The Immune Function of Keratinocytes in Anti-Pathogen Infection in the Skin
2021-01-09JiaNingWangandMinLi
Jia-Ning Wang and Min Li∗
Hospital for Skin Diseases (Institute of Dermatology), Chinese Academy of Medical Sciences and Peking Union Medical College,Nanjing, Jiangsu 210042, China.
Abstract Keratinocytes,located in the outer part of human skin,are the main epidermal cell type and play an essential role in skins defense against infection. Besides creating a physical barrier between the environment and the internal body,keratinocytes exert powerful immune function in anti-pathogen infection in the skin. At the recognition stage,pattern recognition receptors (PRRs) expressed by keratinocytes sense pathogen-associated molecular patterns (PAMPs)existing in pathogens. Toll like receptors (TLRs) are the most important PRRs in keratinocytes. Other PRRs such as dectin-1 and nucleotide-binding oligomerization domain(NOD)-like receptors(NLRs)are also found to participate in this process.Activated PRRs enhance the secretion of cytokines,chemokines and the production of antimicrobial peptides(AMPs).Proinflammatory cytokines tumor necrosis factor-α,interleukin(IL)-1α,IL-6,IL-1β and IL-18,chemokines(C-XCmotif)ligand(CXCL)1,CXCL2,CCL20,CCL2 and IL-8,AMPs human β-defensin(HBD)2,HBD3 and LL37 are the main molecules expressed in this procedure. Thymic stromal lymphopoietin (TSLP), IL-36γ, IL-17 family member IL-17C and anti-inflammatory cytokine IL-10 can also be secreted.Some molecules produced by keratinocytes such as ribonuclease 5 and 7,S100 proteins own antimicrobial properties.Keratinocytes defense responses can be regulated by internal and external factors. This review summarizes recent advances on the innate immune function of keratinocytes against infection,promoting the finding of a new direction for avoiding severe skin infection as well as the potential treatment of keratinocyte-associated inflammatory dermatosis.
Keywords: keratinocyte, immune function, anti-pathogen
Introduction
The skin is the largest organ of human body and act as a barrier. It possesses the function of protecting internal human body from external environment.Epidermis,as the outmost part of skin, consists mainly of keratinocytes(>90%)and is the first line of defense for host.Although keratinocytes are non-bone marrow-derived cells,they are proved to play a critical role in innate immune reactions and participate in the activation of adaptive immunity in the skin. Keratinocytes sense specific microbial components through several receptors and produce effector molecules such as pro-inflammatory cytokines to resist infection. They also attract immune cells such as neutrophils and T cells to the site of infection. We performed a search on PubMed using searching strategy“(keratinocyt*[MeSH Major Topic]) AND (infection[MeSH Subheading]OR innate immun*[MeSH Subheading])”from 2008.1 to 2019.9,to collect related articles for this review.In this review,we would like to illustrate how keratinocytes sense pathogenic microorganisms and initiate subsequent signals as well as how they participate in innate and adaptive immune response in the skin.
Keratinocytes sense pathogens through pattern recognition receptors
In the process of resisting infection, recognition of pathogenic microorganisms is crucial. Keratinocytes can sense pathogen molecules(pathogen-associated molecular patterns[PAMPs])through particular receptors known as pattern recognition receptors (PRRs) in the earliest phase of the response.
PAMPs are a number of highly conserved molecules shared by several pathogens, such as viral nucleic acid,lipopolysaccharide, lipoteichoic acid (LTA), peptidoglycan (PGN), lipoproteins, flagellin, and so on. PRRs are a series of membrane and intracellular receptors that can sense PAMPs and play a role in the early stage of innate immune response. Toll like receptors (TLRs), dectin-1,nucleotide-binding oligomerization domain (NOD)-like receptors(NLRs),and retinoic acid-inducible gene-I(RIGI)-like receptors (RLRs) are illustrated to be expressed in keratinocytes.1-2Many PRRs have synergistic effects(such as TLR cooperation, TLR cooperation with NODreceptors) during the pathogen-recognition process.
