Self-spreadable Octopus-like Electrode Arrays for Long-term Neural Recordings
2020-12-23LuluWangZexinXieChengZhongYongqiangTangFengmingYeLipingWangYiLu
Lulu Wang ,Zexin Xie ,Cheng Zhong ,Yongqiang Tang ,2,Fengming Ye ,2,Liping Wang ,*,Yi Lu ,*
1 The Brain Cognition and Brain Disease Institute,Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences;Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions,Shenzhen 518055,Guangdong Province,P.R.China.
2 Shenzhen College of Advanced Technology,University of Chinese Academy of Sciences,Shenzhen 518055,Guangdong Province,P.R.China.
Abstract:Neural electrodes have been extensively utilized for the investigation of neural functions and the understanding of neuronal circuits because of their high spatial and temporal resolution.However,long-term effective electrophysiological recordings in free-behaving animals still constitute a challenging task,which hinders longitudinal studies on complex brain-processing mechanisms at a functional level.Herein,we demonstrate the feasibility and advantages of using a selfspreadable octopus-like electrode (octrode)array for long-term recordings.The octrode array was fabricated by enwrapping a bundle of eight formvar-coated nickel-chromium microwires with a layer of polyethylene glycol in a custom-made mold.After the electrodeposition of platinum nanoparticles,the microwires at the electrode tip were gathered together and then re-enwrapped with a thin layer of gelatin to maintain their structure and mechanical strength for implantation.Shortly after implantation (within 20 min),the biocompatible gelatin encapsulation swelled and dissolved,causing the self-spreading of the recording channels of the octrode array in the brain.The electrochemical characteristics of the electrode/neural tissue interface were investigated by electrochemical impedance spectroscopy (EIS).Four weeks after implantation,the average impedance of the octrodes (1.26 MΩ at 1 kHz)was significantly lower than that of the conventional tetrodes (1.50 MΩ at 1 kHz,p < 0.05,t-test).Additionally,the octrodes exhibited a better pseudo-capacitive characteristic and a considerably faster ion transfer rate at the electrode interface than the tetrodes.Spontaneous action potentials and local field potentials(LFPs)were also recorded in vivo to investigate the electrophysiological performance of the octrodes.The peak-to-peak spike amplitudes recorded for the octrodes were remarkably larger than those recorded for the tetrodes.The signal quality remained at approximately the same level for the four-week period,while the peak-to-peak spike amplitudes recorded for the tetrodes decreased abruptly.Moreover,the voltage amplitudes recorded by the octrodes at 1-200 Hz were notably larger than those by the tetrodes,suggesting a higher sensitivity in the recording of electrophysiological events.Furthermore,we performed immunochemical analyses on the brain tissues at post-implantation to evaluate the histocompatibility of the electrodes.Tissue responses of the octrodes were alleviated considerably,evidenced by the reduced astroglial intensity and increased neuron density around the implant site as compared to the tetrodes,which may be due to the relatively small size of each decentralized recording channel after self-spreading in vivo.Generally,the fabricated octrodes exhibited a lower electrochemical impedance value at the octrode/neural tissue interface and an increased signal quality during the long-term electrophysiological recording in freely moving mice as compared to the conventional tetrodes.All of these are desirable characteristics in neural circuit dissections in vivo.
Key Words:Neural electrode; Neural interface; Electrode impedance; Tissue response; Electrophysiological recording
1 Introduction
Implantable neural electrodes constitute a powerful tool for the detection of the electrophysiological activity of individual neurons with high-spatiotemporal resolution1,2.Accordingly,these are critical for the dissection of specific neural circuits,and for the investigation of certain neuropsychiatric disorders,including epilepsy3,Parkinson's disease4,autism5,and emotional disorders6,7.To-this-date,the temporally dependent signal deterioration of these electrodes still constitutes a major bottleneck for their functionality in vivo,and has limited the number of longitudinal studies on complex brain processing mechanisms at a functional level in free-behaving animals.
The neural electrode is recognized as a bridge that can transduce electrical signals from biosignals,and is thus used for various experimental purposes8,9.In many cases,electrophysiological studies in freely moving animals have been considered necessary for the understanding of the intrinsic neural basis of specific behavioral phenomena.It has been generally accepted that the inconsistent performance of neural electrodes during long-term implantation is probably attributed to inflammatory responses10,11that result in a dense astroglial encapsulation of the implant that isolates the electrode from the targeted neurons,and thus hinders charge transfer at the electrode/neural-tissue interface12,13.This has led to a dramatic deterioration of the electrochemical performance (decreased capacitance and increased impedance)of the neural electrodes and to a subsequent decrease in the signal-to-noise ratio (SNR)response.
