Propofol vs midazolam sedation for elective endoscopy in patients with cirrhosis:A systematic review and meta-analysis of randomized controlled trials
2021-01-13JohnAlexanderLataGuachoDiogoTurianiHourneauxdeMouraIgorBragaRibeiroAlbertoMachadodaPonteNetoShailendraSinghMarinaGammaroBaldaviraTucciWanderleyMarquesBernardoEduardoGuimaresHourneauxdeMoura
John Alexander Lata Guacho,Diogo Turiani Hourneaux de Moura,Igor Braga Ribeiro,Alberto Machado da Ponte Neto,Shailendra Singh,Marina Gammaro Baldavira Tucci,Wanderley Marques Bernardo,Eduardo Guimarães Hourneaux de Moura
John Alexander Lata Guacho,Diogo Turiani Hourneaux de Moura,Igor Braga Ribeiro,Alberto Machado da Ponte Neto,Marina Gammaro Baldavira Tucci,Wanderley Marques Bernardo,Eduardo Guimarães Hourneaux de Moura,Gastrointestinal Endoscopy Unit,Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo,São Paulo 05403-010,Brazil
Shailendra Singh,Division of Gastroenterology,Department of Internal Medicine,West Virginia University,Charleston,WV 25304,United States
Abstract BACKGROUND Patients with cirrhosis frequently require sedation for elective endoscopic procedures.Several sedation protocols are available,but choosing an appropriate sedative in patients with cirrhosis is challenging.AIM To conduct a systematic review and meta-analysis to compare propofol and midazolam for sedation in patients with cirrhosis during elective endoscopic procedures in an attempt to understand the best approach.METHODS This systematic review and meta-analysis was conducted using the PRISMA guidelines.Electronic searches were performed using MEDLINE,EMBASE,Central Cochrane,LILACS databases.Only randomized control trials (RCTs) were included.The outcomes studied were procedure time,recovery time,discharge time,and adverse events (bradycardia,hypotension,and hypoxemia).The risk of bias assessment was performed using the Revised Cochrane Risk-of-Bias tool for randomized trials (RoB-2).Quality of evidence was evaluated by GRADEpro.The meta-analysis was performed using Review Manager.RESULTS The search yielded 3,576 records.Out of these,8 RCTs with a total of 596 patients(302 in the propofol group and 294 in the midazolam group) were included for the final analysis.Procedure time was similar between midazolam and propofol groups (MD:0.25,95%CI:-0.64 to 1.13,P = 0.59).Recovery time (MD:-8.19,95%CI:-10.59 to -5.79,P <0.00001).and discharge time were significantly less in the propofol group (MD:-12.98,95%CI:-18.46 to -7.50,P <0.00001).Adverse events were similar in both groups (RD:0.02,95%CI:0-0.04,P = 0.58).Moreover,no significant difference was found for bradycardia (RD:0.03,95%CI:-0.01 to 0.07,P = 0.16),hypotension (RD:0.03,95%CI:-0.01 to 0.07,P = 0.17),and hypoxemia(RD:0.00,95%CI:-0.04 to 0.04,P = 0.93).Five studies had low risk of bias,two demonstrated some concerns,and one presented high risk.The quality of the evidence was very low for procedure time,recovery time,and adverse events;while low for discharge time.CONCLUSION This systematic review and meta-analysis based on RCTs show that propofol has shorter recovery and patient discharge time as compared to midazolam with a similar rate of adverse events.These results suggest that propofol should be the preferred agent for sedation in patients with cirrhosis.