TLRs
TLRs are the most important and well-studied PRRs in keratinocytes. They sense PAMPs or danger-associated molecular patterns of pathogenic microorganisms or cells.TLR1-7 and TLR9 are expressed in keratinocytes.TLR1,2, 4-6 are located on keratinocyte cell membrane while TLR3, 7 and 9 express on the membrane of intracellular compartments such as endosomes and lysosomes. Most TLRs form homodimers while TLR2 forms a heterodimer with TLR1 or TLR6 in keratinocytes.1,3
Each TLR has different recognition targets. TLR1, 2,and 6 recognize lipoproteins of viruses,bacteria,fungi,and parasites. TLR2 also recognizes PGN, LTA, fungal components zymosan, and phospholipomannan (PLM).Synthetic lipoprotein Pam3Cys is an important synthetic ligand for TLR1/TLR2 heterodimer while lipoprotein Pam2Cys is a ligand for TLR2/TLR6. TLR4 identifies lipopolysaccharide and O-linked mannosyl chains of Candida albicans (C. albicans) cell wall. TLR5 identifies bacterial protein flagellin. TLR3, located in cytoplasm,recognizes double-stranded viral RNA (dsRNA) and synthetic agonist poly(I:C). Cultured primary keratinocytes express TLR7 and it senses virus single-stranded RNA. In contrast, TLR7 expression was undetected in some experiments.TLR7 expression can be upregulated by keratinocyte differentiation,imiquimod and synthetic poly(I:C).Intracellular TLR9 binds CpG-oligodeoxynucleotide of bacterial and fungal double-stranded DNA.2,4-5
When TLRs are activated, the Toll/IL-1 receptor (TIR)domain of TLRs can act with an adaptor molecule owning the same TIR domain and then several proinflammatory cytokines are produced.There are five members in the TIR adaptor family. Myeloid differentiation factor-88(MyD88) is a main TIR adaptor involved in TLRs signaling, which recruits several kinases and lead to the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) translocation to the nucleus as well as mitogen-activated protein kinase (MAPKs) activation,permitting transcription of the target genes. As a result,cytokines such as tumor necrosis factor (TNF)-α,interleukin (IL)-1, IL-6, and chemokines such as IL-8 as well as antimicrobial peptides(AMPs)are secreted(known as the MyD88-dependent pathway). The other TLR signaling pathway is TIR-domain-containing adaptorinducing interferon-β-dependent pathway. Besides activating NF-κB as the former, the later pathway also activates interferon regulatory factor 3 and results in type I interferon (IFN) production, which is important to viral defense. In keratinocytes, TLRs except TLR3 initiate the MyD88-dependent pathway, while TLR3 and TLR4 activate TIR-domain-containing adaptor-inducing interferon-β pathway.1,3
TLRs play a critical role in anti-bacterial responses.TLR2 recognizes Staphylococcus aureus lipoproteins and LTA as well as promoting the expression of AMP β-defensin 3, IL-1 family cytokines, chemokines (C-X-C motif)ligand(CXCL)1 and CXCL2.Mice lacking TLR2 is more easily to get infected with S.aureus.Phenol-soluble modulin (PSM) peptides from S. aureus mobilize lipoproteins to be recognized by TLR2.6-7Besides, TLR2 heterodimerizes TLR2/1 and TLR2/6 recognize Propionibacterium acnes in human keratinocytes,inducing NF-κB and activator protein 1 activation.8Bacterial flagellin activated TLR5 induces expression of AMP S100A8/S100A9, S100A7, S100A15, and human β-defensin(HBD) 2 in keratinocytes.9TLR5 also recognizes Treponema pallidum flagellins and activates matrix metalloproteinase (MMP) 9 and MMP13 through MAPK/NFκB signaling pathway.10
In addition to bacteria, TLRs act in skin’s defense against fungi and viruses. Upregulated expression of cytokines (such as IL-8) and HBD2 in keratinocytes interacted with Malassezia spp. is probably via TLRs.11TLR2 also identifies C. albicans component PLM, then activating NF-κB and p38MAPK signal transduction pathways to secrete IL-6 and IL-8 in keratinocytes.12PLM, present in the inner layer of C. albicans’ cell wall,owns the function of enhancing C. albicans cell wall homeostasis on β-glucans and chitin synthesis. It is of notable contribution to C. albicans’ pathogenicity and it deregulates yeast phagocytosis and activates inflammasome in macrophages.13TLR4 and TLR2 are important to avoiding Sporothrix spp. infection. They identify Sporothrix schenckii as well as trigger inflammatory response(upregulation of IL-6 and IL-8)in keratinocytes.14Viruses such as herpes simplex virus, Dengue virus, human papillomavirus are sensed and resisted via TLR recognition in keratinocytes.5,15