To alleviate the inflammatory response caused by neural implants,considerable efforts have been devoted to improve the biocompatibility of the electrode/neural interface based on various modification strategies,including surface grafting or coating using tissue-friendly macromolecules or polymers14-16,controlled release of anti-inflammatory drugs or bioactive factors17,and construction of micro- or nanostructures18,19.These studies have demonstrated the advantages at the electrode/neural interface,nevertheless,the minimization of the tissue responses evoked by the implant itself,such as insertion traumas and mechanical mismatches between the electrode and neural tissue,has not been fully resolved.
It has been reported that the mechanical mismatch between the stiff electrode and soft tissue may lead to neural implant micromotions that exacerbate the inflammatory response and deteriorate the signal quality during recording20,21.Decreasing the bulk size of individual electrodes can not only increase spatial precision and minimize insertion trauma,but also reduces the bending stiffness of the implants,and thus promote the mechanical compatibility between the electrodes and surrounding tissues22,23.In addition,owing to their relatively small surface areas,microelectrodes usually result in diminished molecular accumulation and protein adsorption that further alleviate the inflammatory responses in the central nerve system24,25.On the basis of these merits,injectable microelectrode arrays26,27and neurotassels28have been developed that exhibit significantly improved biocompatibility and are able to record the activity of targeted neurons in a stable,temporal manner.However,novel neural electrode arrays fabricated with simplified and costeffective manufacturing methods still require additional investigations.Therefore,in this work we developed a simplified method for the fabrication of self-spreadable octopus-like electrode (octrode)arrays and investigated their advantages during long-term implantation in vivo.
2 Experimental
2.1 Fabrication of electrode arrays
An octopus-like electrode (octrode)array was fabricated by enwrapping a bundle of eight formvar-coated nickel-chromium microwires (diameter 12.7 μm,Stablohm 650,California Fine Wire,USA)with a layer of polyethylene glycol (PEG,MW=4000 g·mol-1,Sigma-Aldrich,USA)in a custom-made mold.The PEG-supporting layer was applied to enhance the mechanical strength of the octrode prior to its use (Supporting information,Fig.S1).Two tetrodes were constructed following the twisting of four microwires,and were enwrapped with a thin layer of PEG and gelatin which served as the control (Supporting information,Fig.S2).
2.2 Electrochemical modification and characterizations
The fabricated electrode arrays were mounted in a threeelectrode cell with a saturated calomel electrode (SCE)as the reference electrode and a large-area platinum electrode as the counter electrode.Enwrapped PEG supporting layers on the electrode tips were dissolved in artificial cerebrospinal fluid(ACSF)prior to testing.An aqueous solution which contained 5 mmol·L-1PtCl4and 100 mmol·L-1HCl was prepared for electrodeposition.Platinum nanoparticles were deposited on the electrodes in the deposition solution at a potential of -0.25 V (vs SCE)which was applied by a potentiostat (Reference 600,Gamry,USA).Electrochemical impedance spectra (EIS)of the electrodes were measured at their open-circuit potentials in ACSF using a potentiostat with a 25 mV AC sinusoid signal in the frequency range of 100 kHz to 1 Hz.The electrode tips were washed with ACSF three times after the test.The microwires at the electrode tip were then gathered together and were reenwrapped with a thin layer of gelatin (Sigma-Aldrich,USA)to maintain their architecture and mechanical strength for implantation.
2.3 Implantation and in vivo characterizations
All experiments were performed in accordance with the protocols approved by the Ethics Committee for Animal Research,Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences.Wild-type male,eight-week-old C57BL/6J mice were used for in vivo studies.A hole was carefully drilled at anteroposterior -2.06 mm,mediolateral-1.25 mm.The fabricated electrode array was slowly lowered into the hippocampus at the dorsoventral location of -2.0 mm,and was cemented in place with dental acrylic.The animals were allowed to recover for seven days before additional recordings were conducted.
