A first-principles investigation on the lone-pair enhanced birefringence and SHG response in K2 SbF2 Cl3
2021-01-11YANGGuangCUIXiuhua
YANG Guang , CUI Xiuhua
(1.School of Continuing Education,Jiaozuo University, Jiaozuo 454000, Henan, China; 2.School of Physical Science and Technology, Xinjiang University, Urumqi 830046, Xinjiang, China)
Abstract: The post-metal cations containing ns2 lone-pair electrons have long attracted increasing interest.The electronic structures and optical properties of K2SbF2Cl3 have been investigated using the first-principles method.The lone-pair electrons distribution was found near to the Fermi level, which was confirmed by the projected density of states, the projected band structures, and the electronic orbitals.The lone-pair electrons distribution originating from the Sb-sp states hybrid with Cl-p states make a main contribution to the total birefringence, and the Sb-F-Cl polyhedra play a key role in enhancing the total SHG response.
Keywords: lone-pair electrons; birefringence; SHG response
In the past several decades, lots of attentions have been paid on the post-metal cations(like Pb2+, Bi3+, Sn2+, Sb3+, et al.)[1-8]containing stereo-chemistry active lone pair electron distribution because the stereo-active lone pair electrons can enhance the birefringence and the SHG response[1-4].For example, the Pb2B5O9Cl owns SHG response as 4×KDP[9], Sn2B5O9Cl has birefringence as large as 0.168 at 546 nm[10]andα-BiB3O6(BIBO)has a SHG response of approximately 3.2 pm/V[11].
Curiously, how can these post-metal atoms own stereo-chemistry active lone-pair electrons distribution? What’s the role played by these lone-pair electrons to the enhancement of the birefringence and SHG response? To resolve these questions, great efforts have been made by using both experimental results and first-principles investigation.Using the first-principles calculation, WALSH et al.proposed a revised lone pair model which states that the interaction of the cation p states with the antibonding cation-s oxygen-p states results in the lone pair asymmetric electron distribution[12-14].By using the first-principles calculations and the real-space atom-cutting method, JING et al.pointed out that the enhancement of birefringence and SHG response in Pb2B5O9Cl has relation with the lone-pair electrons distribution around Pb2+cations[3].
Recently a mid-IR NLO compound K2SbF2Cl3has been synthesized.Its optical bandgap is 4.01 eV, and the SHG response is about 4 times that of KDP[15].Unlike Pb2B5O9Cl whose lone-pair electrons originating from the cation sp states and oxygen p states, the anions in K2SbF2Cl3are F, and Cl atoms.Can lone-pair electron distribution still be found in K2SbF2Cl3? What’s the atomic contribution to the total birefringence and SHG response? In this paper, the electronic structures and optical properties of K2SbF2Cl3have been investigated in detail by the first-principles method.The lone-pair electrons distribution around Sb atoms was confirmed by the projected density of states, the projected band structures, and the electronic orbitals.The atomic contribution to birefringence and SHG response was further investigated using the real-space atom-cutting method.The results show that the Sb-Cl groups give a main contribution to the birefringence, and the Sb-F-Cl polyhedra give a main contribution to the SHG response.
1 Numerical calculation details
The electronic structures and optical properties were investigated using the density functional theory(DFT)implemented in CASTEP code[16-17].During the calculation, the generalized gradient approximation(GGA)with Perdew-Burke-Ernzerhof(PBE)function was adopted[18-19].The optimized norm-conserving pseudopotentials in the Kleinman-Bylander form[20]for all the elements are used, and the valence electrons of these elements are set as K:3s23p64s1, Sb:5s25p3, F:2s22p5,Cl:3s23p5[21-22].The kinetic energy cutoffs are set as 940 eV, and Monkhorst-Packk-point meshes with densities of 3×3×2 points in the Brillouin zone are chosen.Other calculation parameters are defaulted by CASTEP code.The projected band structures are also calculated using the PWMAT code with GGA-PBE function, 3×3×2 Monkhorst-Pack K-points and energy cutoff of 680 eV[23-24].
After the electronic structures were obtained, the refractive indices and the birefringence were further calculated via theOPTADOScode[25-26].The nonlinear optical tensors of K2SbF2Cl3were further investigated using the method described in Refs[27-28].
2 Results and discussion
2.1 The electronic structures and the lone-pair electrons
Using the method described above, the band structure and projected density of states of K2SbF2Cl3have been obtained.Fig.1 illustrates the maximum of the valence band and the minimum of the conduction band are located at Y and S in the Brillouin zone, indicating that K2SbF2Cl3owns an indirect band gap about 3.82 eV.The obtained band gap is slightly smaller than the experimental value(about 4.01 eV).The underestimation of the band gap may have relation with the discontinuities in the calculated exchange correlation energy[29-30].
