lmpact of MitraClip Program on the Volume and Outcomes of Mitral Valve Surgery:A Single-Center Retrospective Study
2020-12-16WeiZhangMDPhDCliftonLewisMDSriniyaMallelaMDAliEbrahimiMDGregoryVonMeringMDandMustafaAhmedMD
Wei Zhang,MD,PhD ,Clifton Lewis,MD ,Sriniya Mallela,MD ,Ali Ebrahimi,MD ,Gregory Von Mering,MD and Mustafa Ahmed,MD ,
1 Internal Medicine Program,Brookwood Baptist Health System,701 Princeton Ave SW,Birmingham,AL 35211,USA
2 The Structural Heart and Valve Center,Princeton Baptist Medical Center,Brookwood Baptist Health System,701 Princeton Ave SW,Birmingham,AL 35211,USA
3 Section of Cardiovascular Medicine,Department of Internal Medicine,Wake Forest School of Medicine,Winston-Salem,NC 27157,USA
4 The Structural Heart Program,Department of Cardiology,University of Alabama at Birmingham,Birmingham,AL 35233,USA
Abstract
Keywords:mitral valve regurgitation; mitral valve surgery; MitraClip; TEE; ouctome
lntroduction
Untreated mitral regurgitation (MR) is associated with poor prognosis.In the USA,more than four million people are experiencing moderate to severe MR.Yet a signif icant number of patients are not able to receive open-heart mitral valve repair or replacement because of high surgical risk.However,multiple transcatheter procedures are emerging.Tested by the Endovascular Valve Edge-to-Edge Repair Study (EVEREST) and ACCESS-EU trials,the MitraClip system (Abbott Vascular Inc.,Menlo Park,CA,USA),also known as percutaneous edge-to-edge transcatheter mitral valve repair,was approved by the US Food and Drug Administration in October 2013 to treat severe degenerative MR in patients with high surgical risk.Its safety and eff icacy have enabled rapid growth of its use since its approval,and so far more than 40,000 patients have been treated with the MitraClip system.Ongoing trials are exploring its application in other types of structural disorders,including functional MR (FMR),tricuspid regurgitation,and recurrent MR after surgery.With more emerging percutaneous devices,both patients and many operators are trending to less invasive approaches particularly for patients with high surgical risk.While surgery remains the standard treatment for MR when it is possible,whether the availability of a transcatheter program would affect mitral valve surgery volume and outcome remains unclear.Since initiation of the MitraClip program in 2015,our institution has seen a rapid growth in MitraClip procedures,with more than 250 cases completed by October 2018.The purpose of the current study was to compare the mitral valve surgery volume and outcomes before and after initiation of the MitraClip program in our institution.
Method
Procedural Volume and Outcomes
The current retrospective study was approved by the Princeton Baptist Medical Center Institutional Review Board,which provided a waiver of individual patient consent.All patients who underwent surgical mitral valve repair (with or without coronary artery bypass graft),surgical mitral valve replacement(with or without coronary artery bypass graft),and MitraClip repair from January 2013 to December 2017 were included in the analysis.Both internal electronic medical records and our institutional data from the Duke Clinical Research Institute were collectively analyzed to achieve the most accurate results.Outcomes included operative mortality,in-hospital mortality,perioperative complications (infection,acute renal failure,and stroke),and length of stay.For comparison before and after initiation of the MitraClip program,we specif cially used the year 2015 as the cut-off year,and data from the 2 years before (2013-2014) and the 2 years after (2016- 2017) were compared.The volumes and outcomes of the MitraClip program were also analyzed from 2016 to 2017.
Statistical Analyses
Baseline characteristics were tabulated by time categories as indicated in Table1.Continuous variables are presented as the mean ± standard deviation,and categorical variables are reported as the frequency count and percentage.Analysis of variance was used to compare the continuous variables,while the chi-square test was used to compare the categorical variables.
All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc.,Cary,NC,USA),and statistical signif icance was def ined by twosided P values less than 0.05.
Results
Procedural Volumes before and after lnitiation of the MitraClip Program
To compare the pre-MitraClip program and post-MitraClip program volumes,we attempted toanalyze the data with and without the year 2015,which was the f irst year of the MitraClip program in our institution.In 2015,there were eight MitraClip procedures,which represents the initiation stage of this new program.Since then,we have seen exponential growth,with 63 procedures in 2016 and 91 in 2017.Meanwhile,the total volume of mitral valve surgery also continued to increase.The mitral valve repair volume increased from 110 (2013-2014) to 154 (2016- 2017),about a 40% increase after initiation of the MitraClip program compared with before initiation of the MitraClip program.The 2-year mitral valve replacement volume also increased from 43 to 60,an increase of about 39.5%(Table1).
