Platelet-Rich Plasma for Androgenetic Alopecia in Women: A Single-Center Case Series Study in Qatar
2021-01-09MohamedAhmedSyedandSamyaSamiAbushaikha
Mohamed Ahmed Syed∗and Samya Sami A.S. Abushaikha
1Department of Clinical Research, Primary Health Care Corporation, P.O. Box 26555 Doha, Qatar; 2Department of Dermatology,Rumailah Hospital, P.O. Box 3050 Doha, Qatar.
Abstract
Keywords: androgenetic alopecia, case series, platelet-rich plasma, Qatar
Introduction
Androgenetic alopecia (AA) presents clinically as the gradual replacement of thick terminal hairs with miniaturized,vellus hairs in well-recognized patterns.1The hair loss patterns differ between men and women, with some overlapping characteristics. In men, the classic pattern of hair loss begins above the temples and on the vertex of the scalp, while the typical pattern in women is diffuse thinning without hairline recession, rarely leading to complete hair loss.1-2
A variety of AA treatments have been investigated,including topical products, oral supplements, low-level laser therapy, and hair transplantation; however, the results are mixed and have generally not been studied rigorously.1There is emerging evidence and growing interest in the use of platelet-rich plasma(PRP)to treat hair loss. PRP is also referred to as autologous platelet gel,plasma rich in growth factors, and platelet concentrate,and is essentially an increased concentration of autologous platelets suspended in a small amount of plasma after centrifugation.3It is hypothesized that growth factors released from platelets may act on stem cells in the bulge area of the follicles, stimulating the development of new follicles and promoting neovascularization.4
The aim of the present study was to describe a singlecenter experience using PRP to treat AA in women. The study objectives were to evaluate the clinical effectiveness and safety of PRP for AA treatment.
Materials and methods
Study population
A retrospective observational study design was employed.Women who presented at the Department of Dermatology of Rumailah Hospital, Hamad Medical Corporation(Doha, Qatar), and met the following criteria were included in the study: (1) >18 years of age and in good general health;(2)Diagnosed with AA by a dermatologist;(3) Minimal or no improvement after treatment with topical minoxidil for at least 6 months. Patients were excluded if they were diagnosed with hematological disorders, thyroid dysfunction, malnutrition, or other dermatological disorders contributing to hair loss.
A total of 20 patients were included between April 2018 and January 2019. The mean age was 41.2 years (range 21-65 years).
The study was approved by the Medical Research Center of the Hamad Medical Corporation(MRC-01-18-468).
Study intervention
Approximately 18 mL of whole blood was taken from a peripheral vein,and 2 mL of anticoagulant was added.The blood was then introduced into a commercially available PRP kit(Dr.PRP Kit).The plasma and red blood cells were separated by centrifugation for 3 minutes at 3000rpm. If the first centrifugation did not achieve an adequate volume of plasma or adequate separation, the blood was centrifuged for an additional 1-2 minutes at 3000rpm.A second centrifugation was done for 6 minutes at 3200 rpm to enrich the concentrated platelet solution.
For each patient, the scalp was divided into four areas(vertex,right,left,and posterior areas)and cleansed with 70% alcohol; local anesthesia was not injected in the treated areas. PRP (0.1mL/cm2) was injected in selected areas of the scalp. Each patient received six treatment sessions at 4-week intervals.
Efficacy assessment
The severity of alopecia tool (SALT) scoring system is a validated tool used to measure the severity of alopecia.4Pre-and post-intervention SALT scores were compared to measure the efficacy of the intervention.
SALT score is the sum of percentage of hair loss in all below mentioned areas.The percentage of hair loss in any of these areas is percentage hair loss multiplied by percent surface area of the scalp in that area.The scalp is divided into four areas namely:(1)Vertex-40%of scalp surface area;(2)Right profile of scalp-18%of scalp surface area;(3) Left profile of scalp - 18% of scalp surface area; (4)Posterior aspect of scalp - 24% of scalp surface area.
All measurements were under taken and recorded at baseline and 1 month after the completion of all six treatment sessions, and the mean pre-intervention SALT score was 27.75±6.35.
Adverse effect assessment
Each treatment session was followed by a 1-hour observation for treatment-related adverse effects. Patients were also instructed to contact the Department of Dermatology if they experienced any adverse effects between sessions. All adverse reactions were recorded.
Statistical analysis
Data are reported as mean±standard deviation for continuous variables. All statistical tests were two-sided,and differences were considered statistically significant at an α level of 0.05. A paired t-test was used to calculate significance levels using the Statistical Package for the Social Sciences statistical software package (version 22).The percentage changes in the mean values were calculated for the total SALT score and for the SALT score in each scalp area. Cohen d was used to calculate the effect sizes.
Results
Clinical efficacy
Between April 2018 and January 2019,20 patients met the inclusion criteria and received PRP injections. Follow-up results were available for all 20 patients at 4-6 weeks after the completion of six treatment sessions.
The mean pre-intervention and post-intervention total SALT score was 27.5±6.35 and 9.41±3.71, respectively(P ≤0.05, Table 1). The post-treatment difference in the total mean SALT score was 18.33±1.64(giving a 33.94%change in the mean SALT score), and the effect size was 3.52.