Fig.1 also presents the total and projected density of states(PDOS)of K2SbF2Cl3.It can find out that the states at the top of the valence band are mainly from the Sb-sp, Cl-p and F-p states.The states at the bottom of the conduction band are mainly the Sb-sp states along with some sp states from Cl, F and K atoms.It is well known that the optical properties have relation with the electron transition among the states nearby the Fermi level[31-34], hence the authors believe the optical properties of K2SbF2Cl3are mainly determined by the Sb-F-Cl groups[15].After detailed checked the states displayed in Fig.1, it is not difficult to draw the conclusion that the states nearby-7.97 eV are mainly the Sb-s states hybrid with the F-p states and Cl-p states, implying that the states nearby-7.97 eV may be the bonding states of Sb s-F p and the bonding states of Sb s-Cl p.At the top of the valence band, from-5 eV to the Fermi level, it has to be noticed that the Sb-sp states along with Cl-p states and F-p states.The states sitting in this region may be the Sb-p states hybrid with the antibonding states of Sb s-F p and the antibonding states of Sb s-Cl p states.Moreover, the states from-5 to-3 eV are mainly the states of F-2p states, along with some Sb-5s5p and Cl-3p states.As for the region of-3 eV to the Fermi level, the states are primarily from the Cl-3p states along with some Sb-5s5p and F-2p states.It is interesting to note that at the Fermi level, a small peak containing Sb-s states hybrid with Cl-p states can be found.
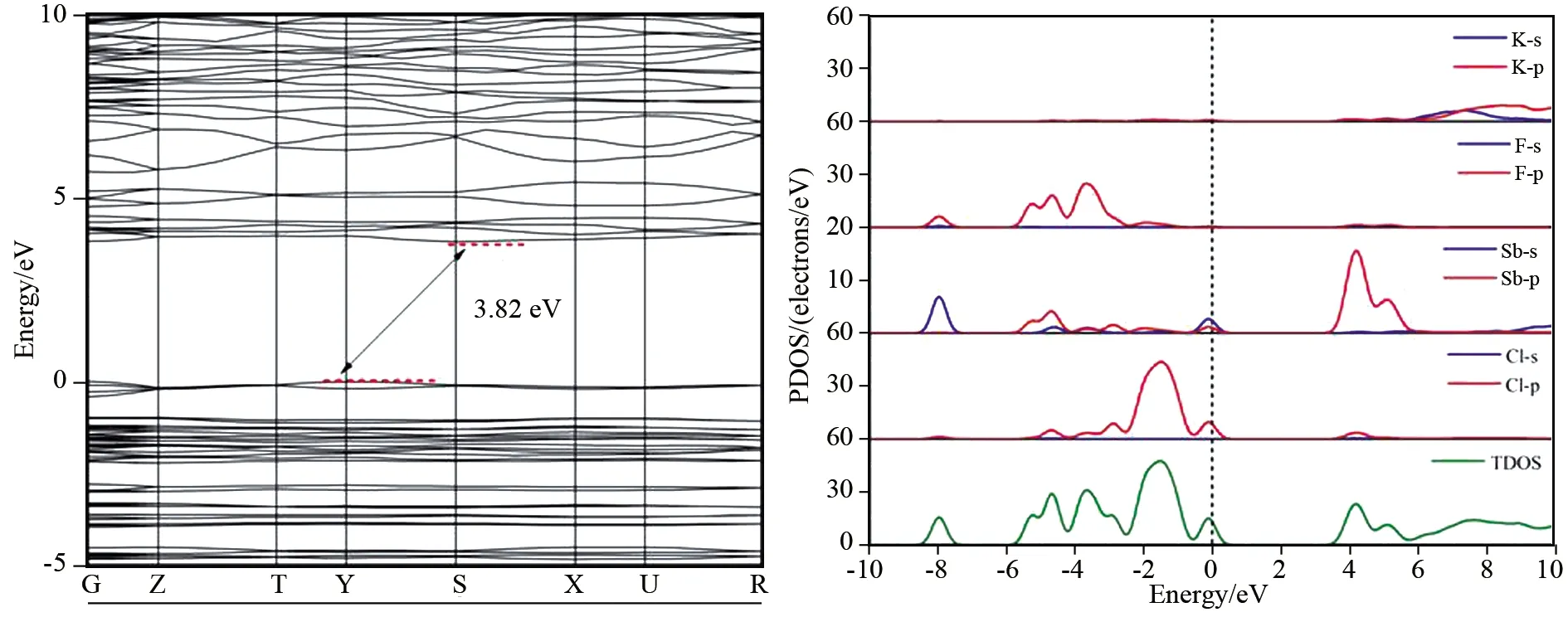
Fig.1 Band structures and the projected density of states(PDOS)of K2SbF2Cl3
In comparison, the projected band structures are also studied.The obtained projected band structures of K2SbF2Cl3are defined in Fig.2.In Fig.2, the red, orange, blue, and pink dots represent the s, px, py, and pzstates, respectively.As illustrated in Fig.2, nearby Fermi level, there are mainly the Sb-s states as well as some Cl-p states.According to the revised model of lone pair electrons[35], it is believed that the states nearby the Fermi level should be the states of the 5s2lone-pair electrons.