After recognition, TLRs are engaged in initiating the expression of many cytokines, chemokines and other functional genes in innate immune responses to resist infection. TLRs also participate in regulating adaptive immune responses through cytokines secretion. TLR2 in keratinocytes initiates anti-infection reactions of immune cells through the secretion of chemokine (C-C motif)ligand (CCL) 20, CCL2, MMP9, and IL-8.16TLR3 functions on epidermal barrier repairment by increasing structural-related gene expression (such as glucocerebrosidase) in epidermis and changing lipid composition,which may promote infection recovery and wound/UV damage repairment.17Recent findings show that TLRs may act on inflammation against microbe through upregulating the expression of Kallikrein-related peptidases (KLKs) inhibitor Lympho-epithelial Kazal-type inhibitor in keratinocytes. KLKs are serine proteases which is necessary for desquamation,owning the function of degrading several adhesive proteins of corneodesmosomes.The expression of several KLKs can be induced by pathogen molecules like S. aureus and poly(I:C), which may lead to the decreased epidermis integrity and pathogenesis of the bacteria. Researchers find TLR1/2,3, 5, and 2/6 upregulate the expression of Lymphoepithelial Kazal-type inhibitor and inhibit KLKs in microbial infected skin lesions,contributing to the defense against infection (such as varicella, pyoderma, and rosacea).18
Several molecules in host and bacteria effect TLRs recognition. The structure of lipoproteins affects TLR2-mediated immune response. Diacylated lipoproteins existing in skin microbiota while deficient in S. aureus,induce immune suppression via TLR2 to help avoiding immune response towards skin normal bacterial flora.Unsaturated fatty acids from skin enhance the TLR2-dependent immune response to S. aureus lipoproteins.Meanwhile, S. aureus produce several molecules to avoid recognition. PSMs of S. aureus act as antagonists for TLR4, helping escape from hosts. Staphylococcal superantigen-like protein 3 from S.aureus avoid the recognition of lipoproteins by TLR2.6
TLR-signaling is negative regulated by some molecules,such as splice variants for adaptors or their related proteins,ubiquitin ligases,deubiquitinases,transcriptional regulators (such as signal transducer and activator of transcription 1 [STAT1]) and microRNAs.3MicroRNA(miR)-146a, a kind of evolutionarily conserved small noncoding RNAs,is an inhibitive element in keratinocytes’TLR2 signal pathway.It is upregulated in TLR2-activated keratinocytes through the NF-κB and MAPK pathways and has a lasting suppression effect on inflammatory cytokines release (such as IL-8, CCL20, and TNF-α) as well as NF-κB target genes.19Normal human flora
Staphylococcus epidermidis LTA can activate TLR2 to express miR-143, which inhibits TLR2 expression and thus decreases opportunistic pathogen P. acnes-induced proinflammatory cytokines (IL-6 and TNF-α) in keratinocytes.6These negative regulators help to maintain the balance of inflammation and defense process in keratinocytes (Fig. 1).
Dectin-1
C-type lectin receptor dectin-1 as one of the important PRRs, is mainly expressed in dendritic cells, monocytes,and macrophages. In dendritic cells, dectin-1 recognizes β-glucans (a cell wall components of bacteria and fungi)and activate spleen tyrosine kinase,leading to NF-κB and MAPK activation as well as cytokine production. In keratinocytes, dectin-1 is found to be located on the cell membrane as well as intracellular while it cannot activate spleen tyrosine kinase-signaling, which differs from other cells.20-21However, dectin-1 is also illustrated to be connected with β-glucan-induced MAPK pathway and IL-8 cytokine production in normal human epidermal keratinocytes.20The activation of dectin-1 in keratinocytes by β-glucan may have the function of enhancing wound healing, for dectin-1 activation induces keratinocyte proliferation and migration.21The function of dectin-1 in the reaction to Mycobacterium ulcerans and Malassezia spp.have been studied.Dectin-1 recognizes and internalize M. ulcerans as well as lead to the release of intracellular reactive oxygen species, CXCL8, CCL2, and LL-37 in keratinocytes.22In antigen-presenting cells, dectin-1 is related to Malassezia spp.recognition and IL-1β secretion.While in keratinocytes, IL-1β cannot be induced by Malassezia spp.23
Several factors participate in dectin-1 expression signals.IFNs and T helper 17 (Th17) cytokines enhance dectin-1 expression. Dectin-1 is upregulated in psoriasis as well.24β-glucan stimulation attenuates dectin-1 expression in normal human epidermal keratinocytes while mycobacterium and zymosan induce dectin-1 mRNA and protein production in keratinocytes.20,22In conclusion, further studies should be made to explore the downstream of activated dectin-1 in keratinocytes.