There were also significant post-treatment improvements in individual scalp areas.The mean pre-intervention SALT scores for the vertex,right,left,and posterior areas were 15.40±4.90, 4.32±1.79, 4.41±1.79, and 3.48±1.64, respectively, and the mean post-intervention SALT scores were 5.32±2.75,1.62±0.85,1.53±0.77,and 0.95±0.90,respectively(all P ≤0.05,Table 1).The difference in the pre- versus post-intervention SALT score and the effect size of the intervention were similar for the right,left,and posterior areas(difference range: 2.53±0.42 to 2.88±0.43; effect size range: 1.91-2.09), and were slightly higher for the vertex (difference: 10.08; effect size: 2.53).
Adverse effects
There were no treatment-related adverse effects such as scarring, progressive worsening of hair loss, or infection.Some participants reported mild headache, tolerable and temporary pain during treatment, mild pruritus and desquamation, and transient edema after PRP treatment.
Discussion
The present results demonstrated that PRP was effective in women,who received six treatment sessions with an intertreatment interval of 4-6 weeks. The total SALT score significantly decreased after PRP treatment by (18.33±1.64).Previous studies that used SALT scores to assess the effectiveness of PRP have reported a decrease of 18.12(from 38.9 to 20.78) with four PRP sessions6and a decrease of 10.82 (from 36.41±16.41 to 25.59±20.54,P<0.001) with 1-5 PRP sessions.7The variation in the number of sessions may have contributed to the difference in the effectiveness of PRP between the studies.
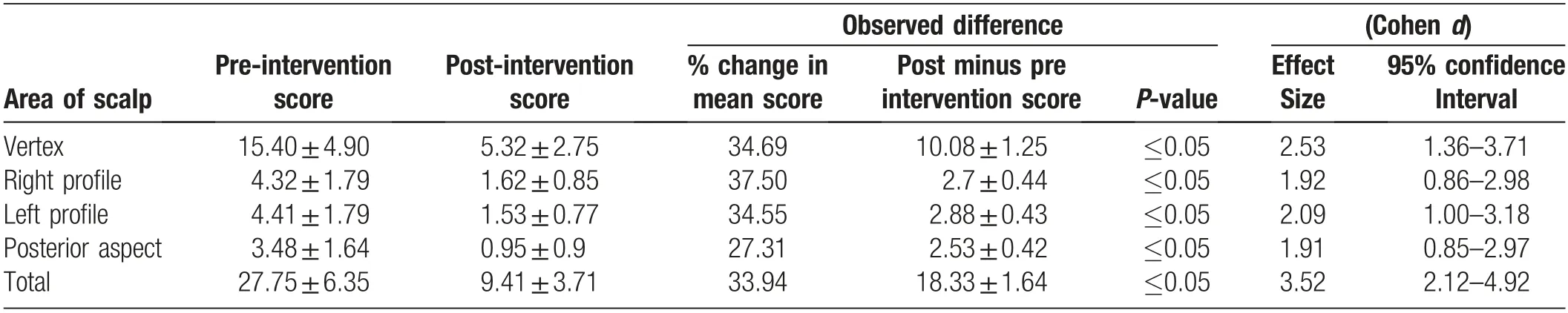
Table 1 Comparison of pre- and post-intervention severity of alopecia tool (SALT) scores for scalp area, mean±SD.
The present study findings provide information on the efficacy of PRP in each area of the scalp.The largest effect size was noted in the vertex, while the effect size was similar across the other areas.No other study has reported the specific findings in each area of the scalp. However,two studies reported that PRP achieved better results than minoxidil therapy.3,8
Several recent studies have reported the efficacy of PRP in treating AA.Recent systematic reviews reported that the majority of these studies demonstrated that PRP is effective as a treatment for AA.9-11However,two studies reported a lack of improvement after PRP treatment.12-13The published studies show that there is a lack of a standardized PRP treatment protocol and standardized evaluation methods.9-10
There were no serious adverse effects of the PRP treatment in the present study; the only adverse effects were mild and transient. These findings are in line with previous studies.9-11This lack of serious adverse effects is an advantage of PRP.
The present study had many strengths,such as the use of a standard PRP kit,the use of a validated assessment tool,and the reporting of results by scalp area. However, the present study also had some limitations: The sample size was small and the follow-up period was short. Given the slow rate of hair growth,a follow-up of 6 months does not enable the evaluation of the long-term efficacy of the intervention,particularly after the intervention is stopped.
Overall,the present study demonstrated the efficacy and safety of PRP injections in treating AA in women,and may provide a viable alternative to existing treatment options of AA.However,the present findings require confirmation in well-designed studies using standardized treatment protocols and evaluation methods.Studies comparing PRP therapy with existing treatments are also needed before PRP can be recommended as the gold standard for the treatment of AA.
杂志排行
国际皮肤性病学杂志的其它文章
- Instructions for Authors
- Intracranial Abnormalities of Infantile Hemangiomas in the Head and Neck Regions:A Retrospective MRI Study
- Efficacy and Safety of Prednisolone Monotherapy Versus Prednisolone Plus Methotrexate in Erythema Nodosum Leprosum(Type 2 Lepra Reaction)
- The Past 70 Years in Control of Syphilis in China: Elimination and Responses to Resurgence
- Hair Matrix Cyst
- General Interest in Rosacea in the United States and China: A Search Engine-Based Pilot Study
