Efficacy and Safety of Prednisolone Monotherapy Versus Prednisolone Plus Methotrexate in Erythema Nodosum Leprosum(Type 2 Lepra Reaction)
2021-01-09ZahidulHasanMohammadRafiqulMowlaDebashishMondolAngkurMdIsmailKhan
Zahidul Hasan, Mohammad Rafiqul Mowla,∗, Debashish Mondol Angkur, Md. Ismail Khan
1Department of Dermatology and Venereology, Chittagong Medical College, Chittagong 4203, Bangladesh; 2Department of Pharmacology, Chittagong Medical University, Chittagong 4203, Bangladesh.
Abstract
Keywords: erythema nodosum leprosum, type 2 lepra reaction, methotrexate, prednisolone, Bangladesh
Introduction
Erythema nodosum leprosum(ENL),also known as type 2 lepra reaction(T2R),is an immune-mediated inflammatory complication affecting about 50% of patients with lepromatous leprosy and 10%of patients with borderline lepromatous leprosy.1-3It is clinically characterized by the occurrence of small or large erythematous, evanescent nodules (hence the name “ENL”) with or without ulceration that are tender on palpation,4and it is associated with systemic symptoms such as fever,malaise,arthritis, iritis, neuritis, and lymphadenitis.5The inflammatory condition associated with T2R may cause significant morbidity and mortality if not treated in a timely manner. T2R is frequently associated with severe economic hardship, and affected patients are at risk of being pushed further into poverty because of disability.In most cases,ENL causes chronic or recurrent episodes of ill health over many years.6
Patients require high doses of corticosteroids to control their disease, leading to complications and a significant number of deaths associated with long-term use of these drugs.7Thalidomide, a possible alternative, is often unavailable because of restrictive regulations. The identification of other agents for controlling T2R and the development of a quantitative measure to assess the severity of ENL have been highlighted as high-priority research areas.8-9
Low doses of methotrexate(MTX)suppress the division of mononuclear cells and inhibit their response to interleukin 2, suppress neutrophil and monocyte chemotaxis in vitro and in vivo, and depress Langerhans cell activity and leukotriene B4 synthesis by neutrophils,10-11mechanisms that also contribute to the manifestations of T2R. Because steroids also exhibit most of these effects,synergistic action between MTX and corticosteroids is expected. A few case studies have shown successful treatment outcomes using MTX in patients with T2R.However, there is a paucity of well-designed studies focusing on the effects of MTX on T2R.12-14Considering this background, the present study was designed to compare the efficacy and safety of MTX with prednisolone versus prednisolone alone for patients with T2R.
Materials and methods
Patients collection
This open-label comparative clinical study was performed at the Department of Dermatology and Venereology,Chittagong Medical College and Hospital from September 2018 to December 2019. A predesigned structured case record form was used to collect the data.
Patients with T2R of both sexes aged 18 to 65 years were included Exclusion criteria: Patients with concomitant febrile illness, erythema nodosum associated with other disorders, or systemic symptoms but no typical ENLassociated skin nodules. Patients diagnosed with chronic hepatic, renal, hematological, or connective tissue disorders;patients with osteoporosis;pregnant/lactating women; severely ill and immunosuppressed patients such as those with human immunodeficiency virus/acquired immunodeficiency syndrome; and patients with internal malignancy. All women of childbearing age who were included in the study were on double contraceptives.
Nineteen available and eligible patients were included in the study. Patients were randomly assigned in a 1:1 ratio(block size of two)to one of the two treatment arms using a computer-generated randomisation list, and ten in the MTX + prednisolone group (Group A) and nine in the prednisolone monotherapy group (Group B). The study was approved by our institute’s ethics committee with ERC number of CMC/PG/2018/447, and all included patients provided written informed consent.
Treatment protocol
Patients in Group A (MTX + prednisolone) received three doses of a 2.5-mg MTX tablet(12hours apart)(7.5mg/week for 6 months)and prednisolone at 40mg/day with tapering over 3 months. The prednisolone was decreased and maintained according to the protocol in Table 1.
Patients in Group B (prednisolone monotherapy)received prednisolone at 40mg/day with tapering over 6 months. The dose was reduced and maintained according to the protocol in Table 1.
