Clinical Analysis of Transcatheter Embolotherapy for Congenital Pulmonary Arteriovenous Fistulas in Children
2020-12-16XueZhouAngLiDanYinXupeiHuangJieTianTieweiLvQijianYiandPingXiang
Xue Zhou ,Ang Li,Dan Yin,Xupei Huang,Jie Tian ,Tiewei Lv ,Qijian Yi and Ping Xiang
1 Department of Cardiology,The Children’ s Hospital of Chongqing Medical University,136 Zhongshan Er Rold,Yu Zhong District,Chongqing 400014,China
2 Children’ s Hospital of Chongqing Medical University,Ministry of Education Key Laboratory of Child Development and Disorders,Chongqing 400014,China
3 China International Science and Technology Cooperation Base of Child Development and Critical Disorders,Chongqing,China
4 Chongqing Key Laboratory of Pediatrics,Chongqing 400014,China
5 The First Aff iliated Hospital of Chongqing Medical University,1 Youyi Road,Yuzhong District,Chongqing 400016,China
6 The Children’ s Hospital of Chongqing Medical University,136 Zhongshan Er Rold,Yu Zhong District,Chongqing 400014,China
7 Department of Biomedical Science,Charlie E.Schmidt College of Medicine,Florida Atlantic University,777 Glades Road,Boca Raton,FL 33431,USA
Abstract
Keywords:Pulmonary arteriovenous f istulas; congenital; transcatheter embolotherapy
lntroduction
Pulmonary arteriovenous f istula (PAVF) is a rare congenital pulmonary vascular malformation.It directly connects pulmonary arteries and pulmonary veins,without an intervening pulmonary capillary bed,providing a right-to-left shunt,and leads to the same pathophysiologic consequences as an intracardiac right-to-left shunt.The clinical diagnoses are not only based on the typical symptoms such as dyspnea on exertion,cyanosis,clubbing,and asymptomatic hypoxemia,physical signs and laboratory examination f indings,but also depend on the type of imaging examinations.With progress in materials science,as well as the maturity of transcatheter embolotherapy in recent years,transcatheter embolotherapy has become the f irst option for PAVF treatment.Therefore,we retrospectively analyzed transcatheter embolotherapy for nine patients with PAVF in the Children’ s Hospital of Chongqing Medical University from July 2004 to July 2019.
Materials and Methods
Study Population and Data Collection
The nine patients with PAVF who underwent transcatheter embolotherapy in the Children’ s Hospital of Chongqing Medical University were retrospectively analyzed from July 2004 to July 2019.Data regarding patient demographics,laboratory results,and transthoracic echocardiography studies at presentation and during follow-up,including posttreatment studies,were obtained from the medical records.
Among the nine patients,four were male and f ive were female,aged from 6 years 5 months to 10 years 7 months,with the mean age at the time of evaluation being 7.58 years (standard deviation 1.68 years).Only two patients were asymptomatic,six patients showed decreased exercise capacity,four patients had cyanosis,three patients demonstrated shortness of breath,and four patients had clubbing.Five patients were found to have a lung mass shadow by CT scan,or unexplained cyanosis or cough.One patient had no symptoms,a bruit was heard during the physical examination,congenital heart disease was excluded by echocardiography,and PAVF was diagnosed by CT.No bruits were heard in the remaining patients (see Table1).
Diagnosis
In all patients routine blood tests,blood gas analysis,transthoracic echocardiography,and chest X-rays were performed before embolization.The patients underwent 64-slice contrast-enhanced CT along with vascular remodeling before transcatheter embolotherapy.During transcatheter embolotherapy,we f irst performed main pulmonary angiography,selective right or left pulmonary angiography,and feeding artery superselective angiography if necessary according to the results.