Key words:Sedation;Midazolam;Propofol;Cirrhosis;Endoscopic;Endoscopy;Metaanalysis
INTRODUCTION
Cirrhosis is an advanced form of fibrosis that affects the liver with the destruction of the organ's lobular and vascular architecture[1].The progression of liver disease causes portal hypertension,which can lead to complications such as esophagogastric varices,portal hypertensive gastropathy,and gastric antral vascular ectasia (GAVE)[2-11].These patients often undergo diagnostic or therapeutic upper gastrointestinal endoscopy,and choosing an appropriate sedative is challenging.Sedation in this group of patients with underlying liver disease and their complications presents increased risks even when performed by well-trained personnel,mainly due to drug metabolism and interactions,baseline hemodynamics,and increased risk of adverse events.The recommended sedation level for elective endoscopies in patients with cirrhosis is mild to moderate that can be administered by anesthesiologists,endoscopists,or registered nurses[12].
The most commonly used sedatives are usually benzodiazepine midazolam and short duration hypnotic agent propofol,while synthetic opioids can be added for their analgesic effect in some cases.Midazolam is the preferred benzodiazepine because of its short induction,recovery time,and amnesic properties[13].However,the half-life of midazolam can be prolonged in patients with cirrhosis,and midazolam can trigger encephalopathy in these patients.Propofol does not need dose adjustment in patients with cirrhosis and has a faster onset of action,shorter effect,and faster recovery times[13].Many studies have compared propofol with midazolam for sedation in cirrhosis showing variable results.Therefore,we aimed to perform a systematic review and meta-analysis of randomized controlled trials (RCTs) to compare sedation with propofol and midazolam in patients with cirrhosis undergoing elective endoscopy.
MATERIALS AND METHODS
Protocol and registration
This systematic review was carried out in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA).The study was registered by The International Prospective Registry of Continuous Systematic Reviews of the National Institutes of Health Research (PROSPERO),under the code CRD42019137659 and was approved by the Scientific Ethics Committee of the Department of Gastroenterology of the Faculty of Medicine of the University of São Paulo.
Information sources and search
The search was carried out using MEDLINE (Pubmed);EMBASE;Cochrane Central Register of Randomized Controlled Clinical Trials/CENTRAL;and Latin-American and Caribbean Health Sciences Literature LILACS electronic databases from their date of inception to November 2019 with no language restriction.A gray literature search was also performed.The terms used for database search were "Sedation OR Sedations OR anesthesia OR Propofol OR Midazolam OR benzodiazepine" AND "Endoscopy OR endoscopic OR panendoscopy" AND "Cirrhosis OR liver OR hepatic."
Study selection,eligibility criteria,and data items
RCTs comparing propofol and midazolam for sedation during elective gastrointestinal endoscopy in patients with cirrhosis more than 18 years of age were included.Studies were excluded if they included patients without cirrhosis,patients with upper gastrointestinal bleeding,decompensated liver disease,neurological or psychiatric diseases;patients who used illicit drugs that could alter their central nervous system;patients that used drugs such as benzodiazepines,anti-depressants,antiepileptics,and patients with ASA class IV-V.Case series and studies that did not provide enough data for outcome analysis or full text were also excluded.The outcomes of our study were procedure time,recovery time,discharge time,and adverse events (bradycardia,hypotension,and hypoxemia).
Study selection and data collection process
All data were extracted from article texts,tables,and figures with any estimates made based on the presented data and figures.Two investigators independently reviewed each included article,and its eligibility was determined based on predetermined inclusion and exclusion criteria.Any discrepancy resolved by discussion and reevaluation by senior authors.The following data were collected:Study model,the total number of included patients,gender,age,etiology of cirrhosis,Child-Turcotte-Pugh score,and adverse events related to the sedation.
Risk of bias in individual studies
The risk of bias in the studies was assessed using the Revised Cochrane Risk-of-Bias tool for randomized trials (RoB-2).We performed a complete analysis using RoB-2 for each of the outcomes in each selected study.In order to simplify the analysis,we assessed the overall risk of bias for each study using the same domains suggested in RoB-2.
