Clinical correlates of hypotension in patients with acute organophosphorus poisoning
2021-01-06NingDongZhexiLuXingliangLiWeiLiLiPangJihongXing
Ning Dong, Zhe-xi Lu, Xing-liang Li, Wei Li, Li Pang, Ji-hong Xing
1 Department of Emergency, the First Hospital of Jilin University, Changchun 130021, China
2 School of Medicine, Pennsylvania State College of Medicine, Hershey 17033, USA
Corresponding Author: Li Pang, Email: pangli211@163.com; Ji-hong Xing, Email: jhxing1@qq.com
KEYWORDS: Acute organophosphate poisoning; Hypotension; Cholinesterase inhibitor;Cardiovascular complication; Shock
INTRODUCTION
Hypotension is a common complication of severe acute organophosphorus poisoning (AOPP).[1-3]The mechanism of development of hypotension in patients with AOPP is not fully elucidated. Some studies have found a direct effect of organophosphorus pesticides(OPs) on the cardiovascular system resulting in the decrease in peripheral resistance and impairment of cardiovascular regulation by the central nervous system(CNS).[4-7]It has also been shown to be an independent predictor of mortality in AOPP patients.[8]Despite the adverse prognostic impact of hypotension, its clinical correlates and associated outcomes have not been investigated in a large and unselected cohort of AOPP patients.
METHODS
Study design
This was a retrospective cohort study of patients with AOPP who were treated at two hospitals in the period between January 1, 2013 and December 31, 2018. The hospital is a referral center for patients with acute poisoning in the Jilin Province. Every year, 500-800 patients with severe poisoning(accidental or intentional) are treated at this hospital.
Diagnosis of AOPP and hypotension
Due to the retrospective study design, the diagnosis of AOPP was based on the documented history of oral intake of OPs and plasma cholinesterase (pChE) level<4,300 U/L at admission, in addition to the characteristic clinical manifestations.
The diagnostic criteria for hypotension were blood pressure less than 90/60 mmHg (1 mmHg=0.133 kPa)and a history of administration of vasopressors, including dopamine or norepinephrine.
Management of AOPP patients
Due to the retrospective nature of this study, the treatment protocol was not standardized. The treatment of individual patients was decided by the treating physician. The typical protocol for AOPP patients was brief ly described here.AOPP patients who arrived within 4 hours after OP intake were subjected to gastric lavage with warm water in the absence of any contradictions. Intravenous and intramuscular injections of atropine were used to achieve and maintain the atropinization of patients until the pChE level increased to>2,300 U/L. The usage of oximes depended on the type of OPs. Hemoperfusion was used for decontamination according to the physician’s recommendation, especially in patients with severe poisoning. Hypotensive patients were treated with hemoperfusion only if they remained hemodynamically unstable after treated with vasopressors. Atropinization was described as heart rate >80 beats per minute, clear chest, and cessation of sweating.[9]
Inclusion and exclusion criteria
The inclusion criteria were: (1) patients aged >18 years who qualified the diagnostic criteria for AOPP; (2)patients hospitalized within 24 hours of exposure to OPs.Patients with any of the following features were excluded:(1) patients with poisoning with other drugs in addition to OPs; (2) patients with a normal pChE level (≥4,300 U/L) at admission.
Data collection
All data were retrieved from the electronic in-hospital database of the two referral hospitals. A specially designed questionnaire was used for data collection, which included age, sex, past medical histories (hypertension, diabetes,chronic obstructive pulmonary disease [COPD], chronic kidney disease [CKD], and liver cirrhosis), laboratory tests(white blood cell [WBC] count, pChE, blood urea nitrogen[BUN], albumin, serum amylase, blood pH, and blood lactic acid), cardiac arrest, and usage of hemoperfusion and ventilation. The main outcome was in-hospital mortality.All data were independently collected by two physicians.Any ambiguity with respect to any variable was resolved by consensus. Patients were categorized into two groups depending on the presence or absence of hypotension. The hypotensive group comprised of patients who developed hypotension either before admission or within 24 hours after admission; the remaining patients were categorized as the non-hypotensive group.
