunt procedure can be performed. If indicated, surgical intervention can be performed (C, 1).
Recommendation 14:For cirrhosis patients with hemorrhagic ascites, the primary treatment is to control the underlying causes. Terlipressin and somatostatin can be used (B, 2).
Recommendation 15:In the case of cirrhotic upper gastrointestinal hemorrhage, terlipressin, somatostatin analogs, proton pump inhibitor or H2 receptor blocker can be used (A, 1).
Recommendation 16:If drugs fail in treating cirrhotic esophageal and gastric variceal bleeding, the Sengstaken-Blakemore tube, endoscopic variceal ligation or tissue glue injection (B, 1), interventional therapies (C, 1) and surgery (C, 2) can be performed.
Recommendation 17:At 5 to 7 d after stopping cirrhotic gastrointestinal bleeding, secondary prevention should be performed with NSBB (A, 1) or carvedilol (B, 1). In patients with gastrointestinal bleeding and ascites, carvedilol is not recommended and the dose of NSBB should be reduced (B, 2).
Recommendation 18:In patients with PHG bleeding, NSBB and iron preparations are recommended (B; 1). In the case of acute hemorrhage, terlipressin or somatostatin analogs can be used (B; 2).
Recommendation 19:In cirrhosis patients with infections, empirical anti-infective therapy should be started as soon as possible. Based on the etiological results, switch to target therapy as soon as possible (B, 1).
Recommendation 20:In the case of sepsis, severe infection or shock, it is recommended to adopt triple therapy including antibacterial agents, ALB, and vasoactive drugs (B, 1).
Recommendation 21:HRS can be treated with terlipressin (1 mg/4-6 h) in combination with ALB (20-40 g/d) for 7 - 14 d, and the therapy can be repeated if HRS recurs (B, 1).
Recommendation 22:For HRS-NAKI patients with a large amount of ascites who do not respond to vasoconstrictors, TIPS can be performed (B, 1). TIPS is not recommended for HRS-AKI patients (C, 1).
Recommendation 23:For HRS-AKI patients who do not respond to vasoconstrictors, renal replacement therapy or artificial liver support can be selected. It is not recommended to perform renal replacement therapy in HRS-NAKI patients. HRS-AKI and HRS-NAKI patients should be preferentially included into the liver transplantation plan (B, 1).
Recommendation 24:There is no effective drug for cirrhotic cardiomyopathy. Patients should be included into the liver transplantation plan (B, 1).
Recommendation 25:There is no effective drug for HPS. Long-term supplemental oxygen is recommended (C, 1). Patients should be included into the liver transplantation plan (B, 1).
Recommendation 26:Anticoagulant therapy or thrombolytic therapy can be adopted for patients with acute PVT and progressive PVT(C, 1). Low-molecular-weight heparin alone or in combination with warfarin may be used (A, 1).
Recommendation 27:In terms of hepatic osteopathy or osteoporosis, bisphosphonates can be used on the basis of calcium preparation and vitamin D (C, 2). Nutritional support is important (B, 1).
PROBLEMS TO BE SOLVED
Test technology
(1) Smart reader of hepatic pathology; (2) Non-invasive monitoring of HVPG; (3) New tools of liver stiffness measurement regardless of ascites, jaundice or inflammation; and (4) Specific and sensitive test of MHE.
Diagnostic method and criteria
(1) Clarification of diagnostic criteria for recompensation and cirrhosis reversion; and (2) Early identification and diagnosis of HRS.
Therapeutic and preventive measures
(1) Evaluation of traditional Chinese medicine in anti-hepatic fibrosis and anticirrhosis; (2) Evaluation of diuretics, ALB, and vasoactive drugs in refractory ascites; (3) Evaluation of antibacterial agents, ALB, and vasoactive drugs in sepsis and severe infections; and (4) Primary and secondary prevention of cirrhotic upper gastrointestinal hemorrhage.