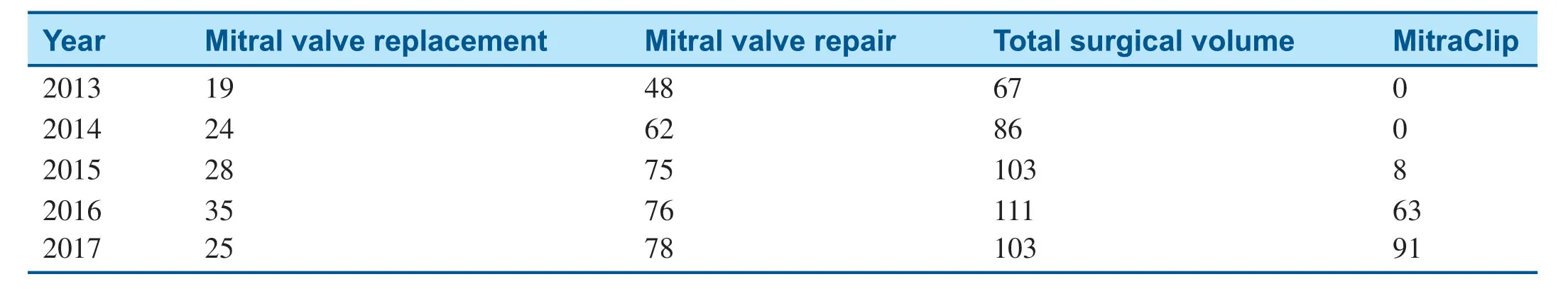
Table1 Volume of Procedures From 2013 to 2017.
Surgical Outcomes before and after lnitiation of the MitraClip Program
The baseline characteristics of patients who underwent mitral valve surgery are shown in Table2.After initiation of the MitraClip program,signif icantly fewer patients who underwent surgery had an ejection fraction less than 40%,and a higher percentage of surgical patients had peripheral arterial disease (Table2).Among patients who underwent mitral valve surgery,in-hospital mortality decreased from 7.2% in 2013- 2014 to 0.5% in 2016- 2017 (Table3),and there was no signif icantchange in the total length of stay (Table4).Mitral valve surgery complications,including infection,renal failure,and stroke,also signif icantly decreased after initiation of the MitraClip program (Table3).
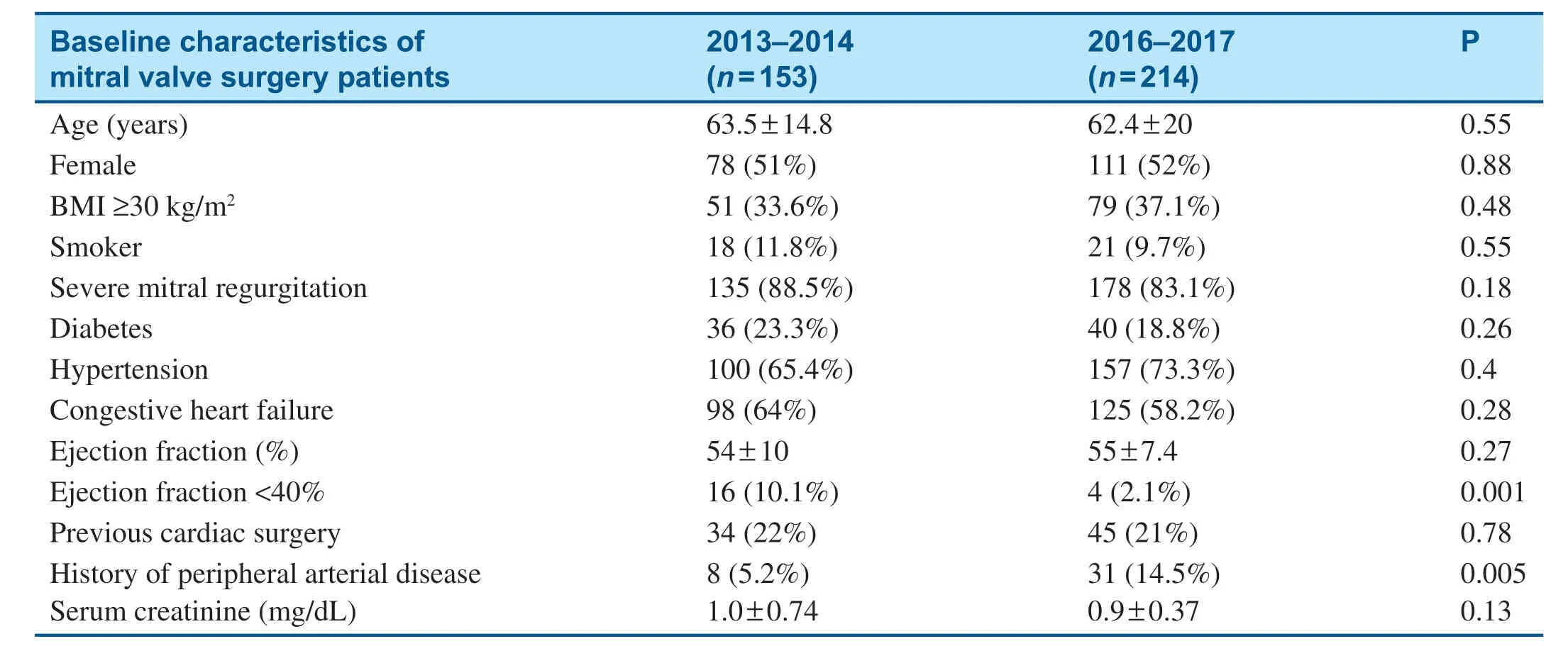