Fig.2 Projected band structures of K2SbF2Cl3. Red, orange, blue, and pink represent s, pz, px, and py states, respectively
To check out the lone-pair electron distribution around the Sb atoms, the orbitals sitting in different energy level are also probed.The orbitals sitting nearby 0,-3,-5, and-8 eV below the Fermi level are exhibited in Fig.3.As identified in Fig.3, it is found that the lobe-like electrons distribution around the Sb atoms in Fig.3A, which is the lone-pair electrons of Sb atoms.The p states of F and Cl atoms are also found in Fig.3A.Noting that except Fig.3A, there are no lobe-like electrons distribution nearby Sb atoms found in Fig.3B, 3C, and 3D, indicating that the post-metal ns2lone-pair electrons can only be found nearby the Fermi level.
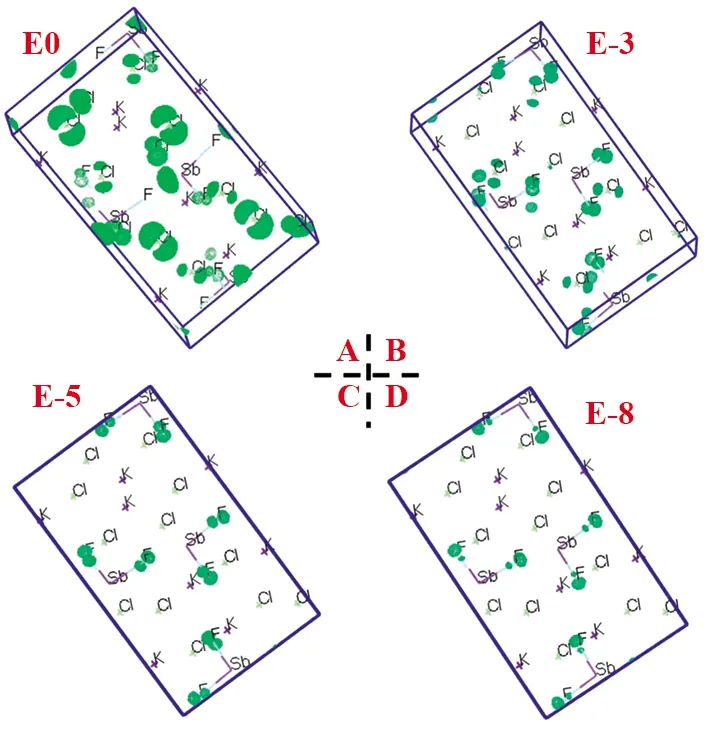
Fig.3 The orbital diagrams nearby the energy level 0, -3, -5, -8 eV below the Fermi level, marked as E0(3A), E-3(3B), E-5(3C), and E-8(3D), respectively
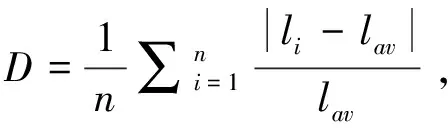
2.2 The linear and nonlinear optical properties
The refractive indices and birefringence of K2SbF2Cl3are further calculated using the OPTADOS code[25-26].The obtained birefringence is 0.132 at 1 064 nm(Fig.2).K2SbF2Cl3crystallizes in the orthorhombic space group P212121which owns only one independent SHG tensor.The obtained SHG tensor d14is-2.304 pm/V.The effective SHG coefficient was further evaluated using the method as described by Refs[36-39].The evaluated effective SHG coefficient is-1.945 pm/V, which is about 5×KDP.The obtained SHG response is in good agreement with the experimental value(about 4×KDP)

Fig.4 Refractive indices and birefringence of K2SbF2Cl3
In order to better study the contribution of atoms/anionic groups, the real-space atom-cutting method is used[27].The cutting radius of K, Sb, F, and Cl is set as 1.4, 1.2, 1.2, and 1.5 Å respectively.As listed in Table 1, the[SbF2Cl3]2-makes main contribution to the total birefringence, while the alkali metal K atoms give very small contribution to the birefringence.As shown in Figs.1-3, the lone-pair electronic distribution from the hybrid Sb s-Cl p states is found at the Fermi level, hence the authors want to know what’s the role played in the lone-pair electronic distribution, and which one is the dominant component to the total birefringence.The contribution from SbCl and SbF polyhedra are further investigated using the real-space atom-cutting method appearance of Table 1, thus we may reasonably arrive at the conclusion that the birefringence of SbCl group(0.140@1 064 nm)is much larger than SbF group(0.023@1 064 nm), and the atomic contribution of Sb is only 0.030@1 064 nm.Therefore, the SbCl groups, arising from the hybrid states among Sb-sp and Cl-p states found at the Fermi level, give more dominant contribution to the total birefringence.