Patient evaluations
All patients in each group were observed for recurrence of leprosy reactions.All patients were evaluated at 7 days and 1,3,4,and 6 months after baseline and then for a further 6 months to check for any episode of recurrence. Each assessment consisted of focused questions about specific symptoms and adverse effects, laboratory investigations,and clinical examinations including measurement of weight and blood pressure. Laboratory investigations at each visit consisted of slit skin smears for the bacterial index, a full blood count, kidney and liver function tests,and measurement of the glucose concentration and erythrocyte sedimentation rate. The body temperature and nodule size were assessed,and the patients were asked about any side effects of the drugs. The reaction severity assessment score (RSAS)15was used for efficacy evaluation. The RSAS assessment included evaluation of skin abnormalities (number of nodules, inflammation of nodules, and edema), fever, forms of neuritis, changes in sensory and motor function assessed using Semmes-Weinstein monofilaments, and voluntary muscle testing.Scoring of the items in the“A-section”of the severity scale was weighted in such a way that a score of ≥3 on any individual item would trigger the diagnosis of a severe outcome event(reaction or nerve function impairment).In the“B-section,”a score of ≥2 triggered the diagnosis of a severe outcome event.Nerve function was assessed at each visit.The severity of T2R-related symptoms was graded as mild, moderate, or severe.

Table 1 Treatment protocol of patients with erythema nodosum leprosum in the two groups.
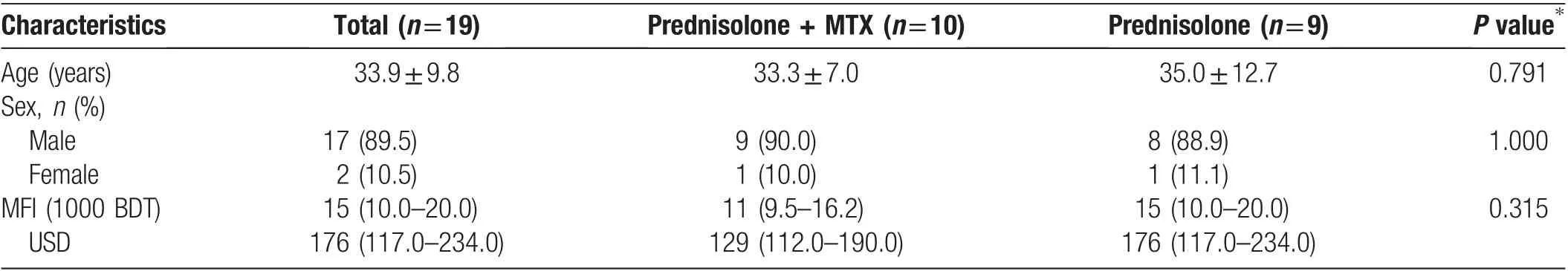
Table 2 Demographic characteristics of the patients with erythema nodosum leprosum.
Data analysis
The data were analyzed by SPSS software (SPSS version 23;IBM Corp.,Armonk,NY,USA).Continuous data are presented as mean ± standard deviation or median and interquartile range. Qualitative or categorical data are presented as frequency and proportion. Proportions were compared using the chi-square test or Fisher exact test as applicable. The unpaired t-test or Mann-Whitney U test was used for between-group comparisons as appropriate.The paired t-test or Wilcoxon signed-rank test was used to compare paired data.Statistical significance was defined as a P value of <0.05.
Results
Of these 19 patients,17(89.5%)were male and 2(10.5%)were female (male:female ratio of 8.5:1.0). The patients’mean age was 33.89±2.25 years (Table 2). Only three patients had a family history of leprosy.The median age at symptom onset was 32 years, and the median disease duration was 1 year. Three patients had comorbidities(diabetes mellitus in two and hypertension in one), but these were not likely to influence the course of T2R. The participants in each treatment arm were similar with respect to the Ridley-Jopling classification; one patient had borderline lepromatous leprosy (5.3%), and the remaining patients had lepromatous leprosy(94.7%).The multidrug therapy status was 4 (21.1%) at diagnosis, 11(57.9%)during treatment,and 4(21.1%)after completion of treatment(Supplemental Table 1,http://links.lww.com/JD9/A4). Weakness was the most prominent complaint(16[84.2%]patients),followed by tingling in 14(73.7%),sensory loss in 13(68.4%),and joint pain in 11(57.9%).The most prominent signs in both groups were nervetenderness (17 [89.5%] patients) followed by edema (8[42.1%] patients).

Table 3 Clinical presentation of the patients with erythema nodosum leprosum.