Table2 Baseline Characteristics of Patients who Underwent Mitral Valve Surgery before (2013- 2014) and after(2016- 2017) Initiation of the MitraClip Program.
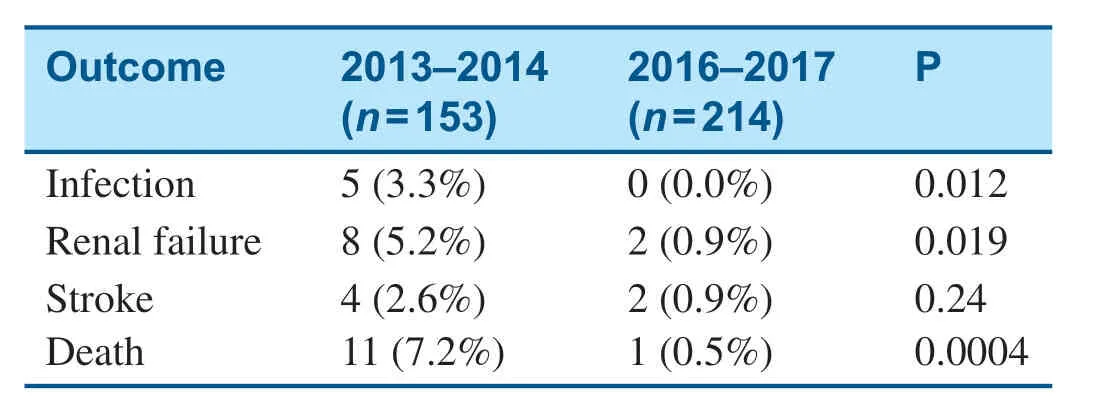
Table3 Surgical Outcomes before (2013- 2014) and after(2016- 2017) Initiation of the MitraClip Program.
MitraClip Outcomes
Table5 shows the baseline characteristics of patients who underwent the MitraClip procedure in 2016 and 2017.All patients who underwent the MitraClip procedure had moderate-severe to severe MR at the baseline (Table5).As shown in Table6,there was a signif icant decrease in f luorescence use from 2016 to 2017 because of the transition to an ultrasound-guided operation (Table6).The average procedural time also signif icantly decreased from 2016 to 2017; meanwhile,the total length of stay remained stable and procedural outcomes remained excellent (Table6).