Statistical methods
Continuous variables were presented as mean (standard deviation) or median (25th-75thpercentile) and betweengroup differences assessed using the Student’st-test for normally distributed variables or Mann-WhitneyU-test for non-normally distributed variables. Categorical variables were presented as frequency (percentage) and compared using Chi-square test or Fisher’s exact test, as appropriate.Missing variables (including serum amylase, WBC, pH,and lactic acid levels, with relative missing rates of 17.9%,3.9%, 4.2%, and 4.2%) were imputed 50 times by chained equations. Univariable logistic regression was used to identify variables associated with hypotension; variables associated withPvalues <0.2 were included in multivariable logistic regression. Multivariable logistic regression was fitted by a backward step-wise method. Survival rates of patients with and without hypotension were compared using the Kaplan-Meier survival method and log-rank test.A univariable Cox proportional hazards model was used to identify parameters associated with mortality. We also used a multivariate proportional hazards model with L2-regulated and 10-fold cross-validation-based iterative determination of covariates to identify the most important parameters associated with mortality. Two-tailedP-values <0.05 were considered indicative of statistical significance. The R packages “survival”,[10]“forestplot”[11]and “glmnet”[12]were used for statistical analyses (R software version 3.6.0).
RESULTS
A total of 1,578 patients with AOPP were identified from the in-hospital databases of the participating hospitals;among them, 707 (44.8%) patients were excluded as they did not qualify the patient-selection criteria: 278 (39.3%)patients were admitted >24 hours after exposure to OPs;362 (51.2%) patients had concomitant poisoning with other drugs; 67 (9.5%) patients had normal pChE at admission.Finally, a total of 871 patients were included in the analysis.The baseline characteristics of patients from the two hospitals were analyzed and showed a signif icant difference with respect to WBC counts (P=0.016), levels of albumin(P=0.021), and levels of lactic acid (P=0.017).
Clinical characteristics of patients with and without hypotension
Out of the 871 patients, 143 (16.4%) had hypotension;all patients had developed hypotension before admission or within 24 hours after admission (Table 1). Hypotensive patients were significantly older (50.0 years old vs. 43.0 years old,P<0.001) and were more likely to be diabetic(10.5% vs. 4.0%,P=0.002) than non-hypotensive patients.Patients with hypotension showed signif icantly lower levels of pChE, albumin, and blood pH. Moreover, patients with hypotension showed significantly higher WBC count,serum amylase, and blood lactate acid. Cardiac arrest was significantly more common in patients with hypotension(0.5% vs. 6.3%,P<0.001). The proportions of patients who received hemoperfusion (48.5% vs. 86.0%,P<0.001)and ventilator support (5.4% vs. 76.9%,P<0.001) in the hypotension group were significantly greater than those in the non-hypotension group. Patients with hypotension experienced significantly higher in-hospital mortality rate than patients without hypotension.shown in Figure 2, three models were fitted according to the variables included in the analysis. Model 1 was fitted with demographic characteristics, past medical history, and treatment parameters. Model 2 was f itted with demographic characteristics and past medical history. Model 3 was f itted with variables that were associated withPvalues less than 0.05 in model 1. We observed a consistent association of hypotension with increased in-hospital mortality in all the three f itted models (Figure 2), as well as with male sex and diabetes. In the analysis of treatment parameters (model 1 and model 3), ventilation support was also associated with an increased risk of in-hospital mortality.