The mean number of nodules was 20 in both groups(Table 3). The mean size of the largest tender nodule at baseline was 20.8mm in Group A and 17.3mm in Group B, and that at the end of 6 months was 3.3 and 5.8mm,respectively (Fig. 1A). The RSAS was evaluated as an outcome measure in both groups at different follow-up schedules. In both treatment groups, the median RSAS significantly decreased from baseline to the end of treatment (P=0.005 and P=0.008 in Group A and B,respectively) (Table 4). There were no significant differences in the RSAS at different time points from baseline to 6 months after the start of treatment(Fig.1B).Episodes of T2R recurrence were observed at 4 months in three(15.78%) patients (one [5.26%] patient in Group A and two[10.52%]patients in Group B)(Fig.1C).A proportion of patients with chronic T2R were already experiencing side effects of prednisolone therapy at recruitment because they had been on prednisolone for varying lengths of time before study recruitment. Of the serious adverse events attributable to prednisolone, one patient developed diabetes requiring insulin and three patients were diagnosed with hypertension (Supplemental Table 2, http://links.lww.com/JD9/A5).
Discussion
This study was conducted with the intent to evaluate the efficacy and safety of oral MTX plus prednisolone versus prednisolone monotherapy in the treatment of T2R.
During our literature search using the Google search engine, we found a dearth of information regarding the efficacy and safety of MTX in patients with ENL. All available reports were case reports involving only very small numbers of patients.12-14The paucity of welldesigned studies makes it difficult to gather evidence for clinical practice. This study was intended to address this issue using a randomized comparsion study. Using a combination of prednisolone and MTX for a period of 6 months, we observed a steady, gradual improvement leading to persistent remission of T2R in all patients. No serious adverse effects were seen.

Figure 1. The treament effect on patients with erythema nodosum leprosum.(A)Comparison of mean sizes of the largest tender nodule in both groups at different time.(B)Comparison of mean reaction severity assessment score in both groups at different time.(C)Recurrence of T2R in both treatment groups (At 4th month). T2R: type 2 lepra reaction; MTX: methotrexate.
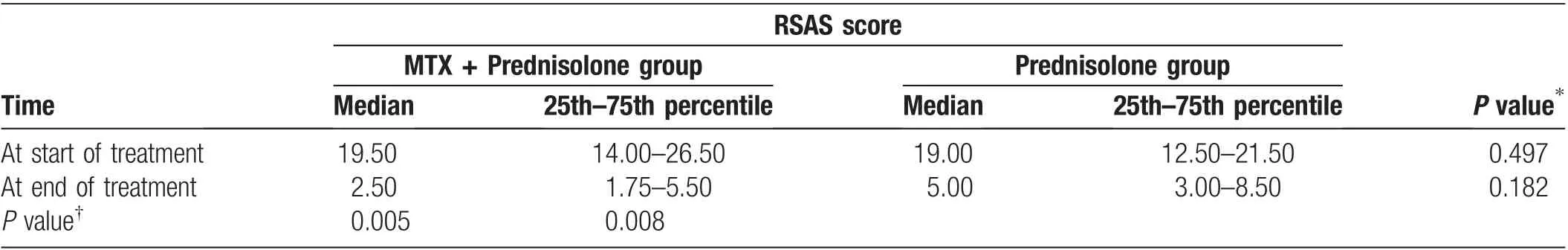
Table 4 Changes of RSAS in both groups in 6 months.
The current guidelines contain no recommendations regarding dose regimens for MTX in combination with prednisolone for ENL. We followed the dosage regimen described in a previously reported case study.13The mean age of the patients in the present study was 33 years.This is similar to other studies, in which the mean age was 34 years.14
The main outcome variable of the present study was the RSAS.This score may play an important role in informing clinical decisions regarding reactions, treatment, and monitoring.15Kar and Babu13reported that a single patient with steroid-resistant T2R in India developed no new ENL lesions after starting additional MTX treatment.In Bangladesh,Hossain14reported that the combination of prednisolone and MTX for 24 to 36 months (mean duration of 30 months)resulted in improvement in all nine patients in their study with persistent remission of T2R.Another study showed that MTX induced and maintained remission in all three patients for 24,24, and 18 months,respectively.12Based on the findings in these case reports,we can hypothesize that prednisolone requires 15 months to bring complex T2R under control and that a similar period of treatment is required to prevent later relapse.The possible role of MTX in controlling T2R and preventing recurrence is unknown. The follow-up period in the present study was short.The study endpoint was 6 months from the start of treatment. During this short period, we observed a significant and uniform reduction in both treatment groups as assessed by the RSAS.