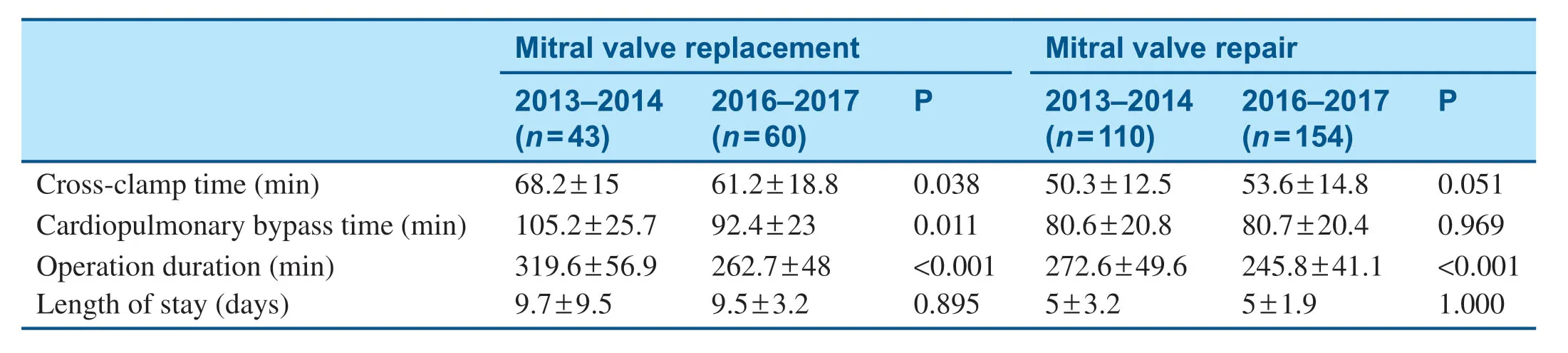
Table4 Procedural Time and Length of Stay before (2013- 2014) and after (2016- 2017) Initiation of the MitraClip Program.
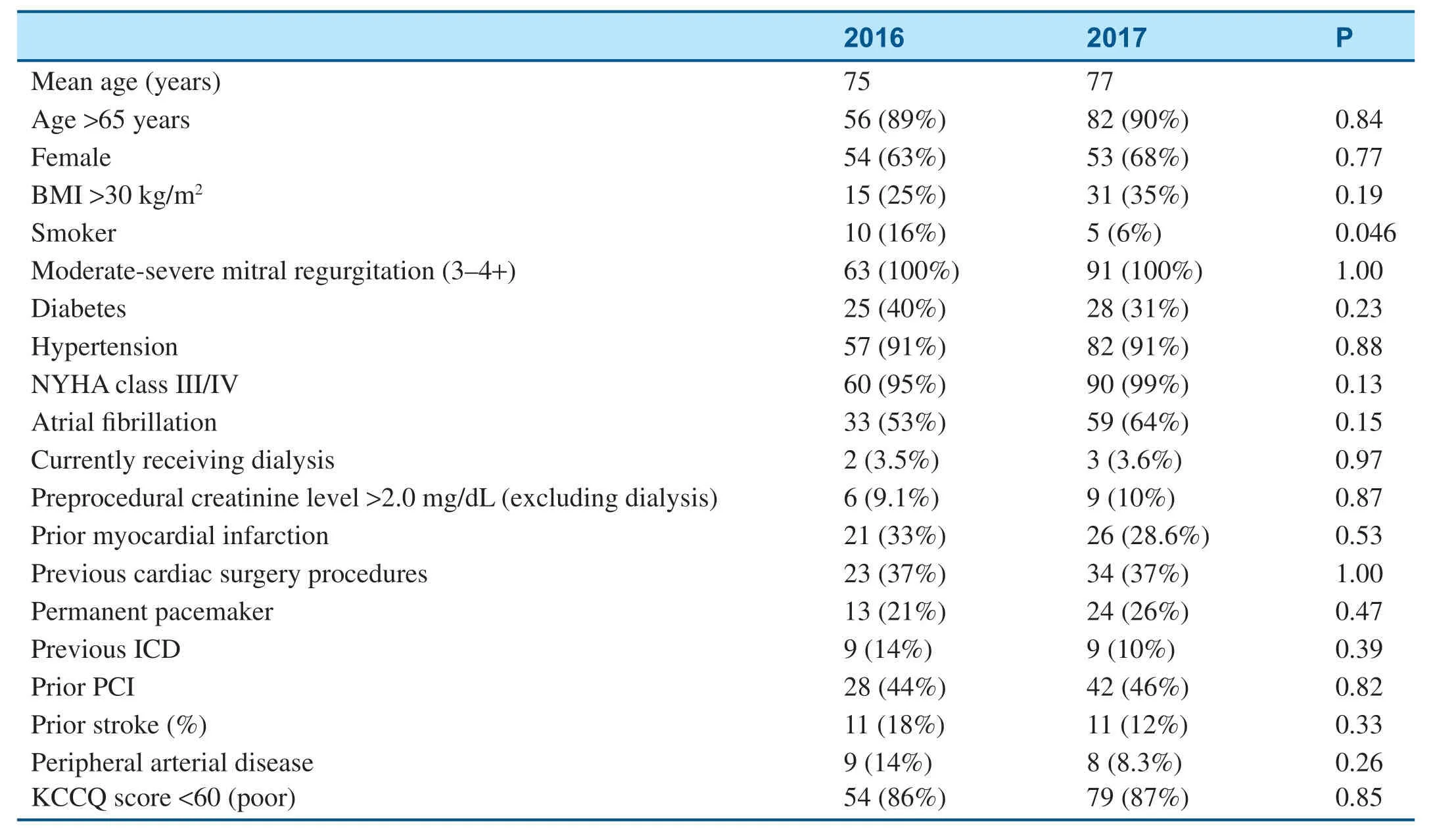
Table5 Baseline Characteristics of Patients who Underwent the MitraClip Procedure.