NLRs and inflammasome
NLRs are cytosolic PRRs and recognize intracellular bacterial components. Nucleotide-binding oligomerization domain-containing protein 1 and 2 (NOD1 and NOD2),as two main NLRs,are found to be expressed in keratinocytes and mediate NF-κB activation. NOD1 senses bacteria PGN fragments while NOD2 recognizes bacterial components such as muramyl dipeptide, γ-Dglutamyl-mesodiaminopimelic acid, and PGN from Gram-positive bacteria.25-26Researches illustrate that keratinocytes’ NOD1 recognizes Pseudomonas aeruginosa and upregulate the secretion of CXCL8(also known as IL-8).26NOD2 acts in defense against S. aureus through getting activated with the involvement of the first binding site of NF-κB, leading to the secretion of IL-17C cytokine and less survival rate of S. aureus in keratinocytes.27TLR2 ligands such as bacterial lipoproteins have a synergistic effect with NOD2 ligand PGN in initiating immune responses.28
The nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain containing 3 (NLRP3)as a member of NLRs, forms a complex named NLRP3 inflammasome with caspase-1 and an adaptor protein ASC(apoptosis-associated speck-like protein containing a CARD domain) in the cytosol. Inflammasome plays an important role in anti-pathogen inflammation. It is also involved in various auto-immune and auto-inflammatory diseases. Recent findings show that in keratinocytes,NLRP3 inflammasome can function as a PRR. It is activated by dsRNA through dsRNA-induced protein kinase in keratinocytes. And the process can be enhanced by type I IFNs. Then the activation leads to caspase-1 cleavage as well as the subsequent formation of IL-1β and IL-18, which are crucial to hosts’ antimicrobial processes.29The two cytokines promote the secretion of TNF,IL-6, and IFN-γ, respectively. NLRP3 inflammasome recognizes fungi in dendritic cells and monocytes, such as C.albicans, Aspergillus fumigatus, and Malassezia spp.23Whether NLRP3 inflammasome in keratinocytes own the same function remains unknown. Absent in melanoma 2 inflammasome is found to detect cytosolic double-stranded DNA such as human papillomavirus 16 DNA and leads to caspase-1 activation in keratinocytes as well.30
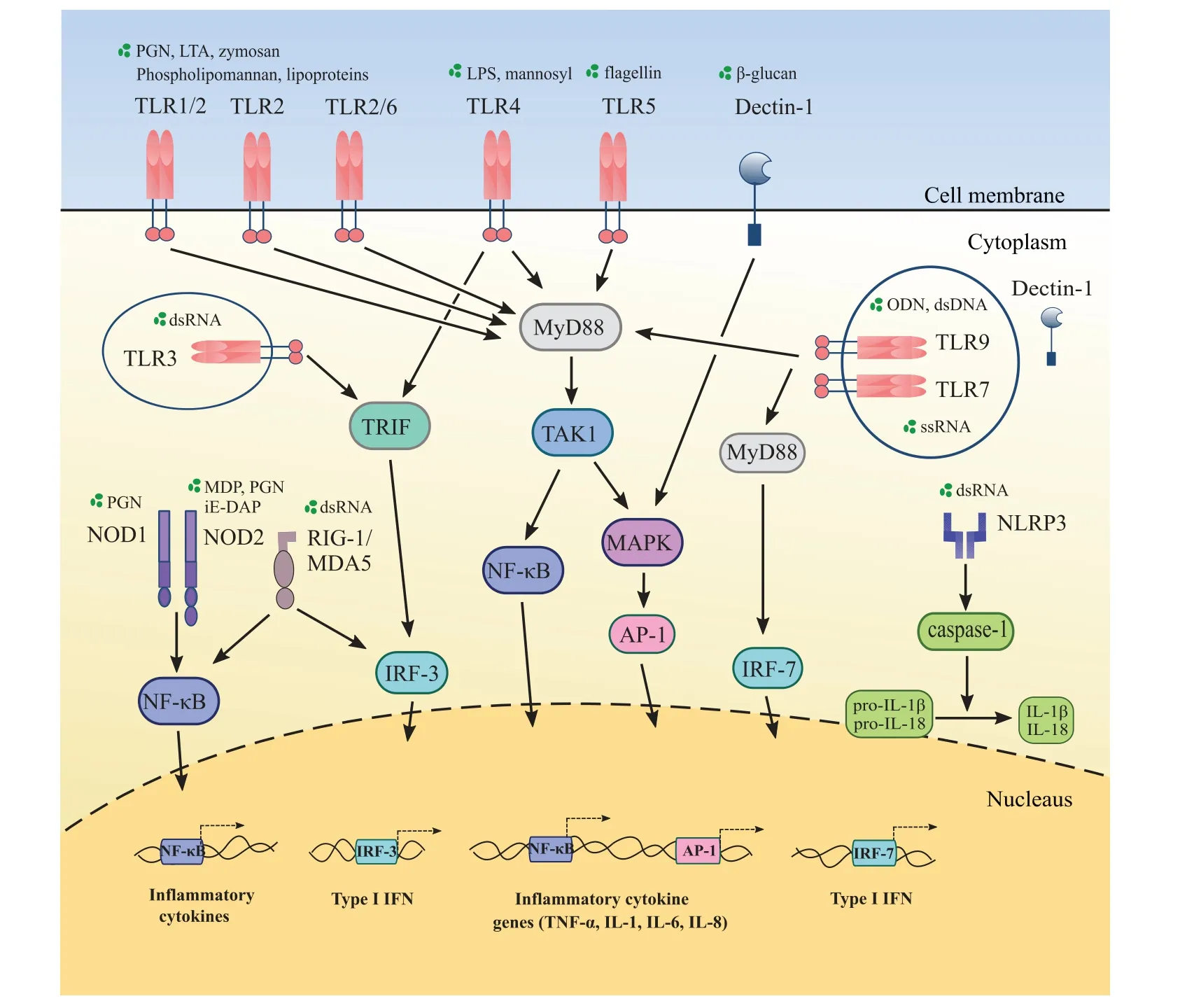