Risk of bias across studies
We evaluated the randomized trials using the criteria from Bias Risk Assessment by the Cochrane Collaboration's tool - ROB2 - Risk of Bias[14].The tool analyzes the risk of bias by classifying it in five different domains:Randomization process,deviations in the intention of the intervention,loss of data on outcomes,methods of measuring outcomes,and selection of reported results.The risk of bias for each specific domain is categorized as "low risk,” "some concerns," or "high risk" for each of the outcomes,according to the criteria described in detail in the Cochrane Handbook[14].
Summary measures,synthesis of results and data analysis
GRADE (quality of evidence):The quality of evidence was assessed with the objective criteria of GRADE (Grading of Recommendations Assessment,Development,and Evaluation) for each of the pre-specified results and outcomes using the software GRADEpro - Guideline Development Tool (Mc Master University,2015;Evidence Prime,Inc.,Ontario,Canada).GRADE is a tool used to assess the quality of evidencebased on criteria that involve assessing the risk of bias,inconsistency,indirect evidence,imprecision,and publication bias.The evaluation of the risk of bias and the quality of the studies was carried out under the supervision of our statistical analysis team.
Statistical analysis:The metanalysis was performed using RevMan 5 (Review Manager version 5.3.5 - Cochrane Collaboration,Oxford,United Kingdom).The risk of difference (RD) with a 95% confidence interval (CI) for dichotomous variables was calculated by using the Mantel-Haenszel Cochran method with the fixed-effects model.For continuous variables,we calculated the mean difference (MD) with 95%CI using random effect with inverse variance.The semi-quantitative values were reported as weighted mean with standard deviation determined by the number of patients in each study.All estimates were made based on an intention-to-treat analysis.Heterogeneity values were estimated according to Chi-square (χ²) and Higgins method(I²).Heterogeneity values greater than 50% were considered high.We used the fixedeffects model if the heterogeneity was <50%.Absolute numbers,means,and standard deviations were used for data analysis.If the means and standard deviations were not reported,they were estimated using mathematical formulas (SP Hozo,B.Djulbegovic,I.Hozo).APvalue of less than 0.05 was considered statistically significant.
RESULTS
Study selection
The initial search identified a total of 3576 citations.After eliminating duplicates,1601 citations were selected for title and abstract review.Out of these,54 studies were selected for full-text review.Eleven articles were then selected to examine for eligibility,out of which 3 were excluded because they were not RCTs.Finally,8 studies[15-22]were included in our meta-analysis (Figure 1).
Study characteristics
Eight RCTs with a total of 596 patients were included;302 in the propofol group and 294 patients in the midazolam group.Individual study characteristics are summarized in Table 1.Four studies[15-18]used sedation with only propofol or midazolam.The other four studies[19-22]also used additional medications for sedation,such as opioid analgesic Pentazocine (Watanabeet al[19]),fentanyl (Ahmedet al[20]and Correiaet al[21]) and Meperidine in the midazolam group (Westonet al[22]).
Risk of bias:Studies by Yooet al[18],Watamabeet al[19],Ahmedet al[20],Agrawalet al[15],and Correiaet al[21]were considered low risk when globally assessed per outcome,while the studies by Khamaysiet al[16]and Westonet al[22]had some concerns,and the study by Riphauset al[17]had a high risk of bias (Figure 2).
GRADEpro:The estimated outcomes of procedure time,recovery time,and adverse events showed very low quality of evidence,and discharge time showed low quality of evidence (Figure 3).
Results of individual studies and synthesis of results
Procedure time:Seven studies[15-17,19-22]with a total of 556 patients (282 propofol group and 274 midazolam group) reported procedure time.No statistical difference was found between the propofol and midazolam groups (MD:0.25,95%CI:-0.64 to 1.13,P= 0.59) (Figure 4).
Recovery time:Six studies[15-18,20,22]with a total of 363 patients (191 in the propofol group and 172 in the midazolam group) reported recovery time after sedation.The recovery time was significantly higher in the midazolam group (MD:-8.19,95%CI:-10.59 to -5.79,P<0.00001) (Figure 5).