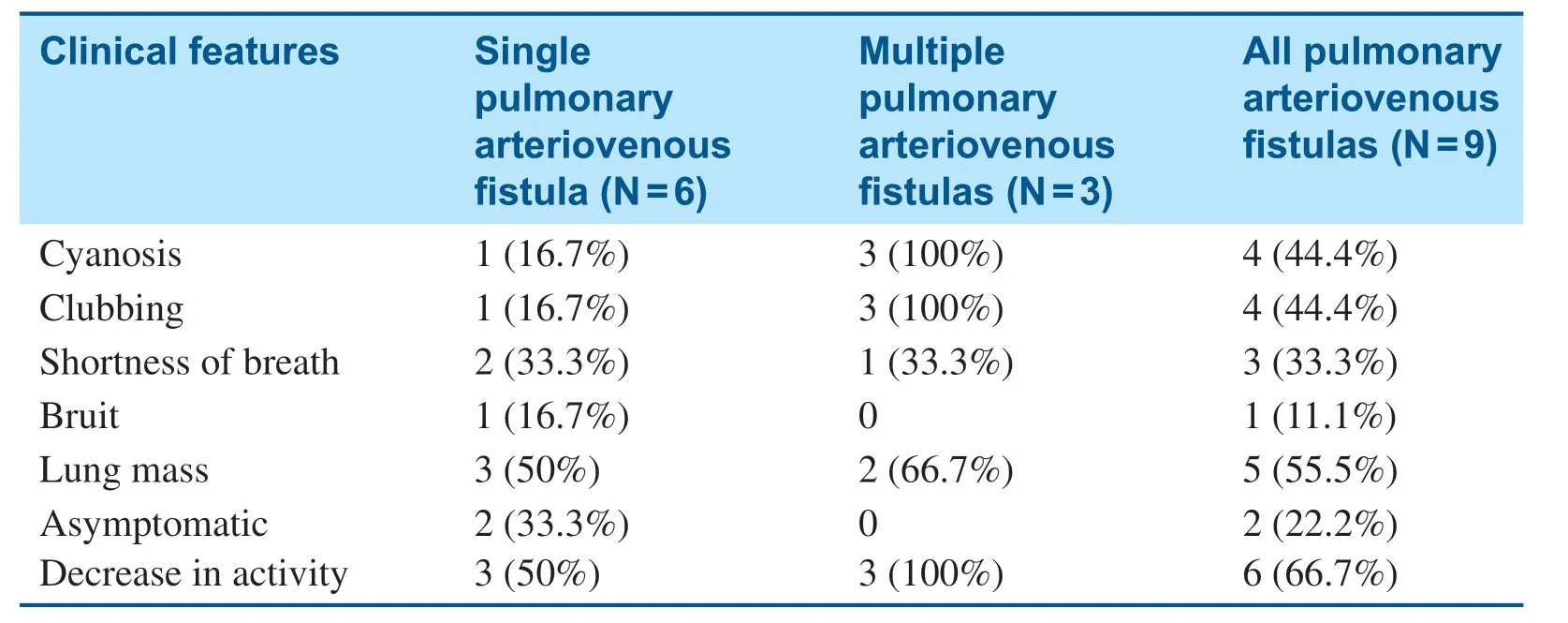
Table1 The Clinical Features of Nine Children with Pulmonary Arteriovenous Fistulas.
Transcatheter Embolotherapy
All patients completed the relevant preoperative examinations.Each embolotherapy procedure was preceded by diagnostic pulmonary angiography and measurement of pulmonary artery pressure.