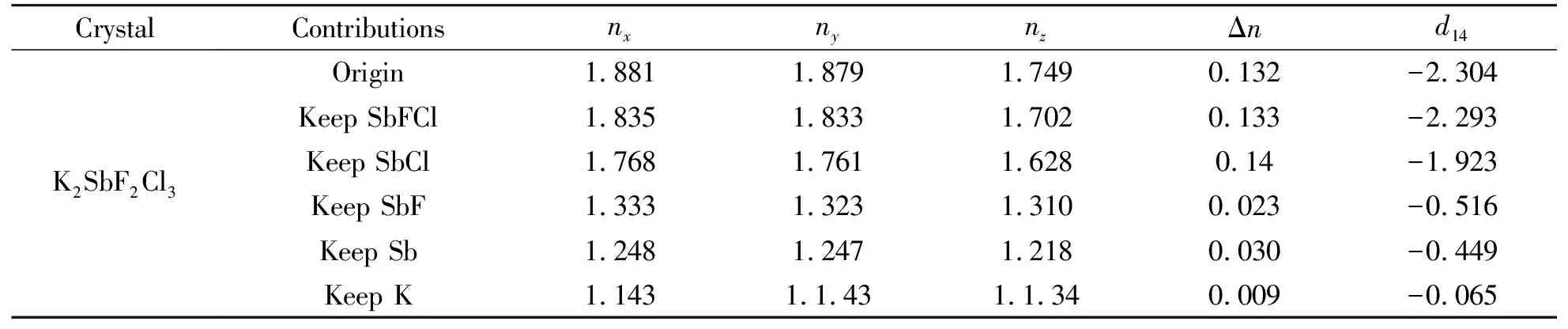
Table 1 The obtained refractive indices and birefringence using the OPTADOS code(marked as origin). The obtained refractive indices and birefringence of different anionic groups using the real-space atom-cutting operator(marked as Keep anionic groups, like Keep SbFCl)
The atomic contribution to the SHG response is also calculated using the real-space atom-cutting method.As indicated in Table 1, the alkali metal K atoms give very small contribution to the total SHG response, and the[SbF2Cl3]2-anionic groups make major contribution to the total SHG response.Unlike birefringence, neither SbCl anionic groups nor SbF anionic groups give main contribution to the total SHG response.The contribution from SbCl and SbF anionic groups are 83.46%, and 22.40% , respectively,which is smaller than the contribution from[SbF2Cl3]2-anionic groups 99.5%.How can these anionic groups give different contribution to the total SHG response? To answer this question, the effective SHG response of each band is further investigated using the band-resolved analysis(Fig.5).As described elsewhere[40],the total SHG coefficient is divided into the contribution from the different processes with virtual-electron(VE)and virtual-hole(VH)processes.As shown in Fig.5, the VE process gives main contribution to the total SHG response.During the VE process, the states nearby the Fermi level and at the bottom of the conduction give main contribution to the total SHG response.Furthermore, the states in the region at-6 to 0 eV below Fermi level also give contribution to the total SHG response.In word, the states nearby the Fermi level(at the top of the valence band, and at the bottom of the conduction band)give contribution to the SHG response.The states nearby the Fermi level are Sb-sp states, F-p, Cl-p states.Hence one can deduce that the Sb, F, and Cl atoms(in[SbF2Cl3]2-anionic groups)have inevitable contribution to the total SHG response.That’s why both SbF anionic groups and SbCl anionic groups(containing lone-pair electron distribution)own SHG response smaller than that of[SbF2Cl3]2-anionic groups.Both SbF and SbCl anionic groups are part of the[SbF2Cl3]2-anionic groups.

Fig.5 VE and VH contribution from each band
3 Conclusion
In this paper, the electronic structures and the linear and nonlinear optical properties of K2SbF2Cl3have been investigated using the first-principles method.The lone-pair electrons distribution was found nearby the Fermi level, which was confirmed by the projected density of states, the projected band structures, and the electronic orbitals.The lone pair electrons distribution originating from the Sb-sp states and Cl-p states give main contribution to the total birefringence, and the Sb-F-Cl polyhedra give main contribution to the total SHG response.