Discharge time:Three studies[16,20,22]with a total of 181 patients (91 in the propofol group and 90 in the midazolam group) reported discharge time after sedation.The discharge was significantly lower in the propofol group compared to midazolam (MD:-12.98,95%CI:-18.46 to -7.50,P<0.00001) (Figure 6).
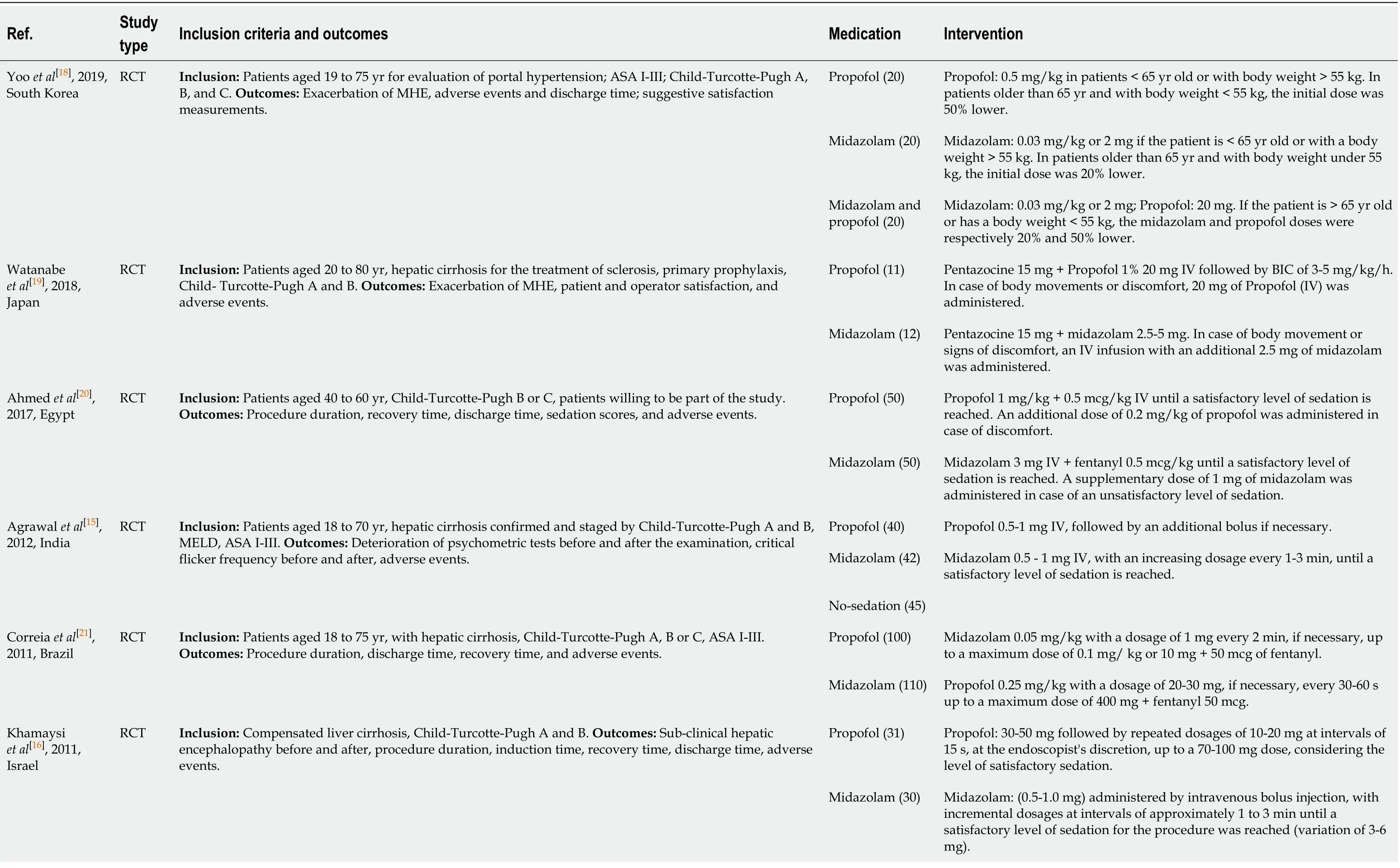
Table 1 Characteristics of included studies

MHE:Minimal hepatic encephalopathy;MELD:Model for End-Stage Liver Disease.