A 5-Fr artery sheath and a 6-Fr venous sheath were inserted after total heparinization (100 U/kg).Left and right cardiac catheterizations were separately performed,and the pressure and oxygen saturation of the superior vena cava,inferior vena cava,right atrium,right ventricle,pulmonary artery,aorta,and left ventricle were measured.On the basis of the results of selective pulmonary angiography,the pulmonary artery catheter was changed from a 6-Fr guide catheter to a 10-Fr guide catheter.Hand bolus injection of contrast medium was performed to clearly def ine the relationship of the catheter and the f istula.After the transport sheath had been placed into the f istula and there were no normal pulmonary artery branches in the distance,a suitable closure device was used on the lesion to occlude the f istula,and then we observed the variation of percutaneous oxygen saturation for 10- 15 min.We punctured another femoral vein and performed selective pulmonary angiography repeatedly to ensure there was a higher success of occlusion,dilated pulmonary arteries far from the closure device were not f illed with contrast medium,and no residual shunt after the closure device was released.If more f istulas were exited,closure devices were released step by step (see Figure1).Routine blood tests,oxygen saturation measurements,chest X-rays,transthoracic echocardiography,and electrocardiograms were performed 1 month,3 months,6 months,and every year after transcatheter embolotherapy.
Statistical Method
Statistical analysis was performed with IBM SPSS Statistics version 22.0.Enumerated data are expressed as the mean ± standard deviation; an independent samplettest or a pairedttest was used.Measurement data are expressed by the rate.The test level wasα= 0.05.
Results
In all patients,pulmonary angiography and selective left and right pulmonary angiography were performed,and PAVFs were diagnosed.Lesions were found in the lower lobe of the left lung in four patients and in the right lung in three patients(located in the upper lobe,the middle lobe,and the three lobes of the right lung,respectively),and two patients had bilateral pulmonary lesions.Among the nine patients,six patients had a single PAVF and three patients had multiple PAVFs.The diameter of the f istula ranged from 2.7 to 7.9 mm and averaged 5.41 mm.
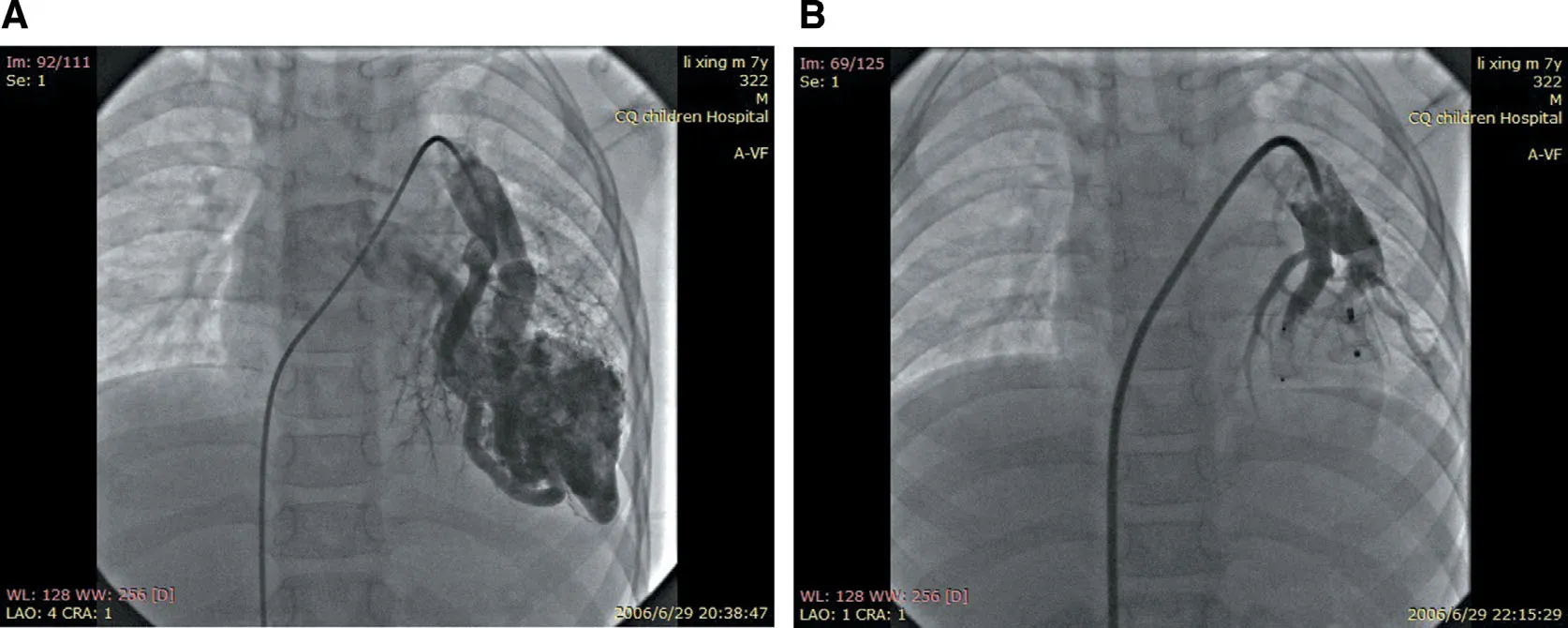
Figure1 Transcatheter Embolotherapy for Pulmonary Arteriovenous Fistula.