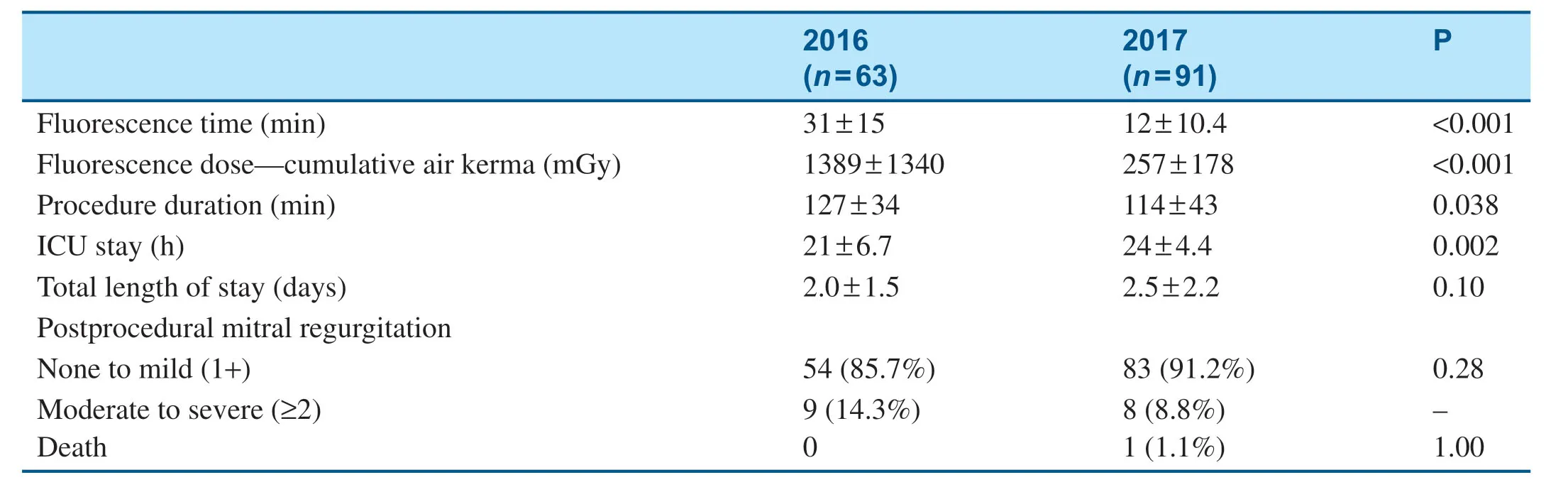
Table6 Fluorescence use,Procedural time and Outcomes of Patients who Underwent the MitraClip Procedure.
Discussion
It is clinically important to know the dynamic changes of the volume and outcomes of mitral valve surgery when a minimally invasive mitral valve program (MitraClip in the current study) becomes available in an institution.Meanwhile,an important question is how the cardiac surgery and cardiology teams come together to ensure the best patient distribution and outcomes.The current study analyzed data from a typical tertiary hospital (505 equipped beds) in the USA.The results show the development of a MitraClip program is associated with increased mitral valve surgery volume and improved surgical outcomes in our institution,which is likely due to improved preoperative risk evaluation and patient distribution by a multidisciplinary heart team.