Adverse events:All included studies[15-22]reported the incidence of adverse events related to sedation during upper gastrointestinal endoscopy (bradycardia,hypotension,and hypoxemia).
Adverse events were similar in both groups (RD:0.02,95%CI:0-0.04,P= 0.58).Also,no significant difference was found when comparing each adverse event individually(Figure 7).
Bradycardia:Eight studies[15-22]with a total of 596 patients (302 in the propofol group and 294 in the midazolam group) reported the incidence of bradycardia related to sedation during upper gastrointestinal endoscopy.Increase incidence of bradycardia was seen in patients receiving midazolam for sedation;however,the difference was not statistically significant (RD:0.03,95%CI:-0.01 to 0.07,P= 0.16).
Hypotension:All studies[15-22]reported the incidence of hypotension related to sedation during upper gastrointestinal endoscopy.An increase in the incidence of hypotension was seen with the use of midazolam;however,it was not statistically significant (RD:0.03,95%CI:-0.01 to 0.07,P= 0.17).
Hypoxemia:All eight studies[15-22]reported the incidence of hypoxemia related to sedation.No statistically significant difference was found between groups (RD:0.00,95%CI:-0.04 to 0.04,P= 0.93).

Figure 1 Flow chart of study selection.
DISCUSSION
Historically endoscopy was performed without sedation,which can be painful and uncomfortable for patients[23].Over time,the application of topical anesthesia was introduced,and some countries still only use topical anesthesia because of low cost,patient preference,or institutional availability[24-26].Administration of analgesics and intravenous sedation during endoscopy was a significant breakthrough worldwide,for both physicians and patients alike because of several advantages such as patient comfort,reduced discharge time,and early recovery after the procedure[27-29].These can be used either alone or in combination for a synergetic effect to comfortably perform the procedure while maintaining an adequate level of sedation.Sedation in endoscopy is safe when we correctly select,individualize,and optimize the medicine dosage for each type of patient.One of the primary considerations is patient comorbidities,including hepatic dysfunction– which can lead to difficulty in clearance,recirculation,and increased half-life of drugs[30-36].Sedation during endoscopy in patients with hepatic dysfunction was highlighted in 1975 when benzodiazepine use was compared in patients with and without liver abnormalities.Patients with cirrhosis can have alterations in the metabolism of benzodiazepines,which can result in impaired psychomotor function and increased recovery time;therefore,it was suggested to use benzodiazepines with caution[31,37-39].Whereas,short-duration hypnotic agent propofol does not need dose adjustment in patients with cirrhosis and has a faster onset of action,shorter effect,and quick recovery time[13].
We studied the optimal approach for sedation during an elective upper gastrointestinal endoscopy in patients with cirrhosis.In our analysis,we included 8 RCTs[15-22]all with adequate designs,with a total of 596 patients.Our analysis showed that propofol had a faster recovery and discharge time.However,procedure time and adverse events were similar between propofol and midazolam group.Our results are consistent with the previous metanalysis by Tsaiet al[40];however,we included three more recent RCTs as well.Despite our study population only composed of patients with liver cirrhosis,our results are similar to a recent meta-analysis by Delgadoet al[41]showing that propofol to be a better approach during upper GI endoscopy for all patients.The use of propofol for endoscopy in patients with cirrhosis has been increasing;however,one of the limitations for widespread use is that propofol is restricted mostly to anesthesiologists in some countries[42-47].

Figure 2 Overall risk of bias.