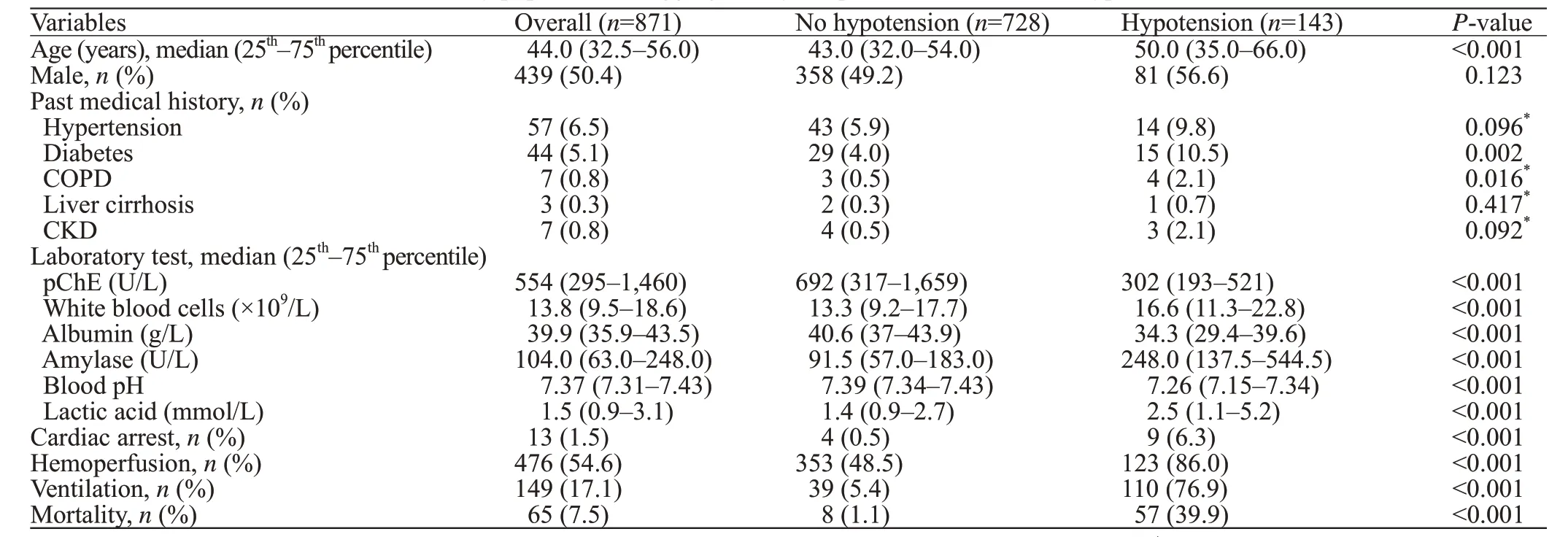
Table 1. Baseline characteristics of the study population disaggregated by the presence or absence of hypotension
Risk factors for hypotension in AOPP
On univariable logistic regression analysis, the following parameters showed an association with hypotension:advanced age; history of diabetes or COPD; cardiac arrest at presentation; decrease in pChE, plasma albumin, or blood pH; and increase in WBC count, serum amylase, and lactic acid level. Variables associated withPvalues less than 0.2 were included in multivariable logistic regression. After variable selection by backward step-wise method, advanced age (odds ratio [OR] 1.25, 95% confidence interval [CI]1.08-1.44), diabetes (OR2.65, 95%CI1.14-5.96,P=0.020),increased WBC count (OR1.06, 95%CI1.03-1.09,P<0.001), serum amylase (OR1.01, 95%CI1.01-1.02,P=0.008), decreased pChE (OR0.91, 95%CI0.84-0.94,P<0.001), plasma albumin (OR0.88, 95%CI0.85-0.92,P<0.001), and blood pH (OR0.64, 95%CI0.54-0.75,P<0.001) were associated with hypotension.
DISCUSSION
In this large cohort study, patients with AOPP who developed hypotension were found to be at a higher risk of in-hospital death. The adjusted hazard ratio for in-hospital mortality in patients with hypotension ranged from 8.77 to 37.06, depending on the existence of confounding factors(Figure 2). Our results are consistent with those of a previous study.[1]These findings indicate the need for appropriate treatment of patients who are at a high risk of hypotension.More studies are needed to develop strategies to decrease the mortality in hypotensive AOPP patients.
On multivariable logistic regression, several parameters were found to be associated with hypotension, including changes in laboratory parameters (WBC count, serum amylase, blood pH, and plasma albumin), advanced age,cardiac arrest, and history of diabetes. The association of advanced age and cardiac arrest with hypotension is entirely plausible, and has been reported earlier.[13]Previous studies have also identified increased serum amylase, increased WBC count, and blood pH as markers of poor prognosis in AOPP patients.[14-16]We believe that these changes in laboratory parameters indicate serious organ damage in
In-hospital mortality and risk factor analysis
Kaplan-Meier survival curves showed a significantly lower survival rate for AOPP patients who had hypotension(log-rank test,P<0.001) (Figure 1). We used the L2-regularized multivariate proportional hazards model to select parameters associated with in-hospital mortality. As severe AOPP other than risk factors for hypotension. Of note, diabetes mellitus was found to be a significant risk factor for hypotension and in-hospital death. The underlying mechanism of this association is not well characterized due to the paucity of evidence.

Figure 1. Kaplan-Meier survival curves of acute organophosphorus poisoning patients with and without hypotension.

Figure 2. Results of L2-regulated multivariable proportional hazards model showing risk factors for in-hospital mortality of AOPP patients.AOPP: acute organophosphorus poisoning; CKD: chronic kidney disease;CI: conf idence interval.