Over the past a few years,technologies have brought multiple novel minimally invasive options for patients with valvular heart disease but high risk for standard-of-care open-heart surgery.Valve-invalve,valve-in-ring,and MitraClip procedures are promoted as the least invasive approaches for mitral valve repair,among which the MitraClip system has been the most well studied and f irst received the CE mark in 2008.Its safety and eff icacy were investigated in the EVEREST trials.EVEREST II is a prospective,multicenter,randomized controlled trial designed to compare percutaneous mitral valve repair with use of the MitraClip device in mitral valve surgery in the treatment of MR.According to the results of EVEREST II,the MitraClip procedure showed superior safety and similar improvements in clinical outcomes compared with mitral valve surgery,despite this approach being less effective at reducing MR than traditional surgery [1].At the 5-year follow up,patients treated with the MitraClip procedure more commonly required surgery for residual or recurrent MR within the f irst 6 months after the procedure,but between 1 year and 5 years,the MitraClip procedure had durability of MR reduction and 5-year mortality rates comparable to those of surgery [2].
The Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation(COAPT) trial is a prospective,randomized,parallelcontrolled,open-label multicenter trial designed to determine the safety and eff icacy of the MitraClip device in treating patients with symptomatic heart failure and clinically signif ciant secondary MR or FMR.The f rist 24-month follow-up results of the COAPT trial were recently published and highlighted at TCT 2018.The results showed that patients treated with MitraClip plus medical therapy had lower rates of heart failure hospitalizations and lower all-cause mortality at the 2-year follow-up [3].The COAPT study data revealed MitraClip as the f rist promising option for patients with heart failure and secondary MR/FMR who continue to be symptomatic despite receiving optimal medical therapy.With these f nidings,an expanded indication for MitraClip to treat secondary MR was approved by the US Food and Drug Administration in March,2019.However,as MitraClip continues to grow with support from large trials and industry,in a study by procedural experts,the durability of MitraClip in EVEREST II was not duplicated,and signif ciantly higher rates of recurrent MR in patients who underwent the MitraClip procedure compared with patients who underwent surgery (with annuloplasty) at the 4-year follow-up were found [4].The MITRA-FR trial reported no differences in mortality or heart failure hospitalizations at 12 months between the MitraClip group and the standard medical therapy group for patients with secondary MR [5].According to the 2014 American Heart Association/American College of Cardiology guidelines,the MitraClip procedure is recommended for patients with absolute contraindications for surgery or with prohibitive risks because of a high number of comorbidities (class 2b),but surgery remains the best therapeutic approach when possible.
As discussed by others,when patients with valvular heart disease are being treated,MitraClip and surgery should not compete with each other [6].In the current study,the initiation of the MitraClip program is associated with signif icantly reduced perioperative complications and in-hospital mortality among patients who underwent mitral valve surgery.During the study period,the institution had the same well-experienced surgical team and anesthesia staff;hence,staff change within the surgical unit does not explain the improved surgical outcomes.We summarize several important factors that could explain the improved quality of care.First,along with initiation of the MitraClip program,a structural heart clinic was formed jointly by a multidisciplinary team consisting of cardiac surgeons,general cardiologists,interventional cardiologists,cardiovascular imaging experts,and administrative and nursing staff.As MitraClip and mitral valve surgery are intended for different patients,a multidisciplinary structural heart team allows the best selection of patients with greater adherence to guidelines and more comprehensive preprocedural,periprocedural,and postprocedural management and therefore improved quality of care.Second,a state-of-the-art electronic medical communication platform was used to allow safe,powerful,high-quality image exchange and effective communication among team members involved in patient care.Third,but a fundamental factor for the change of a hospital system,is the administrative support with strong motivation to reform and improve the structural heart service.Finally,with a shared decision-making process,patients feel more comfortable,get faster and more accurate decisions,and have a short waiting time,and there is overall reduced administrative cost.While we have seen signif icantly improved surgical outcomes,there were also increased numbers of patients undergoing mitral valve surgery at the same time as more patients were undergoing the MitraClip procedure.It is not hard to understand that both referring physicians and patients are more encouraged and willing to work with a facility with a multidisciplinary heart team.We anticipate the importance of collaboration among interventional cardiologists,cardiac surgeons,and other health care professionals will become even greater soon.