Seven RCTs were included[15-17,19-22]in our analysis for procedure time,and we found no statistical difference between midazolam and propofol.Five diagnostic and therapeutic procedures studies[15-17,20,22]showed shorter procedure tie for the midazolam group;however,2 RCTs[19,21],including therapeutic procedures,showed shorter procedure time for the propofol group.The study by Agrawalet al[15]also included patients without any sedation and showed a shorter procedure time in the sedation group,likely due to a reduction in the discomfort that patients felt during endoscopy without sedation.All 6 RCTs[15-18,20,22]evaluating recovery time demonstrated a faster recovery time when using propofol compared to midazolam.Therefore,a statistically significant difference in recovery time was found in the metanalysis favoring the propofol group,although the methods to assess recovery varied slightly in studies.Three RCTs[15,16,22]used blood pressure and heart rate parameters within 20% of the baseline,oxygen saturation greater than 90% in ambient air,ability to tolerate oral fluids,and bedside support capacity without help or regaining basal function.While Yooet al[18]and Ahmedet al[20].evaluated patients for recovery using blood pressure,pulse oxymetry,and heart rate parameters.Different from other studies,Riphauset al[17]used the post-anesthesia recovery score (PARS) which consists of five parameters (1) activity (inability to move the limbs,ability to move two or four limbs with or without command);(2) respiration (evidence of apnea,labored breathing,or normal breathing pattern);(3) circulation (blood pressure compared with baseline:±50% to baseline,± 20% to 50% compared to baseline,± 20% to baseline);(4)consciousness (non-arousable,arousable,or fully awake);and (5) skin color (cyanotic,pink,or normal).0,1 or 2 points are given for each parameter,and complete recovery is indicated by the maximum PARS of 10 points.Our metanalysis,including 3 RCTs[16,20,22],showed that propofol was associated with a faster discharge time than midazolam.Khamaysiet al[16]and Westonet al[22]showed results favoring propofol.While Ahmedet al[20]showed no difference in discharge time between propofol and midazolam.
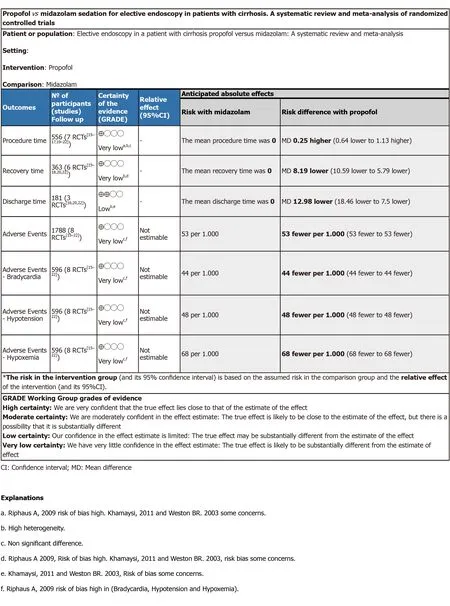
Figure 3 GRADEpro.
Many adverse events in endoscopy are related to sedation.Our study found no statistical difference when comparing adverse events related to the use of propofol and midazolam.Our results were similar to a retrospective study[30]of 1667 patients with cirrhosis,which showed no difference in adverse events between midazolam plus fentanylvspropofol sedation for endoscopy.Another recent multicenter crosssectional study[48]that included 9007 endoscopic procedures in patients with cirrhosis reported that adverse events were infrequent and cardiovascular adverse events were related to unfit patients and those requiring general anesthesia.Cardiopulmonary adverse events in our study were mainly seen in 3 RCTs[17,20,21],which included endoscopic therapeutic procedures (varices treatment) likely because of the prolonged procedure time and the need for higher sedation dose for patient comfort.Given the significance of cardiopulmonary adverse events with sedation,we further evaluated adverse events like bradycardia,hypotension,and hypoxemia individually.

Figure 4 Forest plot comparing procedure time between propofol and midazolam group for sedation during elective upper gastrointestinal endoscopy in patients with cirrhosis.