Eight of the nine patients were successfully occluded immediately after operation.Five patients chose a single occluder (two patients chose a ventricular septum occluder and three chose an Amplatzer duct occluder) and four patients selected two closure devices.Because of vascular distortion in one patient,it was diff icult for the guide catheter to reach the lesion,and embolotherapy was stopped temporarily.However,two coils were successfully used to occlude the f istula through the microcatheter thereafter.Occlusion was therefore successful in all nine patients (Table2).
lmmediate Results after Embolotherapy
Selective pulmonary angiography was performed immediately after release of the occluders.Residual shunts were found in only two cases.One patient had moderate tricuspid regurgitation,and the oxygen saturation was increased from 73- 86% to 90- 99%.
Follow-up
The electrocardiograms showed no changes over continued follow-ups of 1 day,1 month,3 months,and 6 months after embolotherapy.Transthoracic echocardiography showed moderate tricuspid regurgitation in one case; however,it changed to mild tricuspid regurgitation after 1 month.Chest X-rays were performed 24- 48 h after embolization and showed that the lung mass in f ive patients had disappeared.
All patients were followed up for 6 months to 12 years.No residual shunts or other complications occurred,and there were no recurrent cases.Cyanosis remission occurred within 24 h after embolization and disappeared 3 months later.Clubbing f ingers disappeared after 6 months and oxygen saturation was maintained at 93- 100% (Table3).Compared with before embolotherapy,there were signif icant changes in oxygen saturation and total hemoglobin level at the last follow-up (t= 10.06,P = 0.000;t= 3.055,P = 0.0076); however,there was no signif icant difference in the mean pulmonary artery pressure before and after embolotherapy(t= 0.13,P = 0.90).Enlarged hearts were found in f ive patients before transcatheter embolotherapy,and they all returned to normal during the followup (Table2).
Discussion
Pulmonary arteriovenous f istula (PAVF),also called“ pulmonary arteriovenous malformation,” is a direct high-f low,low-pressure artery-to-vein connection.More than 80% of PAVFs are congenital[1].PAVF is a rare pulmonary vascular disease,with an incidence of 2- 3 cases per 100,000 per year[2].The male-to-female ratio ranges from 1:1.5 to 1.8 [3],and in our case the male-to-female ratio was almost 1:1.
PAVF has been classif ied into two groups on the basis of the pathologic f indings:sac type and diffuse type.The sac type is divided into two types:simple PAVF and complex PAVF [4].Simple PAVF comprises one supplying artery and one draining vein,and the sac has no chamber [4].Complex PAVF has two or more feeding arteries supplying the aneurysmal sac and one or two draining veins,and the sac is usually divided into separate compartments [4].Fifty percent to 75% patients have a single PAVF,about 30% of patients have multiple lesions,and the occurrence of bilateral PAVF is 8- 10%.In the Mayo Clinic’ s clinical study,87% of the lesions were located in the lower lobes,37% were located in the upper lobes,and 37% were bilateral [5].In our series,PAVFs were located in the lower lobe of the left lung only in four patients (44.4%),in two patients the PAVFs were bilateral in the lower lobes,one patient had PAVFs in the three lobes of the right lung,one patient had a PAVF in the upper lobe of the right lung,and in one patient the PAVF was in middle lobe of the right lung.