Patients with severe AOPP typically die due to irreversible cardiovascular failure, acidosis, and respiratory failure.[17]With the advances in respiratory management and intensive care, it is possible to maintain the respiratory function of patients with organophosphate poisoning.However, management of hypotension is challenging despite adequate administration of atropine. There is no specific treatment for hypotension in AOPP patients, other than the conventional therapy for shock. However, the conventional therapy is often ineffective in these patients. There is limited evidence available pertaining to the treatment of irreversible hypotension in AOPP patients.
OPs bind irreversibly to acetylcholinesterase in the cholinergic synapses of central and peripheral nerve systems;this results in overstimulation of nicotinic acetylcholine and muscarinic acetylcholine (mACh) receptors in the CNS,neuromuscular junctions, and autonomic nervous system.[18,19]Patients with severe AOPP may develop hypotension due to the overstimulation of muscarinic acetylcholine receptors in the parasympathetic system.[9]In previous studies, patients with severe organophosphate poisoning complicated by hypotension showed low total peripheral resistance and maintained cardiac output.[4,5]The use of catecholamines was ineffective and did not increase the systemic vascular resistance index.[5]This may be attributable to impaired cardiovascular regulation by the CNS through a complex mechanism and depend on the various brain sites and/or actions of different acetylcholine (ACh) receptors.For example, microinjection of mevinphos in the rostral ventrolateral medulla of anesthetized Sprague-Dawley rats induced progressive hypotension that was accompanied by an increase (phase I), followed by a decrease (phase II) in the experimental index of baroreflex-mediated sympathetic vasomotor tone, resulting in a fatality rate of 35%.[20]The activation of mACh receptors in the rostral ventrolateral medulla resulted in a significant increase in mean arterial pressure (MAP) and heart rate (HR),[21]whereas microinjection of ACh into the ventrolateral periaqueductal gray areas and cuneiform nucleus caused a significant depression in MAP with no striking alteration in HR.[22]ACh microinjection or muscarinic receptor activation in the cerebellar cortex was shown to induce marked downregulation of systemic MAP and HR.[6,7]These findings highlight the importance of CNS in the development of hypotension in AOPP patients.
In this context, we highlight the need for further study on the standards pertaining to atropinization in severe AOPP patients. Although atropine shows moderate penetration in the CNS, it is the mainstay of therapy for AOPP. For patients with hypotension, excess doses of atropine may be required to reverse their hypotension; this should take precedence over control of other muscarinic symptoms.[4]Thus, the routine criteria for atropinization may not be suitable for patients with severe AOPP. The correct standard of atropinization is to provide early and sufficient atropine to prevent hypotension.Future studies should evaluate the fundamentals of atropinization in patients with severe AOPP.
Study limitations
This was a retrospective observational study, and hence subject to incomplete or missing information. We did not include the types and doses of organophosphates in the analysis, because these data were either missing(data pertaining to specific types of organophosphates) or ambiguous (dose often mentioned as “half bottle” or “a few sips”). Data pertaining to the timing of occurrence of hypotension were not available for all patients; therefore,this aspect was not included in the analysis. Moreover, we included only data after admission, and many patients had received ventilation and vasopressor treatment prior to their hospital admission. Lastly, the retrospective study design did not permit any causal inferences.
CONCLUSIONS
Hypotension is a serious and common complication of AOPP and is associated with increased in-hospital mortality.Advanced age, history of diabetes, and deranged laboratory parameters were associated with hypotension in AOPP patients. Optimal management of hypotension is a key imperative to improve patient outcomes.
Funding:None.
Ethical approval:The study protocol was approved by the Medical Ethics Committee of the First Hospital of Jilin University (approval number: 2018-146).
Conf licts of interests: None.
Contributors:ND and ZXL contributed equally to this work. LP and JHX were involved in study conception, study design, and manuscript editing; ND and ZXL analyzed the data, reviewed the literature, and drafted the manuscript; XLL and WL prepared the data and validated the statistical results.
杂志排行
World journal of emergency medicine的其它文章
- Trends and challenges of emergency and acute care in Chinese mainland: 2005-2017
- Factors associated with refractory pain in emergency patients admitted to emergency general surgery
- Identifying critically ill patients at risk of death from coronavirus disease
- Effects of viral infection and microbial diversity on patients with sepsis: A retrospective study based on metagenomic next-generation sequencing
- Effects of metabolic syndrome on onset age and long-term outcomes in patients with acute coronary syndrome
- Predictors of recurrent angina in patients with no need for secondary revascularization