Figure 5 Forest plot comparing recovery time between propofol and midazolam group for sedation during elective upper gastrointestinal endoscopy in patients with cirrhosis.

Figure 6 Forest plot comparing discharge time between propofol and midazolam group for sedation during elective upper gastrointestinal endoscopy in patients with cirrhosis.

Figure 7 Forest plot comparing adverse events between propofol and midazolam group for sedation during elective upper gastrointestinal endoscopy in patients with cirrhosis.
In our metanalysis,there was no difference in the incidence of bradycardia between propofol and midazolam.Bradycardia was described as a heart rate (HR) <50 in most studies[16,17,20,22],HR <45 by Watanabeet al[19],25% decrease in initial HR or HR <55 bpm by Correiaet al[21]and a 20% decrease in initial HR by Agrawalet al[15]Patients in only one study (Ahmedet al[20]) were administered atropine 0.3 mg IV to control bradycardia.Hypotension with propofol is well recognized due to a reduction in systemic vascular reduction and depression of myocardial contractility.In our analysis,Agrawalet al[15]and Westonet al[22]notably used high doses of propofol,which could potentially result in the development of hypotension.However,in our analysis,we did not find any difference in the incidence of hypotension between propofol and midazolam.The included studies used various parameters to define hypotension.Agrawalet al[15]defined a blood pressure <20% of the baseline,while Correiaet al[21]considered a 20% decrease in MAP or a systolic blood pressure <90 mmHg or a diastolic blood pressure <50 mmHg as hypotension.Khamaysiet al[16],Riphauset al[17],and Westonet al[22]considered a systolic blood pressure <90 mmHg as hypotension.Ahmedet al[20]considered a decrease in mean arterial pressure (MAP) of 20 mmHg from baseline as hypotension and administrated ephedrine 10 mg and Ringer's lactate 5 mL/kg when it occurred.Watanabeet al[19]considered a systolic blood pressure <80 mmHg as hypotension.Unlike other studies,Yooet al[18]did not report any hypotension in both groups.Similarly,there was no difference in the incidence of hypoxemia seen in the propofol and midazolam group,although the definition of hypoxemia varied in studies.In most included studies[15-17,19-21],hypoxemia was defined as oxygen saturation of less than 90%.Westonet al[22]considered an oxygen saturation <85% as hypoxemia and also measured hypoventilation if the respiratory rate was <8 breaths per minute or by using a capnograph.Yooet al[18]did not specify the values for hypoxemia,or if the patients were receiving oxygen.Seven studies[15-17,19-22]reported the use of oxygen through the nasal cannula at a rate of 2 to 5 L/min with an increase if necessary.
Hepatic encephalopathy is a multifaceted disorder in patients with cirrhosis and more evident in patients with high Child-Turcotte-Pugh and MELD scores.Benzodiazepines can particularly exacerbate hepatic encephalopathy after endoscopy in some patients[49,50],while the risk of encephalopathy reported with propofol is relatively low.Studies by Khamaysiet al[16],Riphauset al[17]and Agrawalet al[15]included in our analysis reported that the risk of exacerbating minimal hepatic encephalopathy was less in the propofol group compared to midazolam.However,studies by Watanabeet al[19]and Yooet al[18]did not present a statistically significant difference in minimal hepatic encephalopathy,with the latter using a software("Stroop") for testing.In our meta-analysis,we could not quantitatively estimate the incidence of hepatic encephalopathy after sedation with propofol or midazolam since it was not uniformly reported.Five RCTs[15-19]that evaluated change in cognition used different tests to assess minimal hepatic encephalopathy prior to and after endoscopy without time standardization.Some of the tests described in the literature[16,17,19]to assess hepatic encephalopathy are Number Connection Tests (NCT),test and combination of psychometric[15],Portosystemic Encephalopathy (PSE)[17]Psychometric tests and Critical Flicker Frequency (CFF)[15],Cognitive Function Score (CFS)[16],Digital Symbol Tests (DST)[15],Line Tracing Tests (LTT)[15],Serial Dotting Tests (SDT)[15],and a test using the app "Stroop"[18](limitation in patients of advanced age,low education level,and high MELD).