Figure 1. Keratinocytes sense pathogens through pattern recognition receptors (PRRs). Keratinocytes express toll like receptors (TLRs),dectin-1,nucleotide-binding oligomerization domain(NOD)-like receptors(NLRs),the nucleotide-binding oligomerization domain,leucine-rich repeat and pyrin doin containing 3(NLRP3),and retinoic acid-inducible gene-I(RIG-I)in cell membrane or cytoplasm.TLR2 forms heterodimers with TLR1 or TLR6 and recognize lipoproteins.Each PRR recognizes different pathogen-associated molecular patterns(PAMPs).TLRs except TLR3 recruit myeloid differentiation factor-88(MyD88)while TLR3 and TLR4 initiate TIR-domain-containing adaptor-inducing interferon-β(TRIF)-dependent pathway.Intracellular NLRs activate the nuclear factor kappa-light-chain-enhancer of activated B cells(NF-κB)in keratinocytes.RIGI and melanoma differentiation-associated protein 5(MDA5),located in cytoplasm,sense double stranded RNA(dsRNA)and activate NF-κB as well as interferon regulatory factor 3(IRF-3).Dectin-1 is expressed in cell membrane and intracellular. It recognizes β-glucan.NLRP3 senses dsRNA and induce interleukin(IL)-1β as well as IL-18 through caspase-1.As a result,several inflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1, IL-6, IL-8, and interferon are secreted.
RLRs
RIG-I and melanoma differentiation-associated protein 5(MDA5) are two major RLRs exist in the cytoplasm of keratinocytes.They are important in defense against virus together with TLR3 and TLR7.RIG-I,as a DExH/D-box contained RNA helicase, identifies dsRNA in cytoplasm and lead to antiviral responses.MDA5 also belongs to the DExH/D-box contained RNA helicase and recognizes longer dsRNA than RIG-I.Both RIG-I and MDA5 activate NF-κB and IFN regulatory factor 3 (IRF-3) to induce subsequent gene expression including type I IFN.2,15In keratinocytes, the expression of RIG-I is upregulated by IFN-γ or TNF-α.RIG-I expression is dependent on IRF-1 protein. Recent studies have found UVB exposure can decrease IRF-1 expression, leading to localized skin immune suppression towards viruses.31
Keratinocytes produce several cytokines and chemokines to regulate immune responses
When PRRs are activated, keratinocytes subsequently secrete several cytokines which are linked to anti-infection response, such as proinflammatory cytokines (IL-1, IL-6,IL-18, TNF, and IFN), anti-inflammatory cytokines (IL-10),chemokines(IL-8,CXCL1,CXCL2,CXCL10,CCL2,and CCL20), and thymic stromal lymphopoietin (TSLP),and so on.4,16Those cytokines are found to be induced by several pathogen molecules through PRRs and function on mediating immune responses in keratinocytes.