The EIS for each implanted electrode array was obtained with a potentiostat (Reference 600,Gamry,USA)with two stainless steel bone screws which served as reference and counter electrodes,respectively.Electrophysiological recordings were performed with a 64-channel neural acquisition processor(Plexon,USA)in freely moving mice.Neural electrophysiological data acquired in all recording channels were sampled at 40 kHz and bandpass filtered in the range of 300-5000 Hz.Individual spikes were detected by setting a threshold at -5× standard deviations (SD),and were measured within a 1400 μs time window.Single units were isolated using the first three principal components,and the SNR of the sorted data were then estimated using methodologies reported in previous studies14,29.The RMS(Root mean square)noise is obtained by subtracting the mean waveform from each individual waveform.
At four weeks after implantation,the mice were sacrificed and horizontal sections (35 µm thick)were prepared.Antibodies against GFAP (Glial fibrillary acidic protein,Abcam,USA)and NeuN (Neuronal nucleus,Abcam,USA)were used to label astrocytes and mature neurons,respectively.Fluorescence images were obtained using an Olympus VS120 microscope.Quantitative analyses were performed with custom routines developed in MATLAB (MathWorks,USA)30-32.The staining intensity of GFAP and NeuN were calculated as a function of distance to the center of the implant site.
3 Results and discussion
3.1 Electrode fabrication and electrochemical characterizations
To minimize the stiffness of the implant and trauma to tissue adjacent to the recording site,a self-spreading,octopus-like electrode (octrode)array was designed and fabricated (Fig.1a,b; Supporting information,Fig.S3).All recording channels(microwires)were gathered together using PEG and gelatin,which significantly strengthened the rigidity of the octrode(Supporting information,Figs.S1a-c,S4).After the gelatincoated octrode tip was inserted into the brain tissue,the supportive PEG layer outside the skull was dissolved using ACSF.The biocompatible gelatin coating swelled quickly and dissolved (Supporting information,Figs.S5-S6),and the gathered microwires self-spread at the implantation site(Supporting information,Figs.S1d,S7,S8).As tetrode technology has been extensively used in neural recordings,an eight-channel tetrode array was also fabricated as a control.
We then used EIS to investigate the electrochemical characteristics of the electrode/neural tissue interface.Prior to implantation,the impedance of the electrodes was reduced by Pt electrodeposition (Supporting information,Fig.S9),and no significant difference was observed between the tetrode and octrode groups.However,after a four-week implantation,the average impedance of the tetrodes tested in vivo was 1.50 MΩ.This value was considerably higher than the impedance of the octrodes (1.26 MΩ,at 1 kHz; Fig.1c).This impedance difference may be attributed to the different ionic transformation properties at the electrode/neural tissue interface.

Fig.1 EIS characterizations of electrode arrays in vivo.
To verify this point,we proposed an equivalent circuit model14,31,33-35(Supporting information,Table S1),which was comprised of circuit elements,including the tissue resistance(RT),electrode-tissue constant-phase element (ZCPE-T),doublelayer constant-phase element (ZCPE-D),and finite-length Warburgh diffusion impedance (ZD).We found that the CPE-q values of the ZCPE-T(CPET-q)and ZCPE-D(CPED-q)in the octrode group were both significantly higher than those in the tetrode group (p < 0.05,t-test),while the CPE-n values of the ZCPE-T(CPET-n)and ZCPE-D(CPED-n)of these electrodes showed no significant difference.This suggests a better pseudocapacitive characteristic of the long-term implanted octrodes as compared to the tetrodes.Additionally,the diffusional resistance(RD)and the diffusional time constant (TD)of the octrodes were both markedly higher than those of tetrodes (p < 0.05,t-test),thus implying a considerably faster ion transfer at the electrode/neural tissue interface.Taken together,the long-term EIS study suggests that the octrode yields a much lower impedance in vivo,which may have benefitted from the superior electrochemical characteristics (high pseudo-capacitance and ionic transfer rate)and biocompatibility of the self-spreading octrode/neural tissue interface.
3.2 Electrophysiological characterizations
To quantitatively compare the electrophysiological performance of octrodes and tetrodes,spontaneous action potentials and local field potentials (LFPs)were recorded in vivo.The quality of electrophysiological signals obtained using tetrode arrays deteriorated considerably after implantation,while the spontaneous neural activities recorded by octrode arrays showed notably higher SNR and peak-to-peak amplitude responses (Supporting information,Figs.S10-S11).At four weeks after implantation,the average SNR in the tetrode group was only 1.62,and was significantly lower than that in the octrode group (SNR=3.45,p < 0.05,t-test; Fig.2a).The percentages of recorded waveforms which were categorized as medium-SNR (SNR > 2)and high-SNR waveforms (SNR > 3)in the cases of tetrodes were only 25% and 8% respectively,while the respective percentages for the octrodes were 100% and 73% (Fig.2a).Furthermore,none of the waveforms recorded by tetrodes were categorized as extra high-SNR waveforms(SNR > 5)compared to 9% for the octrodes.The peak-to-peak spike amplitudes recorded for the octrodes were remarkably larger than those recorded for the tetrodes,while the signal quality remained at the same level approximately for a fourweek period,while the peak-to-peak spike amplitudes recorded for the tetrodes decreased abruptly (Fig.2b,c; Supporting information,Fig.S12).