Despite our rigorous meta-analysis,including only RCTs,our study has several limitations.The quality of our systematic review and meta-analysis is inherently limited by the quality of the included studies.A high degree of statistical heterogeneity was found in some of our estimates.The included studies had patients with different Child-Turcotte-Pugh scores (A-B,B-C,and A-B-C).The doses of sedation used in studies were not consistent.Higher sedation doses of propofol and midazolam were used in the studies by Watanabeet al[19],Ahmedet al[20],Agrawalet al[15],Correiaet al[21],Khamaysiet al[16],Riphauset al[17],and Westonet al[22]as compared to the doses used in the study by Yooet al[18].This variance in doses was likely related to differences in BMI,height,and ethnicity of the patients included in these studies[18].Additionally,some studies also used synthetic analgesics.We could not quantitatively estimate the minimal hepatic encephalopathy after sedation since the tests used in the included studies to assess hepatic encephalopathy were not uniform.
In conclusion,propofol has faster recovery time and a shorter patient discharge time compared with midazolam,with similar adverse events.Therefore,propofol should be the preferred agent for sedation in patients with cirrhosis undergoing upper gastrointestinal endoscopy.
ARTICLE HIGHLIGHTS
Research background
Administration of analgesics and intravenous sedation during endoscopy in patients with cirrhosis has several advantages such as patient comfort,reduced discharge time,and early recovery after the procedure.However,proper selection of sedative medications is essential because of the risk of complications mainly due to underlying hepatic dysfunction– which can lead to difficulty in clearance,recirculation,and increased half-life of drugs.
Research motivation
Many diagnostic or therapeutic upper gastrointestinal endoscopy procedures are often performed in cirrhosis,but choosing effective and safe sedative medications can be a real challenge.Therefore,we wanted to compare commonly used sedation protocols in an attempt to understand the best approach.
Research objectives
To perform a systematic review and meta-analysis of Randomized Controlled Trials comparing sedation with propofol and midazolam in patients with cirrhosis undergoing elective endoscopy.
Research methods
We performed a systematic review and meta-analysis using the PRISMA guidelines.Electronic searches were performed using MEDLINE,EMBASE,Central Cochrane,LILACS databases.Only randomized control trials (RCTs) were included.The outcomes studied were procedure time,recovery time,discharge time,and adverse events (bradycardia,hypotension,and hypoxemia).
Research results
Eight randomized clinical trials were included in the final analysis with a total of 596 patients,of whom 302 belonged to the propofol group and 294 to the midazolam group.Procedure time was similar between midazolam and propofol groups;however,the recovery time and discharge time were significantly less in the propofol group.Adverse events were similar in both groups,and no significant difference was found in rates of bradycardia,hypotension,and hypoxemia.
Research conclusions
Our study showed that propofol has shorter recovery and patient discharge time as compared to midazolam with a similar rate of adverse events.These results suggest that propofol should be the preferred agent for sedation in patients with cirrhosis.
Research perspectives
Sedation medications used during endoscopy can differ in outcomes in patients with cirrhosis.Randomized control trials comparing outcomes and adverse events of multiple sedation protocols in patients with cirrhosis should be carried out in the future.
杂志排行
World Journal of Gastrointestinal Endoscopy的其它文章
- Endoscopic ultrasound-guided fiducial marker placement in pancreatic cancer:A systematic review and meta-analysis
- Which scope is appropriate for endoscopic retrograde cholangiopancreatography after Billroth II reconstruction:An esophagogastroduodenoscope or a colonoscope?
- Improved diagnostic yield of endoscopic ultrasound-fine needle biopsy with histology specimen processing