Proinflammatory cytokines and chemokines are mainly produced after PRRs activation in keratinocytes. They own the function of inducing AMPs production, and immune cells attraction to resist infection. Proinflammatory cytokines IL-1β and IL-18, both belonging to IL-1 family, are secreted primarily dependent on caspase-1 via the recognition of pathogen molecules by inflammasome.29IL-1α, as another member of IL-1 family, is upregulated dependent on TLR2 activation by S.aureus as well as the bacterial cell wall components LTA and PGN stimulation.IL-1α induces antimicrobial protein complex calprotectin (also known as S100A8/A9) expression in both epithelial cells and oral keratinocytes. IL-1α also upregulates the expression of neutrophil targeting chemokines CXCL1, CXCL2, and IL-8 in keratinocytes.7,32IL-36,as a new cytokine related to inflammation,is secreted notably by keratinocytes. IL-36 contains three members(IL-36α, IL-36β, and IL-36γ) in IL-1 family and induces several chemokines and cytokines expression like CCL10,CCL20, CXCL-1, CXCL8 (IL-8), IL-1β.33IL-36γ is mostly upregulated when keratinocytes contacting with TLR ligands.Poly(I:C)induces the transcription of IL-36γ gene and caspase-3/7 activation dependent on caspase 1,which may indicate IL-36γ possesses anti-viral abilities.34Researches showed that under UVB exposure, mice lacking IL-1 receptor are vulnerable to develop epidermal cysts and have downregulated expression of IL-6 and TNF-α with less infiltrating monocytes/macrophages in dermal compartment.35Those findings exhibit the importance of IL-1 cytokine in inflammatory responses.Besides IL-1, TLRs activate pro-inflammatory cytokines IL-6 and TNF-α all through the MyD88-dependent pathway.1
Another cytokine TSLP,a T cell maturation activator,is also secreted by keratinocytes through TLRs activation with the presence of TLR ligands such as dsRNA,flagellin,diacylated lipopeptide or other S. aureus component. In keratinocytes, elevated TSLP promotes Th2-type inflammation and atopic dermatitis (AD) process, probably through suppressing the expression of antimicrobial proteins S100A7 (psoriasin) and HBD2 via Janus kinase signal transducers 2 and activator of transcription 3(JAK2/STAT3)-dependent pathways.36
Cytokines produced by keratinocytes can take part in adaptive immune responses, mainly by activating T cell responses. IL-18 of IL-1 family enhances Th1 adaptive immune responses. IL-1β together with IL-6 enhances Th17 cell differentiation.Th1 cells produce cytokines such as IFN-γ to activate other immune cells including macrophages. Th17 cells play a critical role in defense against pathogens through IL-17 cytokines secretion (which upregulate many inflammatory genes in several cell types including keratinocytes), neutrophils recruitment, and so on.37IL-17A is the first one to be discovered in IL-17 family and is secreted by T cells (such as αβ T cells, γδ T cells,iNKT cells,and LTi-like cells).In recent years,a new family member IL-17C is demonstrated to be secreted by keratinocytes and own the similar functions as IL-17A on inducing inflammatory cytokines,chemokines and AMPs.IL-17C is proved to be induced by pathogens through NOD2 mediated pathways in keratinocytes.It cooperates with TNF on upregulating keratinocytes’ HBD-2 expression.And it is related to reduced survival of S.aureus and decreased sensory neurons apoptosis under herpes simplex virus-2 infection in keratinocytes.38IL-17C also leads to psoriasis like skin lesions together with TNF-α. In addition, TLR2 and TLR5 activation as well as TNF and IL-1β induce IL-17C secretion in epithelial cells.27,39
Anti-inflammatory cytokine IL-10 is secreted by keratinocytes as well. IL-10 secretion exists in other cells like Th2 lymphocytes and mast cells in skin and can regulate keratinocytes’ inflammatory response. Excessive expression of IL-10 suppresses proinflammatory cytokines including TNF-α and IFN-γ. And the expression of HBD2 is inhibited by IL-10 in keratinocytes.40
Chemokines,as a family of small cytokines or signaling proteins, can be secreted by keratinocytes as mentioned above and initiate the activation of other immune cells like T cells to participate in the inflammatory response to defense against infection. For instance, TLR2 mediated secretion of chemokines CCL20, CCL2, and IL-8 contribute to the recruitment of lymphocytes,monocytes,memory T cells and other immune cells to the infection site.16
Keratinocytes secrete AMPs to defense against pathogens
AMPs are small cationic peptide antibiotics widely exist in animals and own the function of anti-pathogens, wound healing, immunomodulation, and so on. HBDs and cathelicidins are two major families of AMPs expressed in keratinocytes.Most AMPs are proved to resist bacterial infection by combining with bacterial membrane and disturbing its structure.41AMPs can inhibit the infection of fungi and several viruses in skin as well.