Studies have reported the use of real-time 3D transesophageal echocardiography to guide the procedure can result in increased reproducibility,reliability,and safety [7].Our data showed that as we transitioned to a more transesophageal echocardiography- guided procedure,the f luorescence time decreased (30.9 min vs.11.9 min),with the total procedure duration shortened as well,which without doubt protects both patients and medical staff from prolonged radiation exposure.Meanwhile,the MitraClip procedural outcomes remain excellent.These results indicate that the MitraClip procedure can be safely and effectively done as a more ultrasound-guided procedure,being less dependent on radiation but more on the imaging specialist.
With accumulation of real-world experience,more upgraded clip devices,improved imaging,and percutaneous mitral prosthetic valve implantation,we expect the procedural eff icacy of percutaneous mitral valve intervention to increase and the associated long-term mortality to decrease.The newer generation of the MitraClip device could be indicated for a larger spectrum of patients.However,conventional surgical valve repair/replacement remains the most effective option when possible,but emerging less invasive approaches provide more options for patients,and in this new era of availability of transcatheter approaches,it is critical to realize the importance of team work among cardiologists,surgeons,imaging experts,nursing staff,and other staff.The transcatheter team and the open-heart team should not compete but should work as a heart team to provide the best evidence-based treatment and thus best the outcomes for patients.While the current study is limited by its single-center retrospective design,further studies with larger sample size with a longer follow-up period are needed to validate our f indings and look for long-term outcomes.
Conf ilcts of lnterest
Dr.Mustafa Ahmed,Advisory Boards:Abbott Vascular Inc.,Edwards Lifesciences,and Medtronic Inc.,Research grants awarded:Abbott Vascular Inc.,Edwards Lifesciences,and Medtronic Inc.The other authors declare that they have no conf ilcts of interest.REFERENCES 1.Whitlow PL,Feldman T,Pedersen WR,Scott Lim D,Kipperman R,Smalling R,et al.Acute and 12-month results with catheterbased mitral valve leaf let repair:the EVEREST II (Endovascular Valve Edge-to-Edge Repair) high risk study.J Am Coll Cardiol 2012;59(2):130- 9.
2.Feldman T,Kar S,Elmariah S,Smart SC,Trento A,Siegel RJ,et al.Randomized Comparison of percutaneous repair and surgery for mitral regurgitation:5-year results of EVEREST II.J Am Coll Cardiol 2015;66(25):2844- 4.
3.Stone GW,Lindenfeld J,Abraham WT,Kar S,Scott Lim D,Mishell JM,et al.Transcatheter mitral-valve repair in patients with heart failure.N Engl J Med 2018;379(24):2307- 18.
4.De Bonis M,Lapenna E,Buzzatti N,Canna GL,Denti P,Pappalardo F,et al.Optimal results immediately after MitraClip therapy or surgical edge-to-edge repair for functional mitral regurgitation:are they really stable at 4 years? Eur J Cardiothorac Surg 2016;50(3):488- 94.
5.Obadia JF,Messika-Zeitoun D,Leurent G,Lung B,Bonnet G,Piriou N,et al.Percutaneous repair or medical treatment for secondary mitral regurgitation.N Engl J Med 2018;379(24):2297- 306.
6.Dreyfus GD.MitraClip and surgery should not compete.Eur J Cardiothorac Surg 2016;50(3):495- 6.
7.Labrousse L,Dijos M,Leroux L,Oses P,Seguy B,Markof M,et al.Guidance of the MitraClip® procedure by 2D and 3D imaging.Arch Cardiovasc Dis 2018;111(6- 7):432- 40.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- WeChat Group of Chest Pain Center for Patients with Acute ST-segment Elevation Myocardial Infarction:Faster Treatment Speed and Better Prognosis
- Clinical Analysis of Transcatheter Embolotherapy for Congenital Pulmonary Arteriovenous Fistulas in Children
- In-Stent Thrombosis after Antiplatelet Therapy Conversion while Awaiting Coronary Bypass
- Current Management Strategies in Patients with Heart Failure and Atrial Fibrillation:A Review of the Literature
- Comparison of Segmentation Algorithms for Detecting Myocardial Infarction Using Late Gadolinium Enhancement Magnetic Resonance Imaging
- Epicardial Adipose Tissue in Patients with Obstructive Sleep Apnea:A Systematic Review and Meta-analysis