CONCLUSION
Liver cirrhosis is a substantial health burden worldwide. To promote diagnosis and treatment of cirrhosis, the CSH and the CSG of CMA developed the Chinese Guidelines for the diagnosis and treatment of cirrhosis complications including esophageal and gastric variceal bleeding, ascites, and hepatic encephalopathy recently[5-7]. However, there is a great need for updates with the development in these areas. In addition, some important topics, especially those on rare complications, have not been covered in those guidelines. Therefore, the present guidelines adds new sections and provides updates, focusing on the early identification of the causes, evaluation of disease severity, comprehensive assessment of complications, etiological treatment, and complication management. Lastly, problems to be solved are addressed.
ACKNOWLEDGEMENTS
We thank all members of the Chinese Society of Hepatology, Chinese Medical Association. The Expert Group members (in order by the Pinyin Romanization of the individual’s last name) include: Ji-Hong An, Guo-Feng Chen, Hong-Song Chen, Jing-Long Chen, Yu Chen, Jun Cheng, Guo-Hong Deng, Hui-Guo Ding, Lei Dong, Xiao-Guang Dou, Zhong-Ping Duan, Hui Gao, Yan-Hang Gao, Tao Han, Ying Han, Ying Han, Jin-Hua Hu, Yuan Huang, Ji-Dong Jia, Jian-Ning Jiang, Ying-An Jiang, Hong-Bin Kong, Yuan-Yuan Kong, Cang-You Li, Jie Li, Jun Li, Qing-Hong Li, Rong-Kuan Li, Shu-Chen Li, Tai-Sheng Li, Wen-Gang Li, Wu Li, Yu-Fang Li, Shu-Mei Lin, En-Qiang Ling-Hu, Bin-Bin Liu, Jing-Fu Liu, Xiao-Qing Liu, Ying-Di Liu, Yu-Lan Liu, Hai-Ying Lu, Lun-Gen Lu, Xin-Hua Luo, Qing-Hua Lu, Xiong Ma, Yue-Min Nan, Yu-Qiang Nie, Jun-Qi Niu, Hui-Ying Rao, Hong Ren, Wan-Hua Ren, Jia Shang, Li Shi, Lei Wang, Xian-Bo Wang, Yu-Ming Wang, Lai Wei, Xiao-Ping Wu, Chao Wu, Jing Wu, Wen Xie, Shao-Jie Xin, Hui-Chun Xing, Jie Xu, Jing-Hang Xu, Xiao-Yuan Xu, You-Qing Xu, Ming Yan, Bao-Shan Yang, Dong-Liang Yang, Ji-Ming Yang, Jin-Hui Yang, Li Yang, Yong-Feng Yang, Yong-Ping Yang, Chang-Qing Yang, Hong You, Yan-Yan Yu, Zheng Zeng, Suo-Di Zhai, Chun-Qing Zhang, Da-Zhi Zhang, Li-Ting Zhang, Liao-Yun Zhang, Ling-Yi Zhang, Lun-Li Zhang, Xin-Xin Zhang, Jing-Min Zhao, Ping Zhao, Shou-Song Zhao, Huan-Wei Zheng, Jun-Ying Zhou, Yong-Jian Zhou, Hui Zhuang, Wei-Ze Zuo. Academic secretaries are: Qian Kang, Jia-Li Pan.
杂志排行
World Journal of Gastroenterology的其它文章
- Altered metabolism of bile acids correlates with clinical parameters and the gut microbiota in patients with diarrhea-predominant irritable bowel syndrome
- Alteration of fecal tryptophan metabolism correlates with shifted microbiota and may be involved in pathogenesis of colorectal cancer
- Relationship between the incidence of non-hepatic hyperammonemia and the prognosis of patients in the intensive care unit
- Endoscopic mucosal ablation - an alternative treatment for colonic polyps: Three case reports
- Tuberous sclerosis patient with neuroendocrine carcinoma of the esophagogastric junction: A case report
- Role of pancreatography in the endoscopic management of encapsulated pancreatic collections – review and new proposed classification