HBD family has four members including HBD1-4.Among which,HBD1,2,and 3 are testified to be expressed in keratinocytes.HBD2 and 3 inhibit cell surface bacteria such as S.aureus.Opportunistic pathogen Staphylococcus epidermidis stimulation upregulates HBD2 and 3 expression through TLR2 signaling.41HBD2 expression induced by IL-1β inhibits S. aureus V8 protease (also named as serine protease A),which is an extracellular protease of S.aureus and function on skin integrity impairment.42Fungi such as C. albicans and Malassezia furfur induce HBD2 expression via TLR2 in keratinocytes.41
Cathelicidin is another family of AMPs. Human cathelicidin hCAP18 (hCAP18/LL-37) consists of two domains: a conserved cathelin-like domain and LL-37.During activation,human cathelicidin hCAP18 is cleaved by proteinase 3 to generate the mature form:LL-37,which has synergistic functions against microbe with HBDs.43Both the two domains of cathelicidin own the prosperities of anti-microbial ability.Towards viruses,LL-37 enlarges dsRNA-induced IFN-β expression and anti-viral response in keratinocytes.44For bacteria such as S.aureus,LL-37 is proved to have eliminating effects towards both extra-and intracellular S. aureus.45C. albicans PLM induces keratinocytes to express LL-37, HBD2, and HBD3 via TLR2.Those AMPs may act in skin’s defense against C.albicans.46Recently, LL-37 is regarded as a potential treatment for polymicrobial infected wounds. Besides direct host defense to pathogens, LL-37 is found to upregulate IL-1 family cytokines (especially IL-36γ through p38 MAPK-signaling pathway), chemokines and TLRs expression in keratinocytes.33
Apart from cathlicidin and HBDs, other molecules expressed by keratinocytes are proved to own antimicrobial properties.Ribonuclease(RNase) 5 and RNase 7, as members of RNase family which catalyze the degradation of RNA, own anti-infection abilities against many pathogens.43For instance, RNase 7 protects skin against S. aureus and can be induced by IL-1β in human keratinocytes and ocular epithelial cells.6S100A7 (psoriasin), S100A8 (calgranulin A), and S100A9 (calgranulin B)are AMPs belonging to S100 protein family.Psoriasin,also known as S100A7, mainly induced by flagellin of Escherichia coli and has bacteria-killing activities.41Psoriasin is also found to be connected with wound healing and benefits skin’s innate immunity.47In keratinocytes, S100A8 and S100A9 form an antimicrobial heterodimeric complex named as calprotectin (S100A8/A9), all of which are upregulated by bacterial flagellin through the activation of TLR5.9S100A8/A9 in keratinocytes resists bacterial invasion and can be upregulated by IL-1α.32Several cytokines and chemokines such as IL-6, IL-8, TNF-α, CXCL1, CXCL2, CXCL3, and CCL20 are induced by S100A8/A9 and those cytokines in turn enhanced S100A8/A9 production.48S100A8/A9 also involves in keratinocytes’ proliferation as well as chronic inflammation. Neuroplastin-β and extracellular MMP inducer are found to form a heterodimer as a receptor for calprotectin (S100A8/A9).
Interestingly,several bacteria can produce peptides with AMPs properties. For instance, Staphylococcus epidermidis produces PSMs γ and δ,which can act together or with IL-37 to inhibit other pathogens.43It makes sense in maintaining the balance of microorganisms on the skin.
The expression of AMPs is regulated by some molecules,most notably cytokines.Proinflammatory cytokines IL-1β,TNF-α,IL-17,IL-22,and so on,induce AMP expression in keratinocytes. IL-17A and IFN-γ upregulate RNase 7 through STAT3.49IL-22,secreted by Th22 cells,acts with the IL-22 receptor on epidermal keratinocytes to activate STAT3 and enhance the expression of HBD2, HBD3,S100A7, S100A8, and S100A9. The effects of STAT3-mediated gene expression are probably through B-cell leukemia (Bcl)-3’s nuclear translocation in keratinocytes(the IL-22-STAT3-Bcl-3 pathway). IL-22 is proved to induce AMPs expression and enhance the thickness of epidermis. Deficiency of IL-22 in keratinocytes may be responsible for chronic inflammatory diseases like hidradenitis suppurativa.50Moreover, IL-22 level is upregulated in skin suffered from inflammatory skin diseases such as psoriasis and AD, indicating that IL-22 as well as its downstream Bcl-3 may involve in the pathogenesis of those skin diseases.51es such as epidermal growth factor(EGF)and platelet-released growth factors enhance AMPs production. EGF are demonstrated to increase AMPs expression with the presence of S.aureus and participate in the expression of RNase 7 and HBD3 in defense against Trichophyton rubrum.49Platelet-released growth factors upregulate keratinocytes’ psoriasin expression through EGF receptor and IL-6 receptor, may promoting wound healing.47S100 calcium-binding protein A11(S100/A11),as a protein in Ca2+-binding family, upregulates the expression of HBD3 and filaggrin (a protein that is important to the integrity of skin barrier) in keratinocytes.52
By contrast, Th2 cytokines inhibit AMP expression in AD patients. Th2 cytokines IL-4 and IL-13 in AD skin decrease S100/A11 production.52IL-4 and IL-13 also induce Bcl-3 expression in keratinocytes and the latter downregulates AMPs (including HBD3 and cathelicidin)functions,showing an opposite effect of Bcl-3 in the IL-22-STAT3-Bcl-3 pathway towards AMPs modulation.53These findings may partly explain why AD patients have weakened skin immune and tend to develop skin infections.