The LFPs were also collected to investigate the influence of the tissue response to electrophysiological recordings (Fig.2d,e,Supporting information,Fig.S13).Voltage drifts at low frequencies (< 1 Hz)are observed only in the tetrode group,while the voltage amplitudes recorded by octrodes at higher frequencies (> 1 Hz)are notably larger than those recorded by the tetrodes.Additionally,the average power spectra also revealed the higher power from 1 to 200 Hz of octrodes compared to tetrodes,thus suggesting a higher sensitivity in the recording of electrophysiological events.Overall,the higher quality of the electrophysiological recordings acquired by the octrode arrays may have benefitted from the high pseudocapacitance and lower impedance of the octrodes in vivo,which is in accordance with the EIS analyses.These merits may be attributed to the tissue-friendly characteristics of the octrode arrays during long-term implantation.

Fig.2 Electrophysiological characterizations on day 28 post-implantation.
3.3 Histology
To evaluate the histocompatibility of the electrodes,we performed immunochemical analyses on tissue sections of mouse brains four weeks after implantation (Fig.3a,b,Supporting information,Figs.S14-S16).Reactivated astrocytes(GFAP labeled)occupied the zone adjacent to the tetrodes,and a much lighter GFAP positive zone was found in the octrode group.It should be noted that no obvious glial encapsulation was observed in the octrode group,which may be attributed to the small size of each decentralized recording channel (diameter=12.7 μm)on the octrode array after self-spreading in vivo.Quantitative analyses of the GFAP intensity profiles of the tetrode and octrode arrays as a function of distance from the center of implantation site are shown in Fig.3c.Statistical results show that the GFAP intensity in the octrode group was significantly lower (p < 0.05,t-test)than that in the tetrode group up to a distance of 150 μm from the center of the implant (Fig.3c).In addition,neuronal loss (neurons labeled by NeuN)was observed around the implants,and this loss was particularly severe in the tetrode group.Quantitative analyses demonstrated that the neuronal density in the octrode group was significantly lower than that of the tetrode group in an annular area (radius 25 to 55 μm,p < 0.05,t-test)around the implant center (Fig.3d).These results suggest that the self-spreadable octrode array can drastically alleviate the inflammatory response (reduced astroglial intensity)and promote neuronal viability (increased NeuN intensity)around the implant site.Benefited from the tissue-friendly characteristics,the octrodes maintained lowimpedance and high-capacitance properties even after long-term implantation that subsequently led to an improved electrophysiological performance in vivo.
Furthermore,it should be noted that,to systematically investigate the characteristics of self-spreadable electrodes in vivo,we compared the performance of 8-channel electrode(octrode)arrays with 8-channel tetrode (bi-tetrode)arrays.However,as the number of recording channels can be customized according to different experimental designs,selfspreadable high-density electrode arrays can be also fabricated following the similar procedure of octrode arrays for in vivo applications (Supporting information,Figs.S17-S18).