PAVF is direct capillary-free connections between pulmonary arteries and veins and leads to a right-toleft shunt.Gas exchange,f iltration,and other processes involving venous blood are impaired.The damaged blood-air barrier results in ineffective gas exchange and leads to hypoxemia [2].The absence of the capillary bed impairs the f litration barrier,and harmful matter such as microemboli and bacteria directly pass into the systemic circulation,which can lead to serious complications such as stroke and cerebral abscess.PAVF can also lead to heart failure,hemothorax,and hemoptysis [6].Unlike systemic arteriovenous malformation,PAVF does not affect cardiac hemodynamics [7,8].The degree of the shunt is what ultimately determines the clinical manifestation [1,8].When the right-toleft shunt is more than 20% of the systemic cardiac output,the patients will have obvious symptoms,such as hypoxemia,dyspnea on exertion,cyanosis,and clubbing,but asymptomatic hypoxemia is most common [8].Therefore,when there is unexplained hemoptysis,cyanosis,clubbing,or bruits,the diagnosis of PAVF should be taken into account.Among the nine patients,two patients (22.2%) in our series were asymptomatic,six patients (66.7%)had decreased activity,three patients (33.3%) had dyspnea,four patients (44.4%) had cyanosis,and four patients (44.4%) had clubbing.
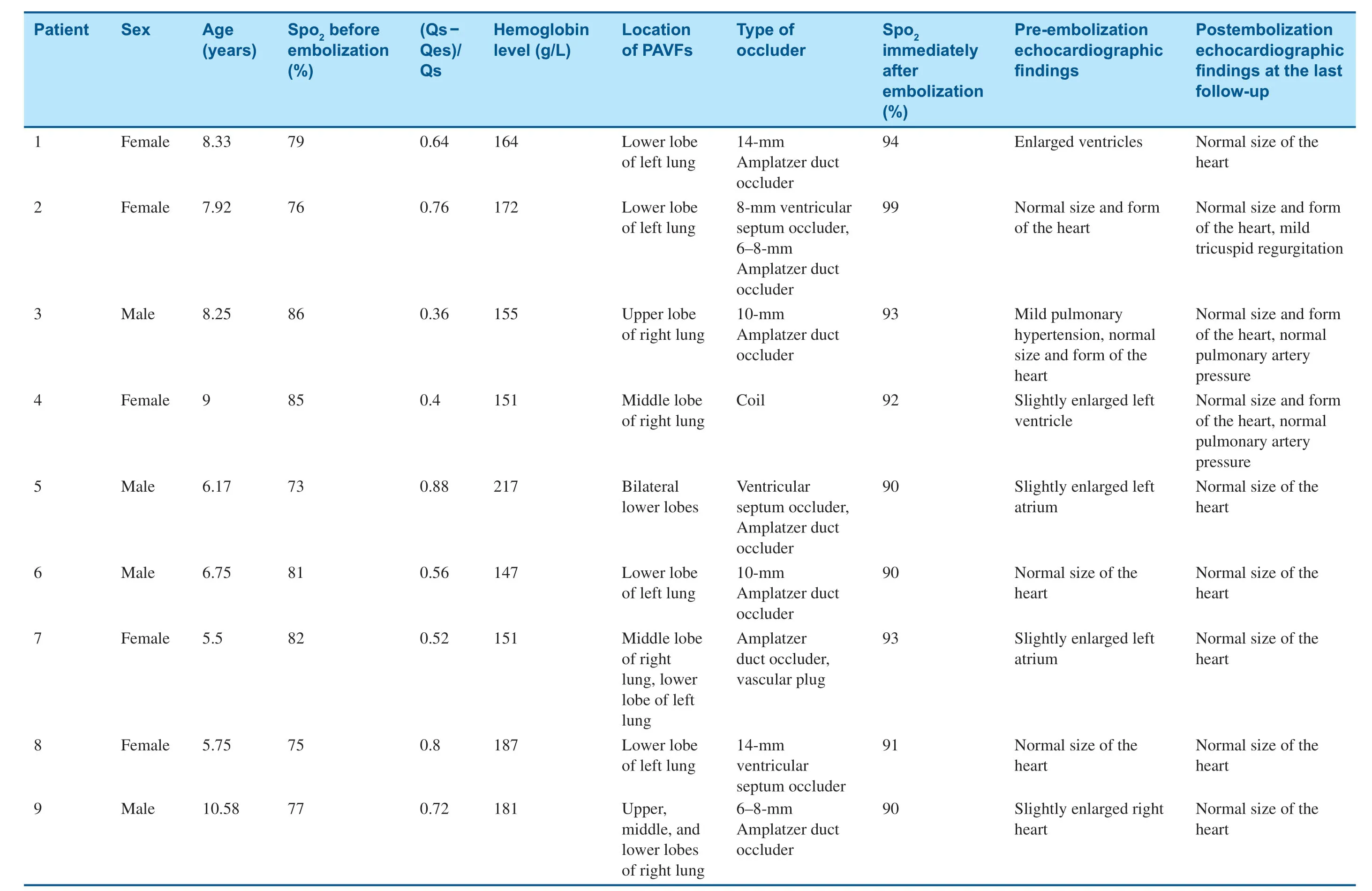
Table2 General Information on,Manifestations in,and Treatment of Nine Patients with Pulmonary Arteriovenous Fistulas (PAVFs).
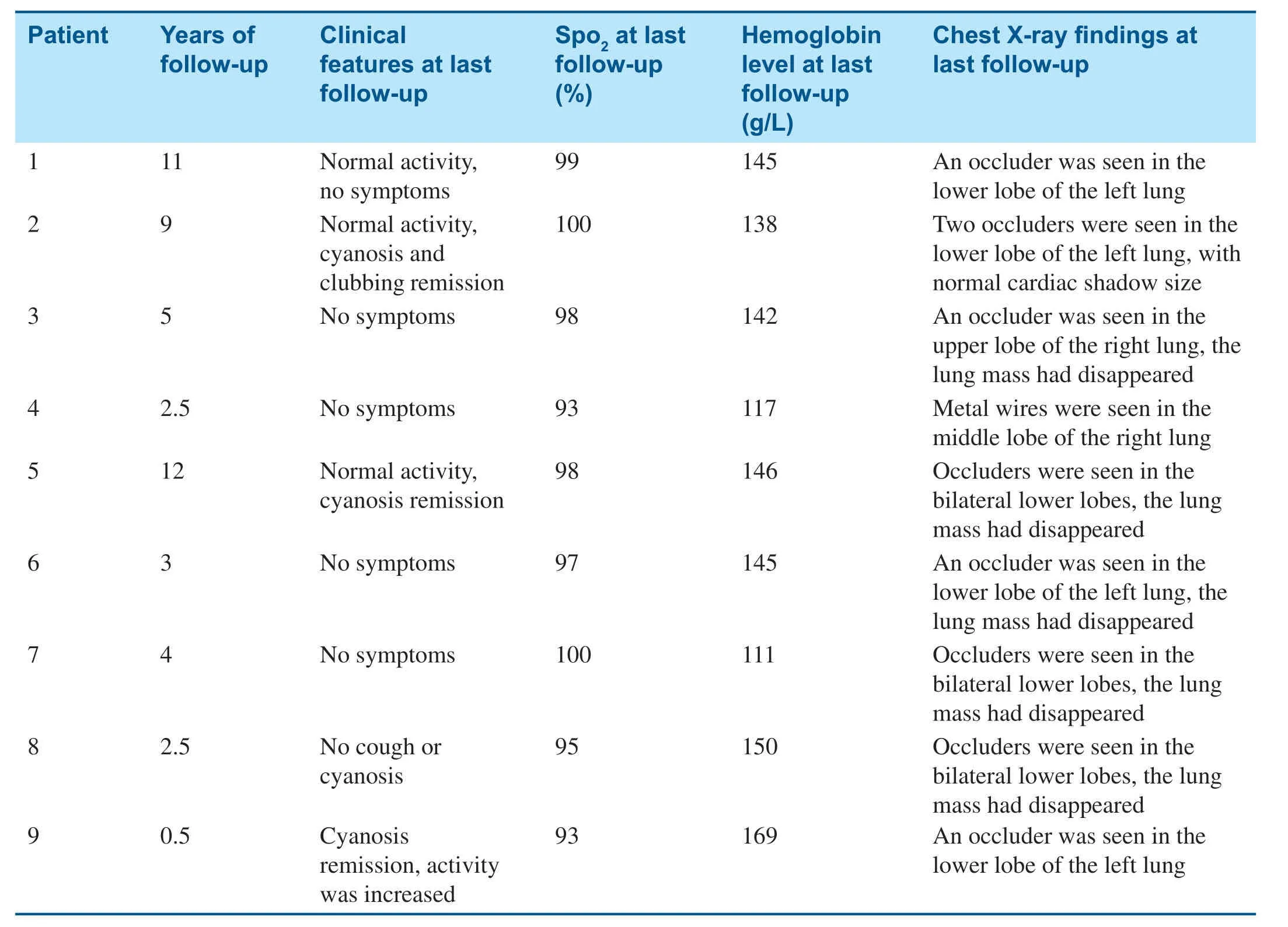
Table3 Manifestations at the Last Follow-up.
PAVF tends to increase in number and size over time,and available data suggest symptomatic patients and asymptomatic patients with lesions less than 2 cm in diameter on chest radiography should be treated [9].The broad indications for treatment include (1) prevention of neurological events,(2)exercise intolerance,and (3) prevention of lung hemorrhage [10].The treatment of PAVF includes surgical resection and embolotherapy.Surgery was the only treatment available until 1978,and traditional surgical procedures include segment resection of the lung and pulmonary lobectomy.During these procedures,not only the lesions but also the neighboring normal lung tissues are resected.For diffuse lesions,surgery cannot be performed and also has the disadvantages of more complications,complex operation,and increased trauma.After Taylor et al.[11] reported the f irst successful percutaneous embolization,it gradually replaced the traditional surgical procedures [12,13].Transcatheter embolotherapy is widely used because it offers the advantages of being less invasive and easily repeated,with a decreased risk of trauma,fewer complications,and a specif ic effect of treatment,and most importantly it can relieve symptoms and simultaneously retain functional tissues.Most PAVFs can be completely occluded through transcatheter embolotherapy,thus relieving the symptoms.For complex PAVF,it is very important to conf irm the origin of the feeding artery before transcatheter embolotherapy.
PAVF embolization is recommended as the f irst-line treatment of PAVFs,and the three major indications for the treatment are (1) prevention of neurological complications,including stroke and cerebral abscess,(2) improvement in exercise tolerance,and (3) prevention of lung hemorrhage [14].Patients with pregnancy,pulmonary hypertension,and kidney failure are not suitable for transcatheter embolotherapy [15].The typical target during the procedure is not the aneurysmal sac itself but the feeding artery.Selective pulmonary angiography needs to be conducted repeatedly to ensure the location of the lesion,the location of normal pulmonary artery branches,and the occluders are placed at the far end of the PAVF without affecting the normal pulmonary artery branches.