Future perspectives
The immune responses of keratinocytes protect human skin from harmful viruses,bacteria,and fungi.As the first line of defense in human immune system,keratinocytes are receiving wider attention than ever.They function through various mechanisms including recognition of harmful microbes, production of AMPs as well as pro-inflammatory cytokines, chemokines (for activating relative signal transduction pathways to induce the expression of effective genes)and regulate the function of other immune cells. At the same time, keratinocytes are the major cell type in epidermis and involved in various skin diseases.Pathogen especially the virus, as a viable cell-demand creature,the replication and invasion of which are largely dependent on keratinocytes. So keratinocytes’ anti-infection function is crucial to internal environment of the human body. Both loss-of-function and gain-of-function of keratinocytes may contribute to the pathogenesis of dermatosis.
There are several physical and chemical factors that influence keratinocytes’ function, and can be used in clinical practice. Q-switched Nd:YAG laser can help to treat C.albicans infection by reducing fungus invasiveness.It inhibits proinflammatory cytokines expression (such as TNF-α and IL-8) as well as induce AMP HBD2 and HSP70B gene expression in HaCaT cells.54High calcium is demonstrated to induce the dsRNA sensors expression,including TLR3,MDA5,and RIG-I of keratinocytes.They also enhance expression of S100/A11 as well as the induction of HBD2 and HBD3 in defense against pathogens.15,52So anti-infection function may be strengthened by regulating calcium concentration in signal pathways of keratinocytes.Other influence factors like injury,as a dangerous condition for skin infection, induce AMP expression and neutrophil chemotactic cytokine IL-8 expression in epidermis, dependent on EGF receptor activation. CCL20, IL-15, and IL-23A are proved to be upregulated in early stage of injury in keratinocytes and may facilitate adaptive immune.55
This review has potential limitations. It mainly focuses on keratinocytes immune responses against bacteria and fungi. Defenses to viruses in keratinocytes remain to be discussed further.In the recognition part,PRRs expressed by keratinocytes and their ligands are introduced, while the detailed immune signaling are discussed briefly.In the following part, effector molecules including cytokines,chemokines and AMPs expressed by keratinocytes are listed.And the immune function of keratinocytes towards several pathogens are elaborated in this review. Keratinocytes may secrete different cytokines towards different pathogens.The inner connection of cytokines,chemokines and the mechanism of AMPs secretion are not very clearly described in this review, which need to be explored and perfected in the future.
Besides keratinocytes,other nonmyeloid cells in the skin like dermal adipocytes, fibroblasts, melanocytes, and sebocytes are proved to participate in innate immune responses against pathogens.43,56-57Recently, adipocytes are illustrated to produce cathelicidin AMP against S.aureus and dermal fibroblasts have the proficiency to locally differentiate into adipocytes to defend against S.aureus.56Melanocytes in epidermis express melanin against C. albicans.57In light of these observations, the skin as an important protective barrier for human body,keratinocytes and many other component cells of which are shown to participate in this anti-infection process.The elucidation of correlated mechanisms and the involved molecules remain to be studied further.
Acknowledgements
This work was supported by the CAMS Innovation Fund for Medical Science (No. 2017-I2M-1-017), the National Natural Science Foundation of China (No. 81773338),and the Nanjing Incubation Program for National Clinical Research Center (No. 2019060001).
杂志排行
国际皮肤性病学杂志的其它文章
- Instructions for Authors
- Platelet-Rich Plasma for Androgenetic Alopecia in Women: A Single-Center Case Series Study in Qatar
- Intracranial Abnormalities of Infantile Hemangiomas in the Head and Neck Regions:A Retrospective MRI Study
- Efficacy and Safety of Prednisolone Monotherapy Versus Prednisolone Plus Methotrexate in Erythema Nodosum Leprosum(Type 2 Lepra Reaction)
- The Past 70 Years in Control of Syphilis in China: Elimination and Responses to Resurgence
- Hair Matrix Cyst