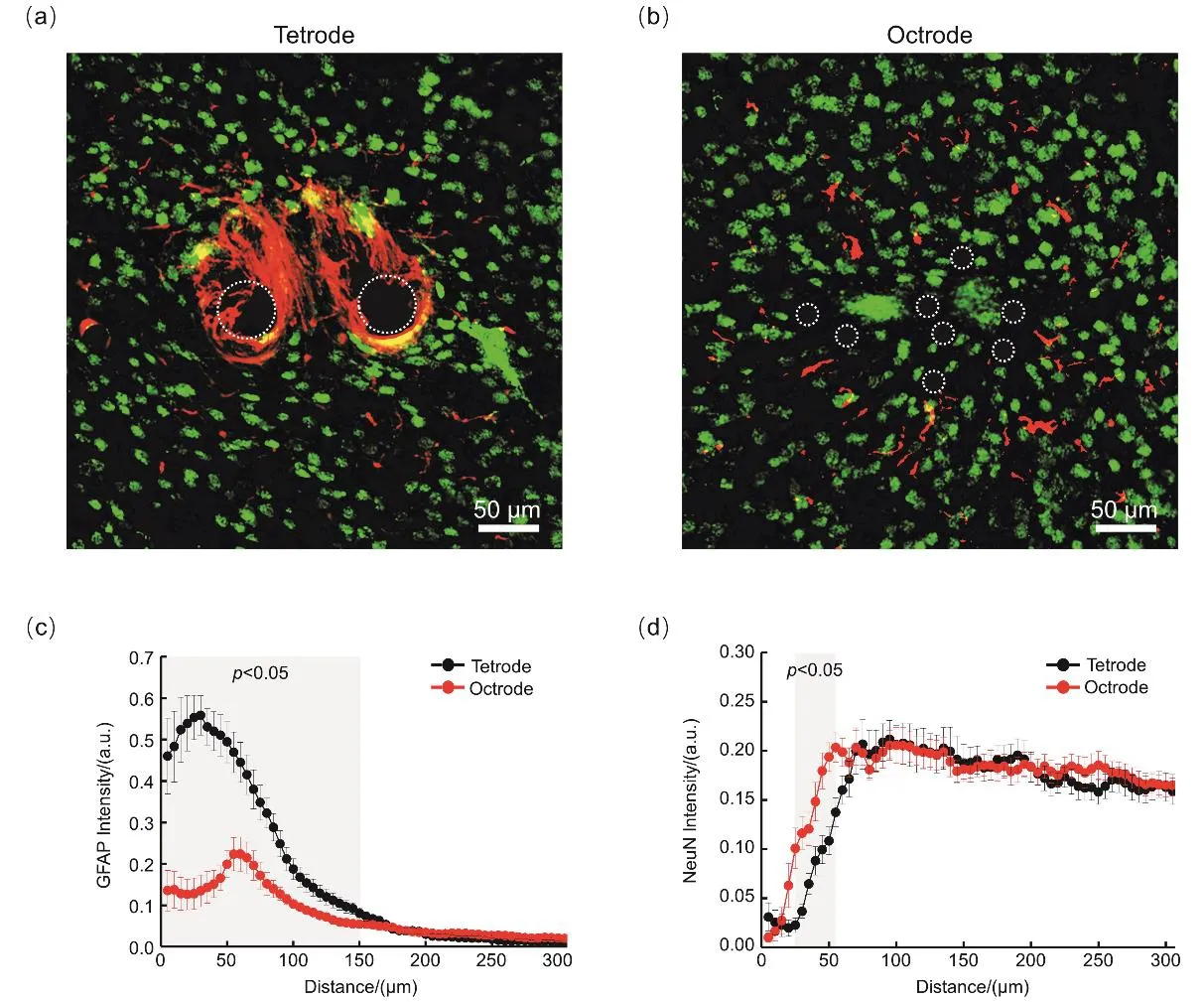
Fig.3 Inflammatory response and neuronal survival around implants at four weeks after implantation.
4 Conclusions
In this study,we demonstrated the feasibility and advantages of use of a self-spreadable octrode array for long-term electrophysiological recordings in vivo.The gelatin encapsulation on the octrode tip was quickly swollen and degraded after implantation,and the recording channels of the octrode array then self-spread in an area with a diameter of approximately 150 μm.The induced tissue trauma and stiffness responses adjacent to the implants were alleviated considerably.These outcomes were attributed to the merits of the relatively small size of the decentralized recording channels.These findings provide evidence for the significantly decreased glial encapsulations and improved neuronal survival after long-term implantation.As a consequence,the octrode arrays exhibited markedly higher capacitance values and ion transfer rates,and lower impedance as compared to tetrode arrays in vivo.Longterm electrophysiological recordings in free-behaving mice acquired with the use of the octrode arrays benefited from these advantages and yielded improved spike SNR and LFP sensitivity responses compared to those evoked from tetrode arrays.All these results are crucial for precisely timed analyses of neuronal activities in vivo,thus suggesting that the self-spreadable octrode array may be of great use in the dissection of the brain circuit functions.Overall,this work has proposed a simplified strategy for the fabrication of self-spreadable multichannel electrode arrays which exhibited low-impedance and tissue-friendly characteristics,and a high-recording quality.Interesting and potential benefits are that the recording channels of electrode can be custom-designed.Accordingly,these electrodes can be conveniently combined with other neural modulation techniques,such as optogenetics,to investigate the complex brain processing mechanisms at the functional level.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