Recurrence episodes occurred in three(15.78%)patients in the present study: two (10.52%) in the prednisoloneonly group and one(5.26%)in the combined prednisolone and MTX group.All three cases of recurrence occurred 4 months after starting treatment. These patients were managed with the addition of acetaminophen and indomethacin (75mg twice daily) for 1 week. An antiulcer agent(omeprazole,20mg twice daily)and vitamin B complex were also given.The two(10.52%)patients in the prednisolone-only group still developed recurrent episodes of T2R and exacerbations after discontinuation of prednisolone.These patients were followed up and treated in our Leprosy Clinic with an increased dose of prednisolone according to the World Health Organization standard protocol. Conversely, the patients in the combined prednisolone and MTX group did not develop recurrence of T2R.
Randomization in this study was effective;there was no significant difference in age, sex, or Ridley-Jopling classification between the patients in the two treatment groups. At recruitment, six of 19 patients were graded as having severe T2R,with ulcerated ENL nodules occurring in 25%of all patients.There was no significant difference in the frequency of extracutaneous manifestations of T2R between the two groups. Bone pain and neuritis were the most common manifestations, followed by peripheral edema. Testicular tenderness was present in half of the male patients. These T2R characteristics were in agreement with other studies.16-17Walker et al.18found that 19.8% of patients had a fever at the time of study recruitment, which is also consistent with our finding(21.1%).
We found no serious clinical or laboratory adverse effects attributable to either treatment regimen. Minor adverse events such as nausea,vomiting,abdominal pain,and headache occurred with the same frequency in both groups and subsided in all patients with symptomatic treatment.However,adverse events directly attributable to prednisolone, such as weight gain, moon face, buffalo hump, muscle wasting, and striae distensae, were more prominent in the prednisolone-only group (without statistical significance, probably because of the small sample size). Moreover, hypertension and diabetes mellitus were observed in three (33.3%) and one(11.1%) patient, respectively, in the prednisolone-only group.Similarly,one study of three patients with resistant T2R revealed no clinical or laboratory adverse events attributable to MTX therapy; however, prednisoloneinduced cushingoid features,striae distensae,gastritis,and weight gain were present in all patients,and hypertension and a deranged blood glucose concentration were present in two patients.12One case series also showed no serious clinical or laboratory adverse effects attributable to treatment.14
T2R is a complicated phenomenon.It is not yet possible to predict which patients will develop T2R, how severe their condition will be, and how long they will require treatment. T2R is often chronic and recurrent in nature.Although most available immunosuppressant medications may work similarly to control the acute symptoms of T2R,prevention of recurrence is far more difficult.Considering the literature and the present study, MTX plus prednisolone may be an effective alternative treatment regimen of T2R. MTX can be used as an add-on therapy for sustainable remission and to limit recurrence, especially when long-term use of steroids is contraindicated.However, more studies of this regimen are needed.
The major limitation of this study is that the sample size was small. This was a single-center study,and all patients were of a single ethnicity.This study also had a short followup;thus,we cannot predict the durability of the response or adverse effects of the regimen over the long term.Moreover,this study was not blinded,and this might have led to biases in recruitment of the patients or analysis of the results.
In conclusion, the present study showed that the combination of MTX plus prednisolone is promising in the management of T2R.This treatment regimen was safe and effective in managing T2R.The steroid-sparing effect of the regimen was not significant,probably because of the small sample size and short study period. Nevertheless,MTX may be added to the treatment of T2R to minimize the toxicity of steroids. Methotrexate may provide a cheaper and more convenient option in the management of T2R to maximize patients’ life expectancy and improve their quality of life, particularly in areas where the use of thalidomide is forbidden. Therefore, a well-controlled multicenter randomized controlled trial is recommended for validation of MTX plus prednisolone in the management of T2R.Additionally,studies with a longer follow-up are required to extrapolate these results to T2R populations in other parts of Bangladesh and the world.
Acknowledgement
The authors thankfully acknowledge the assistance of Mr.Sultan Md Elias Uddin. Program Manager, Leprosy control project, Chittagong.
杂志排行
国际皮肤性病学杂志的其它文章
- Instructions for Authors
- Platelet-Rich Plasma for Androgenetic Alopecia in Women: A Single-Center Case Series Study in Qatar
- Intracranial Abnormalities of Infantile Hemangiomas in the Head and Neck Regions:A Retrospective MRI Study
- The Past 70 Years in Control of Syphilis in China: Elimination and Responses to Resurgence
- Hair Matrix Cyst
- General Interest in Rosacea in the United States and China: A Search Engine-Based Pilot Study