The most critical point to avoid displacement of the occluder is to accurately determine the position and diameter of the diseased blood vessel before the operation.Several closure devices,such as balloons,Amplatzer duct occluders,ventricular septum occluders,and coils,have been used for embolization.In coil embolization,it is important to place the catheter tip as close to the neck of the PAVF as possible.Steel coils are used mainly for smaller PAVFs,where it is hard for the microcatheter to reach the lesions because of vascular distortion.Compared with coils,Amplatzer duct occluders,ventricular septum occluders,and balloons are used for occluding larger-diameter feeding arteries.The advantages of these approaches include good controllability,easier occlusion at the neck of the sac,and occlusion over a shorter length of the vessel,thus reducing the likelihood of occluding vessels supplying the normal lung[16,17].
Multiple studies have demonstrated that transcatheter embolotherapy can reduce right-to-left shunting and increase oxygenation [2,17,18].In our study,occlusion was successful in nine patients,with no residual shunts,symptoms such as cyanosis and clubbing disappeared,and the oxygen saturation was maintained at 93- 100%.To our knowledge,the diameter of the occluder should be 150% greater than the narrowest place of the f istula because of the good expansion ability of the pulmonary artery,especially the feeding artery.
The most common short-term complications of transcatheter embolotherapy are contrast medium allergy and hematoma of the puncture site.Transient symptoms such as angina,bradycardia [19],device migration,myocardial rupture,vascular injury,early def lation of the balloon,and deep vein thrombosis [19,20] may be encountered in transcatheter embolotherapy.Patients treated with transcatheter embolotherapy also have a risk of recanalization,which occurs in up to 20% of PAVFs treated with coils [21].Data have shown recanalization rates may be higher in children [22],and a recent study demonstrated that there was no recanalization 6- 40 months after embolization of 28 PAVFs with Amplatzer plugs and coils [23].In our study,the nine patients had no severe complications,but one patient had moderate tricuspid regurgitation immediately after embolotherapy; however,1 month later,it changed to mild tricuspid regurgitation.We speculated it might be associated with temporary tricuspid valve edema after embolotherapy.There was no recanalization during the follow-up period after embolotherapy.
The limitations of the present study include its retrospective design at a single institution,and because of the aperiodic follow-up of some patients,we could not acquire the exact details about when these patients started to recover or when symptoms disappeared.
Conclusion
PAVF is abnormal connections between the pulmonary artery and pulmonary veins.It can cause many symptoms,such as asymptomatic hypoxemia,cyanosis,clubbing,hemoptysis,and hemothorax.Transcatheter embolotherapy is recommended as the f irst-line treatment for PAVFs as it offers the advantages of safety,decreased risk of trauma,and a specif ic effect of treatment.Further research is necessary to fully prove the safety and the feasibility of embolotherapy for congenital PAVFs in children.Pulmonary angiography should be performed as well to determine the type and location of the feeding artery,with careful operation and close follow-up.Selecting an appropriate plugging position and a suitable occluder is the key to success.
Conf ilcts of interest
The authors declare that they have no conf licts of interest.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- WeChat Group of Chest Pain Center for Patients with Acute ST-segment Elevation Myocardial Infarction:Faster Treatment Speed and Better Prognosis
- lmpact of MitraClip Program on the Volume and Outcomes of Mitral Valve Surgery:A Single-Center Retrospective Study
- In-Stent Thrombosis after Antiplatelet Therapy Conversion while Awaiting Coronary Bypass
- Current Management Strategies in Patients with Heart Failure and Atrial Fibrillation:A Review of the Literature
- Comparison of Segmentation Algorithms for Detecting Myocardial Infarction Using Late Gadolinium Enhancement Magnetic Resonance Imaging
- Epicardial Adipose Tissue in Patients with Obstructive Sleep Apnea:A Systematic Review and Meta-analysis